Pharmaceutical Substances Syntheses, Patents, Applications - Part 71 pot
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (220.65 KB, 10 trang )
P
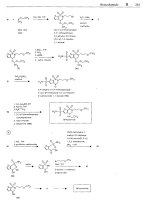
Dolasetron mesilate
D
701
1. sodium borohydride
2.
dihydropyron
1. tBu-OK, toluene. 100°C
2.
HCI. reflux
VIP
HO
(VII)
I,
q
(VIII)
,
CH,CI,, OMAP
0 BCF3
2.
H3C-S03H
M
-
H3C-S03H
I
Dolasetron rnesilote
I
synthesis of intermediate VIlI
+
CH2CIZ
88
F,C
0
CF,
COOH
indole-3- trifluoroocetic
carboxylic acid onhydride
altemotive preporotion via
0-methoxycorbonylglutoroldehyde
dimethyl
molonote
diostereomeric
mixture of
l-methoxycorbonyl-
3-cyclopentene oxide
(trans:cis
N
3:l)
(X)
1.
LiH, OMF
2.
NoOH, MeOH
.
@LCH3
methyl
J-cyclopentene-
1-corboxylote
(M)
VIII
OEH
,
CH2Clz
CI,
X
rn-chloroperbenzoic
ocid
p-methoxycorbonyl-
glutoroldehyde
(XI)
702
D
Domiodol
1.
03, MeOH, H20,
-5
to O°C
2.
H3C-S-CH3
M
XI
A
b
Dolasetron rnesilate
1.
ozone
2.
dirnethyl sulfide
Referencefs):
EP 339 669 (Merrell Dow Pharm. Inc.; appl. 28.4.1989; USA-prior. 29.4.1988).
EP 266 730 (Merrell Dow Pharm. Inc.; appl. 2.1 1.1987; USA-prior. 3.1 1.1986).
Formulation(s):
amp. 12.5 mgl0.625 ml, 100 mg/5 ml;
f.
c. tabl. 50 mg, 200 mg
Trade Name(s):
D: Anemet (Hoechst Marion USA: Anzemet (Hoechst Marion
Roussel; 1997) Roussel; 1997)
Domiodol
ATC: R05CB08
Use: mucoly tic agent
RN:
LD50:
CN:
61869-07-6 MF: C5H,I03 MW: 244.03
79-89 mgikg (M, i.v.); 140-145 mgkg (M, p.0.)
2-(iodomethy1)-
l,3-dioxolane-4-methanol
COOH
H~CVO~~~ H0,S
H3C-0
-
acid
glycerol
1
-benzyl brornoacetaldehyde
(1)
ether
diethyl acetal
HZ, Pd-C
I
+
HO
iodide
Dorniodol
Reference (s):
DOS 2 610 704 (Maggioni; appl. 13.3.1976; I-prior. 2.4.1975)
Formulation(s):
sachet 60 mg; sugar coated tabl. 60 mg; syrup 0.6
%
Trade Natne(s):
I: Mucolitico (Maggioni-
Winthrop)
Domiphen bromide
(Phenododecinium bromide)
ATC: AOlAB06
Use: disinfectant
RN:
538-71-6 MF: C,,H,oBrNO MW: 414.47 EINECS: 208-702-0
LD,,,: 31 mglkg
(M,
i.v.);
18 mgikg (R, i.v.)
CN:
N,N-dimethyl-N-(2-phenoxyethy1)-1-dodecanaminium
bromide
Domperidone
D
703
2-phsnoxyethyl d~methyl-
bromide
(I)
amme
dodecyl bromide
I
Domiphen bromide
I
+
H2N-CH3
b
O/'*W-
/
CH3
dodecylamine
dodecyl(2-phenoxyethy1)omlne
(111)
methyl
brom~de
Reference(s).
1
US
2
581 336 (Ciba; 1952; CH-prior. 1944).
Formulation(s):
tabl. 0.5 mg
L
1
Tmde Name(s):
GB:
Bradosol Plus (Novartis Iodosan Nasale
Oradol (Novart~s-Takeda)
Consumer)-comb.
(SmithKline Beecham)- USA: Bradosol Bromide (Ciba-
3
Bradoral (Zyma) comb. Ge~gy);
wfm
J:
Brado (Novartis-Takeda)-
comb.
Domperidone
ATC: A03FA03
Use: anti-emetic
RN:
57808-66-9
MF:
C22H24CIN502
MW:
425.92 EINECS: 260-968-7
LD,,:
46500 pglkg (M, i.v.);
>8
glkg (M, p.0.);
41700 pglkg (R, I.v.); 5243 mglkg (R, p.0.);
42700 pglkg (dog, i.v.); >I60 mglkg (dog, p.0.)
CN:
5-chloro-1-[1-[3-(2,3-dihydro-2-oxo-1H-benzimidazo-l-yl)propyl]-4-piperidinyl]-1,3-dihydro-2H-
benzirnidazol-2-one
704
D
Domvcridone
r
+
KOCN
4
potassium
cyonote (11)
ethyl 4-amino-
piperidine-1
-
corbaxylate
48%
aq.
HBr
H
b
Hs.
Roney-Ni
+
v
Darnperidone
US
4
066 772 (Janssen; 3.1.1978; prior. 21.7.1975, 17.5.1976).
DE
2
632 870 (Janssen; appl. 21.7.1976;
USA-prior.
21.7.1975).
Formulation(s):
eff. gran. 10 mg; f. c. tabl. 10 mg; suppos. 30 mg; susp.10
mglml;
tabl. 10 mg
Trade Name(s):
D: Motilium
(Byk
Gulden; Motilium (Sanofi Peridon (Fisons;
1979) Winthrop;
1982)
Italchimici)
F:
Motilium (Janssen-Cilag;
I:
Fobidon (Biomedica J: Nauzelin (Kyowa
Hakko;
1983)
Foscama) 1982)
PCridys (Robapharm) Gastronorm (Janssen)
GB:
Domperamol (Servier)- Mod (Irbi)
comb. Motilium (Janssen; 1982)
Donepezil hydrochloride
D
705
Donepezil
hydrochloride
('-2020)
ATC: N06DA02
Use: cognition disorders,
acetylcholinesterase inhibitor
RN: 12001 1-70-3 MF: CZ4H2,NO3
.
HCl MW: 415.96
CN:
2,3-dihydro-5,6-dimethoxy-2-[[l-(phenylmethyl)-4-piperidinylmethyl]-1
H-inden-I-one hydrochloride
base
RN: 120014-06-4 MF: C24H,9N03 MW: 379.50
BuLi,
iPr2NH. THF
OHC
ethyl acetate
H~C-O@=
e
HCI
H3C-0
'
1
-benzyl-4-(5.6-dimethoxy-
1-oxoindan-2-ylidenemethy1)-
pipendine
(I)
I
Donepezil hydrochloride
I
Reference(s):
EP
296 560 (Eisai Co.; appl. 22.6.1988; J-prior. 22.6.1987).
Imura, J.
et
al.: J. Labelled Compd. Radiopharm. (JLCRD4)
27,
835-839 (1989).
Fomulation(s):
tabl. 5 mg, 10 mg
Trade Name(s):
D:
Aricept (EisaiIPfizer)
GB:
Aricept (EisaiPfizer) USA: Aricept (EisaiPfizer)
Dopamine
ATC: COlCA04
Use: sympathomimetic
RN: 51-61-6 MF:
C8HIlNOz MW: 153.18 EINECS: 200-110-0
LD,,: 59 mgkg (M, i.v.)
CN:
4-(2-aminoethy1)-l,2-benzenediol
hydrochloride
RN: 62-31-7 MF: C8H1,NOz.HCl MW: 189.64 EINECS: 200-527-8
LD,,: 156 mgkg (M, i.v.); 4361 mglkg (M, p.0.);
4800
pgkg (R, i.v.); 2859 mglkg (R, p.0.);
79 mgkg (dog, i.v.)
706
D
Dopexamine
hornoveratryl-
arnine
(cf. popaverine
synthesis)
Doparnine
I
Reference(s):
Schopf; Bayerle: Justus Liebigs Ann. Chem. (JLACBF) 513, 196 (1934).
alternative with
HCI:
DE 247 906
(K.
W. Rosenmund et al.; 1909).
Hahn, G.; Stiehl,
K.:
Ber. Dtsch. Chem. Ges. (BDCGAS)
69.
2640 (1936)
FR-appl. 2 332 748 (P. Fabre; appl. 28.1 1.1975).
combination with "nitro"-preparations (for treulmerrt of cardiogenic shock):
DOS 2 649 162 (Natlermann; appl. 28.10.1976).
Formulation(s):
vial 50 mg, 200 mg, 250 mg, 500 mg (for inf. sol.)
Trade Name(s):
D: Dopamin AWD (ASTA Dopamin Solvay (Solvay
Mcdica AWD)
Arzneimittel)
Dopamin Fresenius
F: Dopamine 200 Lucien GB
:
(Fresenius-Klinik)
(Lucien)
Dopamin ratiopharm
Dopamine Nativelle
I:
(ratiopharm)
(Procter
&
Gamble)
J:
USA:
Dopamine Pierre Fabre
(Pierre Fabre)
Intropin (Arnar-Stone);
wfm
Revivan (Astra-Simes)
Inovan (Kyowa Hakko)
generics
Dopexamine
ATC: COICA14
Use: cardiotonic
RN: 86197-47-9 MF: C22H,,N202 MW: 356.51
CN:
4-[2-[[6-[(2-phenylethyl)amino]hexyl]amino]ethyl]-1,2-benzenediol
dihydrochloride
RN: 86484-9 1-5 MF: C,,H,,N,O, .2HCI MW: 429.43
0
Ad
HOOC
0
hornoveratrylarnine
chlaride
monoethyl adipate
ethyl
6-0x0-6-[2-(3,4-dimethoxy- 6-0x0-6-[2-(3.4-dimethoxy-
phenyl)ethylarnino]hexanoote
(I)
phenyl)ethylarnino]hexonoic
acid
(n)
Dornase
alfa
D
707
C
Dopexomme
i
i'
Referencels):
1
EPX!
O6l
(Fisons, appl. 22.7.1982; GB-prior. 5.8.1981,9.10.1981, 17.1 1.1981).
k
1
synthesis
of
11:
/
Kametani,
T.
eta].: Yakugaku Kenkyu (YKKKA8) 37,23 (1966); C.A. (CHABAS)
65,
15320 (1966).
t
%
Formulation(s):
amp. 50 mgJ5 ml for inf
I
1
Tmde Namefs):
D:
Dopacard (Ipsen Pharma; F: Dopacard (IpsenBlotech)
GB:
Dopacard (Speywood; as
f
as hydrochloride)
hydrochloride)
1
Dornase
alfa
f
(rhDNase)
ATC: R05CB 13
Use: cystic fibrosis therapeutic
RN:
143831-71-4 MF: unspecified MW: unspecified
CN:
deoxyribonuclease (human clone 18-1 protein moiety reduced)
Domase alfa is produced by genetically engineered Chinese Hamster ovary cells containing DNA encoding for
thenative human proJein deoxyribunuclease
I.
It is purified by tangentral flow filtration and column
chromatography.
Reference(s):
WO
9
007 572 (Genentech; appl. 12.7.1990; USA-prior. 23 12.1988, 8.12.1989).
Shak,
S.
et
al.:
Proc. Natl. Acad. Sci. USA (PNASA6) 87(23), 9188 (1990).
Formulation(s):
amp. 2.5 mgl2.5 ml
Tmde Namefs):
D: Pulmozyme (Roche) GB: Pulmozyme (Roche) USA: Pulmozyme (Genentech)
Donolamide
(L-671152;
MK-507)
ATC: SOlECO3
Use: antiglaucoma, topical carbonic
anhydrase inhibitor
RN:
120279-96-1 MF: CI0Hl6N2O4S3 MW: 324.45
CN:
(4S-trans)-4-(ethylamino)-5,6-dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-2-sulfonamide
7,7-dioxide
bans-base
RN:
120279-89-2 MF: C10H,,N,04S3
MW:
324.45
708
D
Dorzolamide
manohy drochloride
RN:
130693-82-2
MF:
C,,H,,N2O4S3. HCl
MW:
360.91
maleate
(1:l)
RN: 147600-19-9
MF:
C,,H,,N2O4S3. C4H404
MW:
440.52
crotonic
ocid
4H-thieno[2,3-b]-
thiopyran
(21)
Z-thiophene-
thiol
COOH chloride
3-(2-thienyl-
.
2. tin@')
thio)butanoic chloride
acid
(I)
thiopyron-2-sulfonic
ocid
oxone,
H3C
\IPC*I!-NH~
S
s
CH,Oti
sodium
II
0
0
OH
0
borohydride
5.6-dihydro-4-hydroxy-
6-methyl-4H-thieno-
[2.3-blthiopyran-2-
sulfonarnide 7,7-dioxide (IV)
1.
Tos-CI, pyridine
2.column chiomatogrophy,
seporation of the trans isomer
+
H2N-CH3
b
V
ethylamine
0
resolution with di-p-toluoyi-D-tartaric ocid
1
-propon01
b
0
H*I,CH3
(V)
Darzalomide
Dorzolamide
D
709
1
CIS0,H
o,\
/p
o,\
,p
2
Pa5
H3cp
+
H3C-CN H3c'"'03
3
NH,
i
b
VII
Ritter
OH
reaction
HNKCH3
@
preparation of the optically active thienothiopyran intermediate
6
CH,
K
0
@)
stereoselective synthesis of intermediate
I
2-thiophene- (+)-(R)-B-
thiol lithium methyl-
salt propiolactone
(S)-3-(2-thienyl-
thio)butanoic
acid
Reference(s):
a
US 4 797 413 (Merck
&
Co.; appl. 10.1.1989; USA-prior. 12.12.1984, 19.9.1985, 14.5.1986).
b
EP 617 037 (Merck
&
Co.; appl. 17.3.1994; USA-prior. 22.3.1993, 10.2.1994).
c
JP 06 107 666 (Kanegafuchi Chern.; appl. 19.4.1994; J-prior. 28.9.1992).
d
US 4 968 815 (Merck
&
Co.; appl. 6.1 1.1990; USA-prior. 16.4.1990).
US 4 968 814 (Merck
&
Co.; appl. 6.11.1990; USA-prior. 18.4.1990).
combination with calcium antagonists:
W09 323 082 (Alcon Lab.; appl. 12.5.1993; USA-prior. 13.5.1992).
cotnbination with P-adrenergic antagonists:
EP509 752 (Merck
&
Co.; appl. 14.4.1992; USA-prior. 17.4.1991, 13.2.1992).
EP457 586 (Merck
&
Co.; appl. 16.5.1991; USA-prior. 17.5.1990).
EP 375 319 (Merck
&
Co.; appl. 18.12.1989; USA-prior. 19.12.1988).
Formulation(s):
eye drops 22.3 rng/ml (as hydrochloride)
Trade Name(s):
D:
Trusopt (Chibret)
GB:
Trusopt (Merck Sharp
&
USA: Trusopt (Merck; 1995 as
Dohrne; as hydrochloride) hydrochloride)
710
D
Dosulcpin
Dosulepin
(Dothiepin)
ATC: N06AA16
Use: antidepressant, thymoleptic
RN: 113-53-1 MF: C,,H,,NS MW: 295.45 EINECS: 204-031-2
LD,,,: 3
1
mgkg
(M, Lv.)
CN: 3-dibenzo[b,e]thiepin-1
l(6H)-ylidene-N,N-dimethyl-I-propanamine
hydrochloride
RN: 897-15-4 MF: C,,H2,NS
.
HCI
MW:
331.91 EINECS: 212-978-8
LD,,:
29.2 mglkg (M, i.v.); 209 mglkg
(M,
p.0.);
24 mglkg
(R,
i.v.); 260 mglkg (R, p.0.)
polyphosphoric acid
_____+
11-0x0-6.1
l-di-
magnesium chloride
hydrodibenzo-
[b-elthiepin
RE
618 591 (Spofa; appl. 6.6.1962; CS-prior. 8.6.1961).
Forn~ulation(s):
cps. 25 mg, 50 mg, 75 mg.; sup. 25 mg
Trade
Nume(s):
D: Idom (Kanoldt)
GB:
Prothiaden (Knoll; as
F: Proth~aden (Knoll, as hydrochloride)
hydrochloride)
I: Protiaden (Roots Italia)
Doxapram
ATC: R07ABOl
Use: central respiratory stimulant
RN: 309-29-5 MF: C2,H3,N202 MW: 378.52 EINECS: 206-216-3
LD?,,: 268 mglkg (M,
i.p.1
CN:
1-ethyl-4-[2-(4-morpholinyl)ethyl]-3,3-diphenyl-2-pyrrnlidinone
monohydrochloride monohydrate
RN: 7081 -53-0 MF: C2,H7,N,O,
.
HCI
.
H20 MW: 432.99
LD,,,: 85 mglkg
(M,
i.v.);
270 mglkg
(M,
p.0.);
72 mglkg
(R,
i.v.); 261 mg/kg
(R,
p.0.);
40 mglkg (dog, i.v.); 150 mgtkg (dog, p.0.)