Pharmaceutical Substances Syntheses, Patents, Applications - Part 209 pot
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (244.26 KB, 10 trang )
r
Torasemide
T
2081
Reference(s):
Kingsbury, W.D. et al.:
J.
Med. Chcm. (JMCMAR)
34
(I), 98 (1991).
EP
321
122
(SmithKline Beecham; appl. 30.1 1.1988; USA-prior. 1.12.1987).
WO
9 205 785 (SmithKline Beecham; appl. 23.9.199
1;
USA-prior. 28.9.1990).
Formulation(s):
vial 4 mg (as hydrochloride)
Trade Namers):
D:
Hycamtin (SmithKline GB: Hycamtin (SmithKline USA: Hycamtin (SmithKline
Bcecham) Beecham) Beecham)
F:
Hycamtin (SmithKline
1:
Hycamtin (SmithKline
Beecham) Beecham)
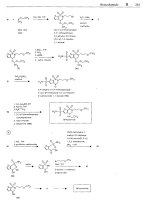
Torasemide
(AC
4464;
BM
0201
5)
Use:
antihypertensive,
loop diuretic
RN: 56211-40-6
MF:
C,,HmN40,S MW: 348.43
CN:
N-[[(1-Methylethyl)amino~carbony1]-4-[(3-methylphcnyl)amino]-3-pyridinesulfonamide
sodium
salt
RN: 72810-59-4
MF:
C,,H,N40,S
.
xNa
MW: unspecified
4-hydroxy-
pyridine
m-taluidine
(111)
3-sulfanomido-
4-(3-rnethylani1ino)-
pyridine
(N)
111,
Cu,
Q5OC
Torasemide
H H
isopropyl
Torasemide
isocyanate
(V)
I
2082
T
Toremifene
Reference
(s):
a,b
Delarge, J.: Arzneim Forsch. (ARZNAD)
38
(I),
la (1988).
DE
2
516 025 (A. Christiaens; appl. 12.4.1975; GB-prior. I7.4.l974).
stable crystalline
modification:
DE 3 623 620 (Boehringer Mannheim; appl. 17.8.1985; D-prior. 17.8.1985).
injections containing
torasemide:
DE 3 623 620 (Boehringer Mannheim; D-prior. 12.7.1986).
rupidly disintegrating pellets:
WO 09 810 754 (Boehringer Mannheim: appl. 9.9.1997; D-prior. 12.9.1996).
polynorphism and control
of
the serum solubility of orally administered
torasemide:
US
5
914 336 (Boehringer Mannheim; 22.6.1999; USA-prior. 2.6.1998).
tablets containing
torasemide:
WO 9 300 097 (Boehringer Mannheim; appl. 25.6.1992; J-prior. 25.6.1991).
use for treatment
of
brain edema:
DE 4 113 820 (Boehringer Mannheim; D-prior. 27.4.1991).
Formulation(s):
amp. 10.631 mg/2 ml, 21.262 mg/4 ml, 212.62 mgl20 ml (as sodium salt); tab1 2.5 mg,
5mg,
10 mg
Trade Narne(s):
D: Torem (Berlin-Chemie) Unat (Roche; 1999)
Toremifene
(FC-
I
157a)
ATC: L02BA02
Use: antiestrogen, antineoplastic
RN:
89778-26-7 MF: C2,H2,CIN0 MW: 405.97
LD,,,: 1700 inglkg (R, p.0.)
CN:
(Z)-2-[4-(4-chloro-1,2-diphenyl-1-butenyl)phenoxy]-N,N-dimethylethanamine
citrate
(1:l)
RN:
89778-27-8 MF: C2,H2,CIN0. C,H807 MW: 598.09
LD,,,:
3
glkg (R, p.0.)
4-hydroxybenzo- 2-(dimethylamino)-
4-[Z-(dimethylamino)ethoxy]-
phenone ethyl chloride
benzophenone
(I)
1.
LiAIHI
+
hcH0
2. recrystollization from acetone
cinnomaldehyde
Tosufloxacin
T
2083
1.
AC20
2.
NoOH
3.
recrystollizotion
from
toluene
u
b
H,c'~+o
FH3
J+J')Q
I
Toremifene
I
Reference(s):
EP
95 875 (Farmos; appl. 20.5.1983; SF-prior. 27.5.1982).
US
4 696 949 (Farmos; 29.9.1987; appl. 29.1.1986; SF-prior. 27.5.1982,9.5.1983).
Formulation(s):
tabl. 20
mg,
60
mg
(as citratc)
Trade Name(s):
D:
Fareston (ASTA Medica
I:
Fareston (Schering-Plough) USA: Fareston (Schering-Plough)
AWD)
J:
Fareston (Nippon Kayaku;
GB:
Fareston (Orion)
as
citrate)
Tosufloxacin
Use: quinolone antibacterial, gyrase
inhibitor
RN: 108138-46-1 MF: C,,H1,F3N4O3 MW: 404.35
CN:
(f)-7-(3-amino- 1 -pyrrolidinyl)- 1
-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-
carboxylic acid
monotosylate
RN: 115964-29-9 MF: C,,H,,F,N,O,
.
C71180,S MW: 576.55
LD,,:
196 mg/kg
(M,
i.v.);
>6
glkg (M, p.0.);
270 mg/kg
(R,
i.v.);
>6
glkg
(R,
p 0.);
>3 gkg (dog, p.0.)
.
1.
CF3COOH,
HCI
C,
N,
CJ
0
CI
N,
CI
2.
SOCl
n-C,HgLi
F
w0,,CH3
A
FwCI
-
monoethyl
rnolonote.
0
u
n-butyllithiurn
ethyl
2,6-dichloro-
5-fluoronicotinate
2.6-dichloro-5-fluoro
nicolinoyl
chloride
2084
T
'
Tralonide
ethyl 2.6-dichloro-5-fluoro- triethyl orthoformote 2.4-difluoro-
(w
nicotinoylacetote
(I)
aniline
2. KOH
1. 3-ominopyrrolidine
ethyl 7-chloro-1-(2.4-di-
Tasufloxocin
1luorophenyl)-6-fluoro-l,4-
dihydro-4-0x0-1,8-
nophthyridine-3-carboxylate
Reference(s):
DE 3 514 076 (Toyama; appl. 31.10.1985; J-prior. 26.4.1984).
US 4 704 459 (Toyama; 3.11.1987; appl. 17.1.1986; J-prior. 23.1.1985, 18.2.1985,7.3.1985, 3.4.1985, 8.5.1985,
14.6.1985).
Chu, D.T.W. et al.: J. Med. Chem. (JMCMAR) 29,2363 (1986).
Narita, H. et al.: Yakugaku Zasshi (YKKZAJ)
106,
802 (1986).
synthesis qf
ethyl
2,6-dichloro-5-fluoronicotinate:
JP 82/72 981 (H. Matsumoto et al.; appl. 7.5.1982).
alternative syntllesis:
EP
302 372 (Abbott; appl. 8.2.1989; USA-prior. 4.8.1987).
BE 904 086 (Toyama; appl. 14.6.1985; J-prior. 23.1.1985).
Formulation(s):
tabl. 75 mg, 150 mg (as tosylate)
-
Trade Name(s):
J: Osex (Toyama; 1990) Tosuxacin (Dainabot;
1990)
Tralonide
ATC: H02AB; R03BA
Use: glucocorticoid
RN: 21 365-49- 1 MF: C24H2,CI,F20, MW: 489.39
CN: (6a,
1
1
P,
16a)-9,11
-dichloro-6,21-difluoro-
16,17-[(1
-methylethylidene)bis(oxy)Jpregna-1,4-diene-3,20-
dione
Tramadol
T
2085
1.
K,CO,. CH,OH
2.
CH,SOZCI, pyridine
3. LiBr. CH3COCH3
b
1.
potassium
corbonate
2. methonesulfonyl chloride
3.
lithium bromide
21 -acetoxy-3,20-dioxo-6a-
fluoro-l6a,l7-isopropylidene-
dioxy-1,4,9(1 1)-pregnotriene
(from fludroxycortide,
q.
v.)
2. ClZ, CHC13. pyridine
I
1.
potassium fluoride,
2.
chlorine
F
Tralonide
DOS 2 225 324 (Syntex; appl. 25.5.1972; USA-prior. 26.5.1971).
starting material:
US 3 282 929 (American Cyanamid; 1.1 1.1966; prior. 6.7.1964).
alternative synthesis:
US
3
409 613 (Syntex; 5.1 1.1968; prior. 28.7.1966).
ZA
680 282 (Syntex; appl. 15.1.1968).
medical use:
US
3
934 013 (Syntex; 20.1.1976; prior. 21.2.1975).
Trade Name(s):
USA: Talidan (Lilly); wfm
ATC: N02AX02
Use: analgesic
RN: 27203-92-5 MF:
C16H2,N02
MW:
263.38 EINECS: 248-319-6
LD,,: 228 mglkg (R, p.0.)
CN:
cis-(lt)-2-[(dimethylamino)methyl]-l-(3-methoxyphenyl)cyclohexanol
hydrochloride
RN: 36282-47-0 MF: C,,H2,N02
.
HCl MW: 299.84 EINECS: 252-950-2
2086
T
Tramazoline
H3C\ H3C\
Mg.
THF
-
Z-dimethylamino-
B
r
MgBr
rnethylcyclohexanane
J-bromo-
anisole
Tramadol
u
Reference(s):
GB 997 399 (Griinenthal; appl. 1.4.1964; D-prior. 2.4.1963),
Formulation(s):
amp. 50 mglml, 100 mg/2 ml; cps. 50 mg; drops 100 mglml; eff. tabl. 50 mg;
s.
r. tabl. 100
mg,
150 mg, 200 mg; suppos. 100 mg; tabl. 50 mg (as hydrochloride)
Trade Name(s):
D: Amadol (TAD)
TRADOL-PUREN (Isis
Puren)
Trama (Kade)
Trama AbZ (AbZ-Pharma)
Trama beta (betapharm)
Tramadol (ASTA Medica
AWD; Dolorgiet;
ratiopharm; Stada;
ct-
Arzneimittel)
Tramadura (durachemie)
Tramagetic (Azupharma)
Tramagit (Krewel
Meuselbach)
Tramal (Griinenthal)
Trama-Sanorania
(Sanorania)
Tramdolar (Hexal)
Tramedphano (medphano)
Tramundin (Mundipharma)
Tramazoline
F: Topalgic (Hoechst HoudC)
GB: Tramake (Galen)
Zamadol SR (ASTA
Medica)
Zyndol SR (Searle)
I: Contramol (Formenti)
Fortradol (Bayer)
J:
Crispin (Kowa)
USA: Ultram (Ortho-McNeil)
ATC: ROlAA09
Use: vasoconstrictor, rhinological
therapeutic
RN: 1082-57-1 MF: CI3HL7N3
MW:
215.30 EINECS: 214-105-6
CN:
4,5-dihydro-N-(5,6,7,8-tetrahydro-
1-naphthaleny1)-1 H-imidazol-2-amine
monohy drochloride
RN: 37 15-90-0 MF: C13H,,N3
.
HC1 MW: 251.76 EINECS: 223-064-3
LD,,,: 11.6 mglkg (M, i.v.); 130 mglkg (M, p.0.);
190 mglkg (R, p.0.)
HzNKNH iodide
s
5-amino-
ammonium
letralin rhodonide
NH2
H2NyNH
1-
+
(1)
ethylene- Tramozoline
Trandolapril
T
2087
Reference(s):
DE 1 173 904 (Thomae; appl. 5.8.196 1).
DE
1
191 381 (Thomae; appl. 24.6.1963; addition to DE 1 173 904).
DE 1 195 323 (Thomae; appl. 24.6.1963; addition to DE 1 173 904).
Formulation(s):
eye drops 0.6 mglml; nasal sprayldrops 1.2 mglml
Trade Name(s):
D: Biciron Augentropfen Rhinospray (Boehringer
I:
Rinogutt Spray (Fher)
(Alcon) k.1
J:
Towk (Tanabe)
Ellatun Nasentropfen
GB:
Dexa-Rhinspray
(
Alcon)
(Boehringer 1ng.)-comb.
Trandolapril
(RU-44570)
ATC: C09AA10
Use: antihypertensive (ACE inhibitor)
RN: 87679-37-6 MF: CxH,,N,O, MW: 430.55
-
LD,,:
>2 g/kg (R, i. v.); >5 g/kg (R, p. 0.);
3 g/kg (M, i. v.); 3.99 g/kg (M, p. 0.);
2
glkg
(dog, p. 0.1
CN:
[2S-[1[R*(R*)],2a,3aa,7a~]]-1-[2-[[1-(Ethoxycarbonyl)-3-phenylpropyl]amino]-l-oxopropyl]octahydro-
1H-indole-2-carboxylic acid
hydrochloride
RN: 87725-72-2 MF: C2,H,,N20S
.
HC1 MW: 467.01
1.
onod.
oxidation,
CH,OH
1
H2.
Pt02.
CH3COOH
2'
H3c-'F
5'-
cN
,
CH~CI~
CH3
+
1
2.
trimethylsilyl
cyonide
H
octohydroindole
octohydroindole-2-
actohydroindole-
carbonitrile
(I)
2-corboxylic
ocid
HOBt,
DCC, DMF.
N-ethylmorpholine
-b
111
(
q
N-[1
(S)-ethaxycorbonyl-
3-phenylpropyll-
L-olonine
(cf.
spiropril
and
irnidapril
syntheses)
2088
T
Tranexamic acid
D:
Gopten (Knoll)
Tarka (Knoll)-comb. with
verapamil hydrochloride
Udramil (Hoechst Marion
Roussel; Pohl-Boskamp)-
comb. with verapamil
hydrochloride
(Dl)
Tranexamic acid
(Acide
tranexamique)
Trandolapril
Udrik (Hoechst Marion Zeddan (Mediolanum)
Roussel; Pohl-Boskamp)
J:
Preran (Hoechst)
F:
Gopten (Ebewe, A; Knoll) USA: Mavik (Knoll Pharmac.)
Odrik (Roussel) Tarka (Knoll Pharmac.)
GB: Gopten (Ebewe, A; Knoll)
Odrik (Roussel)
I:
Gopten (Ebewe, A; Knoll)
Reference(s):
DE
3
15 1 690 (Hoechst AG; appl. 29.12.198 1
;
D-prior. 29.12.198 1)
Formulation(s);
cps. 0.5 mg, 1 mg, 2 mg; tabl.
2
mg,
4
mg
Trade
Narne(s):
ATC: B02AA02
Use: antifibrinolytic, hemostatic
RN: 1197-18-8 MF: C,H,,NO,
MW:
157.21
EINECS:
214-818-2
LDSI,: 1350 mgkg (M,
i.v.);
>10 glkg (M, p.0.);
1200 mglkg
(R,
i.v.);
11 10 mgkg (dog, i.v.);
>5
gkg (dog, p.0.)
CN:
trans-4-(aminomethyl)cyclohexanecarboxylic
acid
CH3
COOH COOH
HZ.
Raney-Co H2/Pt or H2/Ru
+
1
C
N
C
N
4-cyano-
benzoic acid
4-aminomethyl-
benzoic acid
cis-trans
isamerizatian by heating of
the alkali metal salts to 210-240
OC
and
CooH
subsequent separation via salts
of
sulfonic ocid
4-ominomethyl- Tranexomic acid
cyclohexane-
corboxylic ocid
cis/trans mixture
(I)
Tranilast
T
2089
-
-
-
-
-
Reference(~):
syntheses:
Einhorn, A,; Ladisch,
C.:
Justus Liebigs Ann. Chem. (JLACBF)
310,
194 (1900).
Levine,
M.;
Sedlecky,
R.:
J. Org. Chem. (JOCEAH)
24,
115 (1959).
DAS
1
443 755 (Daiichi Seiyaku; appl. 23.12.1964; J-prior. 24.12.1 963).
DAS
I
793 841 (Daiichi Seiyaku; appl. 23.12.1964; I-prior. 24.12.1963).
GB 1202 189 (Kureha; appl. 13.6.1969; J-prior. 14.6.1968, 12.9.1968, 28.12.1968, 17.2.1969).
US
3499 925 (Daiichi ~eiyaku; 10.3.1970: ~-~rior. 23.3.1964, 6.7.1964).
DOS 1 568 379 (Daiichi Seiyaku; appl. 13.4.1966; J-prior. 13.4.1965).
DOS 2 227 504 (Kowa; appl. 6.6.1972).
DAS 2 344 043 (Teijin; appl. 31.8.1973; J-prior. 7.9.1972,30.3.1973).
GB
1
409 938 (Asahi; appl. 29.1 1.1973; J-prior. 29.1 1.1972).
DAS 2 359 251 (Asahi; appl. 28.1 1.1973; J-prior. 29.1 1.1972).
GB
1
410 108 (Asahi; appl. 2.10.1973; J-prior. 2.10.1972).
DAS 2623 130 (Kureha; appl. 22.5.1976; J-prior. 27.5.1975).
Formulation(s):
amp. 250 mg15 ml, 500 mg15 ml; cps. 250 mg, 500 mg; f.
c.
tabl.
Trade Namefs):
D:
Anvitoff (Knoll)
Cyklokapron (Pharmacia
&
Upjohn)
Ugurol (Bayer)
F:
Exacyl (Sanofi Winthrop)
Spotof (CCD)
GB: Cyklokapron (Pharmacia
&
Upjohn)
I:
Amcacid (Bonomelli); wfm
J:
Amcacid (Bonomelli-
Hommel); wfm
Emorhalt (Sigurti); wfm
Trancx (Malesci); wfm
Transil (Malesci)-comb.;
wfm USA:
Ugurol (Bayer);
wfm
500 mg; tabl. 250 mg
Carxamin (Sankyo Zoki)
Hexatron (Nihon Shinyaku)
Rikavarin
(Toyo)
Spiramin (Mitsui)
Tranexamic Acid (Mohan)
Transamin (Daiichi)
Cyklokapron (Pharmacia
&
Upjohn); wfm
Tranilast
ATC: R06
Use: antiallergic
RN:
53902-12-8 MF: C,8H17NOI MW. 327.34
LDlo. 680 mglkg (M, p.o.1;
1
I00 mglkg
(R,
p.0.);
660 mdkg (dog, p.0.)
CN:
2-[[3-(3,4-dimethoxypheny1)-1
-oxo-2-propenyl]amino]benzoic
acid
anthmnilic 3.4-dimethoxy-
I
Tronilast
acid cinnamoyl chloride
Reference(s):
DOS 2 402 398 (Kissei; appl. 18.1.1974; J-prior. 18.1.1973).
US
3
940 422 (Kissei; 24.2.1976; appl. 17.1.1974; J-prior. 18.1.1973).
US 4 070 484 (Kissei; 24.1.1978; prior. l8.l.1973).
Formvlation(s):
cps. 100 mg, eye drops 0.5
%;
gran. 10
%
Trade Name(s):
3:
Rizaben (Kissei; 1982)
Tranylcypromine
ATC: N06AF04
Use: psychoanaleptic, antidepressant
RN: 155-09-9 MF: C9Hl,N MW: 133.19 EINECS: 205-841-9
LD,,,: 64 mgkg (M, p.0.)
CN:
trans-(+)-2-phenylcyclopropanamine
sulfate
(2:l)
RN: 13492-01-8 MF: C,H,,N. l/2H2S04 MW: 364.47 EINECS: 236-807-1
LD,,: 37 mglkg (M, i.v.); 38 mg/kg (M, p.0.)
styrene ethyl ethyl 2-phenyl-
diazoocetate cyclopropone-
carboxylote
trans-2-phenyl-
cyclopropane-
carboxylic acid
(I)
1.
N0N3
2.
HCI
1
-
thionyl
chloride aide
trans-2-phenyl- Tranylcyprornine
cyclopropone-
corbonyi chloride
US
2 997 422 (Smith Kline
&
French; 22.8.1961; prior. 9.1.1959).
DOS 2 649 700 (Nelson Res.
&
Dev.; appl. 29.10.1976; USA-prior. 3 1.10.1 975).
US 4 016 204 (Nelson Res.
&
Dev.; 5.4.1977; appl. 31.10.1975).
Burger, A,; Yost, W.L.:
I.
Am. Chem. Soc. (JACSAT)
70,
2198 (1948).
Formulation(s):
drg. 10 mg; tabl. 10 mg (as sulfate)
Trade Name(s):
D: Jatrosom (Procter
&
GB: Parnate (SmithKline I: Parmodalin (Sanofi
Gamble) Beecham) Winthrop)-comb.
F: Tylciprine (ThCraplix); Parstelin (SmithKline USA: Parnate (SmithKline
wfm Beecham)-comb. Beecham)
Trazodone
ATC: N06AX05
Use: antidepressant, anxiolytic
RN: 19794-93-5 MF: C19H22ClNS0 MW: 371.87 EINECS: 243-317-1
LD
j,,:
9
1
mglkg (M, i.v.); 6 10 mgkg (M, p.0.);
91 mglkg (R, i.v.); 690 mg/kg (R, p.0.)
CN:
2-[3-[4-(3-chlorophenyl)-l-piperazinyl]propyl]-1,2,4-triazolo[4,3-a]pyridin-3(2H)-one
monohydrochloride
RN: 25332-39-2 MF: Cl,H22ClN50~ HCI MW: 408.33 EINECS: 246-855-5
LDs(,: 91 mgkg (M, i.v.); 584 mglkg (M, p.0.);
91 mglkg (R, i.v.); 690 mglkg (R, p.0.);
>40 mglkg (dog, i.v.); 500 mglkg (dog, p.0.)