Ebook Gynecologic oncology clinical practice and surgical atlas: Part 2
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (26.94 MB, 862 trang )
MetastasestotheGynecologicTract
S.DianeYamadaandNitaK.Lee
Metastases to the genital tract may occur as a result of recognizable widely
disseminateddiseasefromanothersiteorasanisolatedlesion.Inthelattercase,
it may be difficult to distinguish between a primary tumor of the gynecologic
tractormetastasestothegynecologictractfromanongynecologicsite.Because
treatment planning and appropriateness of surgery may be dictated by the
primarysiteofthetumor,itisimportanttomakethedistinctionbetweenprimary
and metastatic disease. This chapter focuses on common sites of metastases to
the gynecologic tract, characteristic clinical presentations, and radiologic and
pathologicconsiderationsthatmaybeclinicallyhelpfulintreatmentplanning.
EPIDEMIOLOGY
KeyPoints
1. Metastatic disease to the gynecologic organs most commonly arises from
colorectal,breast,gastric,andappendicealprimarymalignancies.
2. Within the reproductive tract, the ovaries and vagina are the organs most
commonlyaffectedbymetastaticdisease.
3.Malignantmassesorlesionsinthegynecologicorgansshouldbeconsidered
as potential sites of metastases if an established primary malignancy is of
advancedstageordemonstratespoorprognosticfactors.
Metastatic disease to the genital tract from nongenital tract malignancies is
relatively uncommon but is influenced by geographic differences in cancer
incidence. For instance, in Asian countries where gastric cancer is more
common,metastaticdiseasetothegenitaltractismoreprevalent.InJapan,18%
to29%oftumorsfoundinthereproductiveorgansmaybenon-gynecologicin
origin; in Thailand, where cholangiocarcinoma is quite prevalent, 7% of all
metastases to the genital tract may arise from the gallbladder or extrahepatic
biliary tract.1 A single-institution review from the United States of 445,000
accessionedcasesidentified325metastatictumorstothegenitaltractovera32yeartimeperiod;149(45.8%)werefromextragenitalsitesincludingthecolon
andrectum,breast,stomach,andappendix.Additionalprimarysitesincludedthe
bladder, ileum, and cutaneous melanoma. The remaining sites of metastases
originatedfromotherareaswithinthegenitaltractsuchastheendometrium.2
The ovaries and vagina are, by far, the structures most commonly involved
withnongenitaltractmetastases.Althoughpercentagesmayvarybygeographic
area, the most common primary sites of disease metastatic to the ovaries
typically arise from the gastrointestinal (GI) tract (large intestine and stomach,
pancreas, biliary tract, and appendix) and breast. These sites comprise 50% to
90%ofthemetastaticcancerstotheovaries(Table17-1).Althoughthehistology
of a metastatic breast cancer may look uniquely like breast cancer, metastases
from other sites, such as the pancreas and appendix, are mucinous and can be
difficult to distinguish from a primary mucinous tumor of the ovary.
Endometrioid-appearinghistologiesintheovarycanarisefrommetastaticcolon
cancer, and clear cell histology can be confused with signet ring cells from a
gastric cancer or a metastatic clear cell renal carcinoma. In the case of breast
cancer,metastasestotheovarymayremaincompletelyoccultandaredetected
onlyatautopsyorwhentheybecomesymptomatictothepatientoridentifiedon
examination by her physician. With mucinous tumors, the metastases in the
ovary can become quite large, leading to significant symptoms and typically
dominatingtheclinicalpictureforthepatientandtheclinician.
Table17-1MetastaticTumortotheOvaries
Reproductive tract lesions are most likely to reflect metastatic disease when
there is an established nongynecologic primary malignancy, especially if the
primary tumor is advanced or has poor prognostic factors. This is true of
metastaticbreast,pancreatic,andcoloncancer.InthecaseofsomemetastaticGI
tractmalignancies,however,theprimarytumormaynotbefoundformanyyears
afterthemetastasis.Theclassicsignetringcelladenocarcinomaoftheovaryis
calledaKrukenbergtumor,whichrepresentsfewerthan6%to7%ofallovarian
tumorsinWesterncountries.Thesignetringmorphologywasinitiallydescribed
in 1896 by a German pathologist and gynecologist, Friedrich Krukenberg.
However, the extragenital origin of the Krukenberg tumor was not described
until 6 years later. The stomach is the primary site of malignancy in 70% of
casesofKrukenbergtumor.Therouteofspreadtotheovariesisbelievedtobe
lymphatic due to the copious lymphatic plexus surrounding the gastric mucosa
and submucosa. This lymphatic plexus, which communicates with the
lymphatics along the ovarian vessels, provides a direct conduit for even small
gastriccancerstospreadtothehilumandcortexoftheovary.3
Primary appendiceal neoplasms, including low-grade mucinous neoplasms,
signetringadenocarcinomas,andmucinouscarcinoidtumors,alsomayremain
occult until they present with symptomatic ovarian masses or disseminated
mucin consistent with pseudomyxoma peritonei. The rupture site of a primary
low-grade appendiceal neoplasm may be small and contained with fibrotic
mucus.4 When this occurs, the resulting ovarian metastases are frequently
bilateral and occur as a result of implantation of tumor cells and mucin on the
surface of the ovaries, which can then invade into the stroma. If there is
unilateral involvement of the ovary, it is more frequently on the right side,
adjacenttotheappendix.5
Mostpatientswithcolorectalcancer,similartothosewithbreastcancer,will
have their primary malignancy detected before the diagnosis of metastatic
diseasetotheovaries.Incolorectalcancers,only3%ofpatientsinitiallypresent
with an ovarian mass. In general, the majority of primary colon cancers occur
distally in the sigmoid or rectum. In patients who develop ovarian metastases,
most have a primary lesion in the colon that has full-thickness invasion of the
bowel wall, direct invasion into adjacent structures, multiple positive lymph
nodes, and/or involvement of other non-ovarian sites such as the omentum or
liver.6 Although ovarian involvement can occur by direct extension, other
processes such as angio-genesis and stromal cell–cancer cell interaction have
been proposed for the predilection of colorectal cancer to metastasize to the
ovaries. In patients with pancreatic cancer, 4% to 6% will have ovarian
metastasesduringthecourseoftheirdisease.4Inasmallseriesofpatientswith
metastatic pancreatic cancer, all patients had other sites of intraperitoneal
disease, such as the omentum and bowel mesentery, when the ovarian
involvementwasdetected.7
Carcinomas of the extrahepatic bile ducts and gallbladder are far more
commoninAsiancountries.Ovarianmetastasesmaypresentinaheterogenous
manner,withnearlyequalnumberofpatientspresentingatthetimeofprimary
tumordiagnosisandbeforeorafterdetectionoftheprimarytumorsite.Thevast
majority of metastases are bilateral and mucinous, but the tumor may be
infiltrativeorprimarilypresentonthesurfaceoftheovariesandcanbecystic,
solid,ormixedinmorphology.8
Afterthegastrointestinaltract,breastcanceristhemostcommonsiteoforigin
of metastatic disease, especially to the ovaries. Because there are genetic
mutations in BRCA1, BRCA2, and the DNA mismatch repair genes that
predispose women to develop ovarian cancer, distinguishing a primary ovarian
malignancyfromametastaticbreastorcoloncancerinwomenwhoharborthese
geneticmutationsmaycreateadiagnosticdilemma.Nearly10%ofwomenwho
develop breast cancer before the age of 50 years will harbor a mutation in
BRCA1orBRCA2thatwillplacethematriskforovariancancer.9Distinguishing
advanced primary ovarian cancer from metastatic breast cancer is critical in
providing recommendations for the appropriateness of cytoreductive surgery,
chemotherapy,orhormonaltherapy.
Inareviewof79womenwithahistoryofbreastcancerwhopresentedwith
carcinomatosis and underwent surgery, the majority of patients (75%) were
diagnosed with primary ovarian, tubal, or peritoneal cancers.10 Although not
statistically significant, the authors suggested a trend favoring a new primary
ovarian cancer in women with longer intervals since their breast cancer
diagnosis and higher CA-125 values. In autopsy studies, 10% of patients with
breast cancer have ovarian metastases.11 The most significant risk factor for
ovarian involvement is advanced-stage breast cancer. In a series of 31 patients
with stage IV breast cancer who underwent laparoscopy for either an adnexal
mass or therapeutic bilateral salpingo-oophorectomy, 21 patients (68%) were
diagnosed with metastatic breast cancer.12 Conversely, women diagnosed with
early-stage breast cancer are more likely to have benign adnexal disease than
metastaticdiseaseintheirovaries.Inaseriesof129womenwithbreastcancer
who underwent surgery for an adnexal mass, 88% were found to have benign
ovarian cysts; of the remaining patients with malignant lesions, the majority
wereprimaryovariancancersratherthanmetastaticbreastcancer.10
Metastatic melanoma and renal cell carcinoma frequently pose diagnostic
problems.Whenmetastatictotheovariesoruterus,themajorityofpatientswith
melanoma have disseminated disease in other areas. The ovaries represent the
majority (75%) of metastases. Usually, there is a history of removal of a
cutaneous lesion or an ocular lesion. The time span to the development of
metastatic disease that involves the ovaries and becomes clinically significant
maybemanyyears.
Metastasestotheuterus,cervix,vagina,andvulvaareexceedinglyrare,with
individual reports scattered throughout the literature. Primary sites that can
metastasize to the uterine corpus or cervix include breast, stomach, colon,
rectum, melanoma, lung, and kidney.13 In patients with a history of breast
cancer, distinguishing between a primary uterine malignancy and metastatic
breastcancercanbechallengingifthepatienthasreceivedhormonaltherapyfor
her breast cancer. Tamoxifen is associated with known uterine pathology,
including hyperplasias, highly irregular polyps, and primary endometrial
cancers,allofwhichmayalsopresentwithvaginalbleeding.Ingeneral,women
withmetastaticbreastcancertotheuterushaveapoorprognosis,astheuterusis
rarelytheonlysiteofdisseminateddisease.14Isolatedmetastasestothevagina
havebeendescribedinbreast,renal,pancreatic,biliarytract,andcoloncancer.
Reportsofmetastasestothevulvaareevenmoreunusual.
DIAGNOSIS
KeyPoints
1. Metastatic lesions to the reproductive organs typically present with similar
symptoms of primary gynecologic cancers and include abnormal bleeding,
pelvicpain,andbloating.
2. Ultrasound imaging may identify solid and bilateral ovarian masses that are
suggestiveofmetastaticdisease.
3.Inwomenwithpelvicmassesorvaginallesions,elevatedserummarkers,such
as carcinoembryonic antigen (CEA) or CA–19-9, may suggest a
nongynecologicprimarymalignancy.
Wheneverapatienthasahistoryofcancerandpresentswithamassorlesionin
thegynecologictract, metastaticdiseasemustbeconsideredin thedifferential.
Patients with metastatic disease to the ovaries are frequently younger than
patients with primary ovarian cancer. On average, patients with Krukenberg
tumorsareinthe40-to50-yearagerange.15Symptomsassociatedwithovarian
involvement can include abdominal bloating, abdominal or pelvic pain, and
weightloss.Gastriccancers,becauseofluteinizationoftheovarianstroma,may
produce virilization or, on occasion, irregular vaginal bleeding.16Occasionally,
thepatientmaybeasymptomaticandhaveamassdiscoveredonroutinephysical
examination.17Thiscanoccurwithmetastaticbreastcancer.In30%ofcasesof
metastaticdiseasetotheovaries,themassmaybetheinitialpresentingfeature
before the diagnosis of the actual primary tumor site.16 Any metastatic tumor
that involves the uterus, cervix, vagina, or vulva may lead to symptoms of
irregularorpostmenopausalbleeding,discomfortduetothepresenceofamass,
orpain.
Symptoms or the finding of an unexplained mass in the reproductive tract
should trigger a diagnostic work-up in the form of imaging and appropriate
laboratory studies. Ultrasound or CT is usually the initial imaging study
performed. Features of ovarian tumors on ultrasound that suggest a metastatic
origin include bilateral involvement of the ovaries, a solid appearance, and a
differentialinthesizeoftheovaries.Thesefeaturesoccurin80%ofpatientsof
Krukenberg tumor. When there is a combined solid and cystic component, or
cystic features only, distinction from a primary ovarian cancer becomes
challenging(Figure17-1).OnCTscanorMRI,manyofthesamefeaturesfound
onultrasoundwillbepresent,includingaprimarilysolidcomponentorsolidand
cystic components with septations (Figure17-2). In the face of bilateral cystic
ovarian masses and copious fluid on imaging studies, a low-grade appendiceal
neoplasmresultinginpseudo-myxomaperitoneishouldbesuspected.
FIGURE17-1.TransvaginalultrasoundofKrukenbergtumorinvolvingtheleft
ovary,metastaticcholangiocarcinoma.
FIGURE17-2.CTscan,Krukenbergtumorinvolvingovaries,metastatic
colorectalcarcinoma.
CTscanmayidentifytheprimarysiteofdiseaseifasuspiciousmassisfound
elsewhere in the GI tract. In addition, a radiographic abnormality on upper GI
seriesmaysuggestanunderlyinggastriccancer.However,tumorsinthegastric
mucosa may be quite small when they metastasize and remain undetected for
many years. Endoscopic examination may miss a small tumor that has
extensivelyinfiltratedintothesubmucosa.Tumorsarisingfromthepylorusmay
alsobedifficulttodetectgiventheirlocation.
Serummarkersmayhelptodistinguishtheprimarysiteofdisease.CA-125is
elevated in 70% of advanced-stage ovarian cancers. Although CA-125 may be
elevated in a patient with a Krukenberg tumor, it may not be elevated to the
degreeofepithelialovariancancers.18CEAisamarkerforcolon,appendiceal,
andgastriccancers,whereasCA–19-9canbeamarkerforpancreaticcancer.A
CA-125toCEAratioofgreaterthan25hasalsobeenusedtohelpdistinguish
ovarianfrommetastaticcolorectalcancerwithanoveralltestaccuracyof94%.19
Metastatic lesions to the ovary from the breast frequently present as solid
masses or generalized ovarian enlargement in postmenopausal women. This
occurs more frequently in women who have stage IV breast cancer. When the
masses are cystic and solid, however, differentiating a primary ovarian cancer
from a metastasis is more difficult. Positron emission tomography (PET)-CT,
whichisoftenusedinbreastcancerstagingandrestaging,candetectincidental
lesions in the pelvis that require further investigation. The sensitivity and
specificity of PET for metastatic lesions to the ovary is not well established.
Although the standardized uptake value (SUV) is higher in breast cancer
metastasestotheovariescomparedwithGIcancers,theseresultsarebasedon
verylimitednumbersofpatientswithoverlappingSUVlevelsandshouldnotbe
usedtodistinguishprimarycancersfrommetastaticdiseaseatthistime.20
PATHOLOGY
KeyPoints
1. Mucinous adenocarcinomas of the ovary may represent metastases from a
primarymalignancyinthegastrointestinaltractorpancreas.
2. Krukenberg tumors are characteristically bilateral, solid, and exhibit a
bosselatedoutersurface.
3. Immunohistochemical profiling, including CK7, CK20, CDX2, and S100,
maydistinguishaprimarygynecologicmalignancyfrommetastaticdisease.
Themajorityofprimaryovariancancersareofpapillaryseroushistology.Inany
instance where the ovarian cancer is a mucinous or endometrioid histology, it
may be challenging to distinguish a primary malignancy of the ovary from a
metastatictumor.Metastaticcolorectalcancerscanmimicprimaryendometrioid
ovariancarcinomas,whereasmetastaticappendicealandpancreaticcancerscan
beconfusedwithprimarymucinousormucinousborderlinetumorsoftheovary.
Frequently,however,primarymucinousorendometrioidcancersoftheovaryare
unilateral, not bilateral. When the tumor is bilateral, or small (< 10 cm) and
unilateral,metastaticdiseaseshouldbesuspected.TheclassicKrukenbergtumor
haspathologiccriteriadefinedbytheWorldHealthOrganizationtoincludethe
presenceofmucin-producingsignetringcells,stromalinvolvement,andovarian
stromal sarcomatoid proliferation.21 The intracytoplasmic mucin of the signet
ring cells typically stains with mucicarmine or a periodic acid-Schiff stain.
AlthoughKrukenbergtumorshavetypicallybeenclassifiedasmetastaticgastric
cancers,morerecentlythetermhasbeenappliedtoallmetastaticGIcancersand
can include colon or pancreatic cancers as well as metastatic tumors of any
nongenitaltractorigin.Whentheyresultfrommetastaticgastriccancer,theyare
grosslysolidwithasmoothnodularorbosselatedoutersurface(Figure17-3A).
When cut, the surface is white or tan with areas of red or brown discoloration
and a firm or gelatinous appearance (Figure17-3B).Histologically,thetumors
haveaninfiltrative,irregulargrowthpatternwithsingle-cellinvasion,signetring
cells,andsurfacemucin(Figure17-4A,4B).22Metastaticmucinouscancerstend
to have a multinodular growth pattern that involves the ovarian surface. The
presenceofnecroticdebrisor“dirtynecrosis,”ahigherdegreeofnuclearatypia
in the well-formed glands, and desmoplasia are features of metastatic colon
cancer and can be used to distinguish a metastatic lesion from a primary
mucinous or endometrioid cancer (Figure17-5A-C).11 The size of the ovarian
metastasis does not seem to be a distinguishing factor in metastatic colorectal
cancer,asthelesionscangetquitelarge(>10cm)andbeunilateral.6
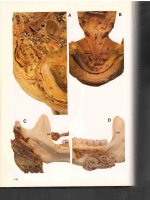
FIGURE17-3.A.Krukenbergtumor,gastriccancer,grosssection.B.
Krukenbergtumor,gastriccancer,cutsurface.
FIGURE17-4.A.Krukenbergtumor,metastaticgastriccancer,AE1/AE3stain,
×40.B.Krukenbergtumor,metastaticgastriccancer,AE1/AE3stain×400.
FIGURE17-5.A.Krukenbergtumor,coloncancer,grosssection.B.Metastatic
coloncancerinthestromaofthefallopiantubedemonstratingdesmoplasia.C.
Metastaticcoloncancerinvolvingtheovary,lowpower.D.Metastaticcolon
cancerinvolvingtheovary,highpower.
Pancreaticductaladenocarcinomasandcholangio-carcinomas(Figures17-6A
and17-6B)areanothersourceofmucinoustumors.Fourto6%ofpatientswith
pancreatic cancer will have metastases to the ovaries. They more commonly
arise from the tail of the pancreas and may be confused with primary ovarian
cancers, especially when there is diffuse peritoneal and omental involvement.
The ovaries may become quite large (average size, 12.5 cm) and contain large
mucinouscystswithsmallerglandsintheinterveningstroma.23
FIGURE17-6.A.Metastaticcholangiocarcinomatotheovary,lowpower.B.
Metastaticcholangiocarcinomatotheovary,highpower.
The classification of appendiceal mucinous tumors has gone through some
evolution.Tumorsthatareconfinedtotheappendixhavebeencalledadenomas
or low-grade appendiceal mucinous neoplasms. If there is a breach in the
muscularis mucosa, the tumor has been called a mucinous tumor of uncertain
malignant potential. Tumors with high-grade cytology or with destructive
invasion of the appendiceal wall are called appendiceal adenocarcinomas or
invasive adenocarcinomas.24 Primary appendiceal carcinomas are quite rare,
representing fewer than 1% of all GI tract cancers. The primary tumor in the
appendixmaybesmall,ortheappendixmayrupture,leadingtoobliterationof
the tumor altogether. In pseudomyxoma peritonei, there is diffuse gelatinous
material present in the peritoneal cavity. Mucinous low-grade appendiceal
neoplasms may produce secondary neoplasms in the ovary. Most commonly,
thereisbilateralinvolvementoftheovaries;ifunilateralinvolvementisfound,it
isusuallyrightsided.Oncutsectionoftheovary,thereismucinousmaterialin
the stroma of the ovary (pseudo-myxoma ovarii) and multiple cysts lined by
low-grade, bland-appearing mucinous cells with minimal nuclear atypia. In a
small number of cases, primary appendiceal carcinomas in the form of signet
ringhistologycanmetastasizetotheovariesasaKrukenbergtumor.
Application of an immunohistochemical profile using cytokeratin stains and
othermarkerscandistinguishtheprimarysiteofdisease(Table17-2).Primary
ovarian carcinomas are typically cytokeratin (CK) 7 positive and CK 20
negative. In contrast, colon cancer is typically CK 7 negative and CK 20
positive. Other GI primaries such as appendiceal, gastric, and small intestinal
cancers,likeovariancancer,canbeCK7positive.CA-125isexpressedinthe
majority of papillary serous ovarian cancers but is also positive in half of
mucinous ovarian cancers, making it a less useful immunostain for mucinous
tumors. Although CEA is positive in most mucinous tumors, this can occur
regardless of whether the origin is ovarian or GI, also making it a less useful
distinguishingmarker.CDX2isanucleartranscriptionmarkerthatisfrequently
positive in lower GI tract cancers, which can be helpful in differentiating a
metastaticGIprimaryfromaprimarymucinousovariancancer.25Otherstudies
ofmucinousmarkerssuchasMUC2andMUC5AChaveshownvariableresults.
S100 is a marker for melanoma that is characteristically diffusely positive in
metastatic lesions. Although immunostain profiles can be helpful, they are not
mutually exclusive, and the patient’s entire clinical picture must be taken into
accountwheninterpretingtheresultsofstains.
Table17-2ImmunostainProfileofPrimaryVersusMetastaticCarcinomas
InvolvingtheOvaries
In autopsy studies, metastatic breast cancer has been found in the ovaries in
10%ofpatients.Lobularcarcinomashaveagreaterpropensitytospreadtothe
ovaries than infiltrating ductal carcinomas; however, given the higher overall
frequencyofductalcarcinomas,mostmetastasestotheovarieswillbeductalin
nature.Theovariesmaybequitesmallwheninvolvedwithmetastaticdisease.If
thehistologyoftheovariancancerispapillaryserous,itismorelikelytobea
separateprimaryovariancancer.Lobularcarcinomascanpresentwithsolidnests
of tumor that may appear similar to adult type ovarian granulosa cell tumors.
Ductalcarcinomascanbechallengingtodifferentiatefromadenocarcinomasof
the ovary or peritoneum and often require further work-up, including specific
immunohistochemical stains. Breast carcinomas tend to have a similar
cytokeratin profile to ovarian cancers and are often CK 7 positive and CK 20
negative.CA-125,WT1,andgrosscysticdiseasefluidprotein15(GCDFP-15)
maybehelpfulindistinguishingprimaryovariancancerfrommetastaticbreast
cancer. In primary ovarian cancers, CA-125 is positive in 92% of cases, and
WT1 is positive in 76% of cases, whereas GCDFP-15 tends to be negative in
comparison with the breast cancer lesions.26 More recently, PAX8, a
transcription factor necessary for organogenesis in the thyroid, kidney, and
Mullerian system, has been studied with WT1 to distinguish ovarian cancers
from breast cancers. PAX8 staining performed better than WT1 and was
diffuselypositivein87%ofknownovariancancers,whereasnoneofthebreast
cancersstainedpositiveforPax8.27Althoughitisarareoccurrence,whenbreast
cancermetastasizestotheuterus,itofteninvolvesthemyometriumandstroma
whilesparingtheendometrialglands.
Renalcellcarcinomametastatictotheovariesisquiterare,butwhenitoccurs,
itisinvariablyoftheclearcelltype.Therefore,makingadistinctionbetweena
primaryovarianclearcellcancerfromametastaticrenalcellcarcinomabecomes
adilemma.Inprimaryclearcellcancersoftheovary,thereisaheterogeneityin
the appearance of the cells, as there is an admixture of flattened cells, hobnail
cells, and cuboidal cells. Primary clear cell cancers of the ovary may also be
associatedwithendometriosis.Inmetastatictumors,thereismorehomogeneity
totheclearcells,andtheremaybeasinusoidalvascularpattern.PositiveCK7
immunostaining may help to distinguish a primary ovarian cancer from a
metastaticrenalcellcancer,whichtendstobeCK7negative.28
Cutaneous malignant melanoma can metastasize to nearly any organ in the
body.Approximately20%ofsuchpatientsdeveloprecurrentdisease.However,
metastases to the genital tract are exceedingly rare and represent only 2.5% of
cases. Although the most frequent sites of recurrence are the primary site of
diseasefollowedbytheregionallymphnodes,visceralspreadcanoccur.Inthe
genitaltract,theovariesarethemostcommonmetastaticsite,butmetastasesto
theendometriumandmyometriumoftheuterushavealsobeendescribed.
Only30%ofovarianmetastasesarepigmented.ImmunostainssuchasS-100,
HMB-45, and MART1 are positive in most melanomas and may be helpful in
identifyingtheprimarysitesofdisease.11
TREATMENTANDPROGNOSIS
KeyPoints
1.Surgicalresectionofmetastaticlesionsshouldbeconsideredinsymptomatic
patientswithgoodperformancestatusandisolatedmasses.
2. Limited evidence suggests a potential survival benefit with resection of
metastaticdiseaseincolorectal,pancreatic,andbreastcancers.
The treatment of patients with metastases to the genital tract depends on the
performancestatusofthepatient,whetherthepatienthasaknownprimarysite
of disease, and whether the metastatic lesions are isolated or diffusely
disseminated.Overall,thesurvivalofpatientswithmetastasestotheovariesis
poor. Nearly 80% of patients succumb to their disease within 2 years. Despite
this,metastatictumortotheovariescancreatesignificantsymptomstowarrant
removalforpalliation.Inonerecentretrospectiveseriesofpatients,therewasa
significantdifferenceinsurvivalifpatientshadisolatedmetastasestotheovaries
or disseminated disease. In patients with isolated ovarian metastases, median
survivalwas30.7monthsascomparedwiththosewithextensive,disseminated
disease,forwhomthemediansurvivalwas10months(P =.02). Patientswith
colon cancer had longer survivals compared with patients with gastric cancer
(29.6monthsvs.13months,respectively),andpatientswhounderwentsurgery
and were left with microscopic disease also experienced longer survival as
compared with those left with visible residual disease.29 In another study of
metastatic pancreatic cancer to the ovaries, the mean patient age was only 49
years.Thosewhounderwentresectionoftheirovarianmetastaseshadamedian
survival of 16.5 months, as compared with 8.5 months in those who received
chemotherapy alone. In patients who received only chemotherapy, the ovarian
metastases did not respond to treatment.23 Patients with known ovarian
metastasesfromcoloncanceralsoshowedalackofresponsetochemotherapy,
whichmaysuggestthattheovariesareasanctuaryformetastaticdisease.30Ina
Korean study of patients with a history of gastric cancer who developed
Krukenberg tumors, the median age was also only 41 years. Patients who
underwenteitherbilateralsalpingo-oophorectomyorhysterectomywithbilateral
salpingo-oophorectomy had median survivals of 10.9 months if there was no
gross residual disease or 7.5 months if left with gross residual disease. There
were 2 patients in this series of 34 patients who survived longer than 4 years
after complete resection of their Krukenberg tumors. Additionally, there was a
significantdifferenceifthepatienthaddiseaseconfinedtotheovaries(median
survival,13.1months)ascomparedwithdiseaseinthepelvis(mediansurvival,
7.5 months) or intra-abdominal disease (median survival, 3.6 months).31
Therefore,areasonableapproachinapatientwithgoodperformancestatusand
isolatedmetastaticdiseasewouldbetoconsidersurgerytoremovetheovaries.
ManyofthesametreatmentstrategiesobservedinmetastaticGIcancerhold
trueformetastaticbreastcancer.Severalretrospectivestudieshaveidentifieda
trend toward longer progression-free survival in women who can be optimally
debulkedeveninthesettingofmetastaticbreastcancer.Thistypicallyoccursin
the setting of isolated metastatic disease and in patients without evidence of
othermetastaticdisease.Gargetal32showedthatin19patientswhowerefound
to have abdominal carcinomatosis due to breast cancer, those undergoing
successfulcytoreductivesurgery(5patients)hadalongermediansurvivaltime
thanthosewithlargervolumeresidualdisease(14patients;34.4vs.3.9months;
P=.0001).32Inanothersmallstudyof29patients,62%underwentnonoptimal
surgery or biopsies and the remainder underwent complete resection of
metastaticdisease;mediansurvivalwas2yearsintheformergroupandhadnot
been reached at 2 years of follow-up in the latter group.33 The subsequent
development of metastatic disease in other sites such as the pelvis and liver
occurredinthemajorityofpatientsaftersurgery.Thisshouldbeconsideredin
counselingpatientsabouttherisksandbenefitsofundergoingsurgeryandeffect
on palliation of symptoms. As chemotherapy regimens improve for
nongynecologiccancers,however,thesurvivalofthesepatientsmaybeexpected
toimprove.
Management of metastases to other areas of the genital tract will clearly
depend on the patient’s overall prognosis and symptoms. If a patient develops
significantproblemswithvaginalbleedingfromuterineorcervicalmetastases,it
wouldbereasonabletoperformahysterectomy.Acompletehysterectomyalso
allowsfordefinitivediagnosisofthedisease.Metastasestothevaginaandvulva
must be managed individually and with attention paid to minimizing
complicationsinthefaceofadiseasewithpoorprognosis.Primaryresectionand
radiation have been described in the management of disease involving the
vagina, but the numbers of patients treated are too small to provide any
meaningfulconclusionsabouttheeffectivenessoftreatment.
FUTUREDIRECTIONS
In conclusion, patients who develop metastatic disease to the genital tract
frequentlyhaveprimarymalignanciesarisingfromtheGItractorbreast,andthe
primary disease is usually advanced stage. Ovarian metastases are frequently
bilateral;mucinoushistologies,inparticular,maybedifficulttodistinguishfrom
primarymucinousovariancancers.Surgerytocyto-reducethemetastaticlesions
appeartobeassociatedwithlongersurvivalifthemetastaticdiseaseisisolated
and able to be completely cytoreduced. As molecular markers improve, it may
become easier to distinguish primary gynecologic malignancies from
nongynecologic malignancies. Although it would be preferable to have serum
markers that could adequately determine the primary site of disease, often the
decision to operate or treat with chemotherapy or hormonal therapy tailored to
the primary site of disease becomes a function of patient performance status,
symptoms,andthepresenceofothersitesofdisease.
REFERENCES
1.KhunamornpongS,SuprasertP,ChiangmaiWNA,etal.Metastatictumorsto
theovaries:astudyof170casesinnorthernThailand.IntJGynecolCancer.
2006;16:132-138.
2. Mazur MT, Hsueh S, Gersell DJ. Metastases to the female genital tract.
Analysisof325cases.Cancer.1984;53:1978-1984.
3. Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor. Arch
PatholLabMed.2006;130:1725-1730.
4. Hart WR. Diagnostic challenge of secondary (metastatic) ovarian tumors
simulating primary endometrioid and mucinous neoplasms. Pathol Int.
2005;55:231-243.
5.YoungR,GilksB,ScullyR.Mucinoustumorsoftheappendixassociatedwith
mucinous tumors of the ovary and pseudomyxoma peritonei: a
clinicopathologic analysis of 22 cases supporting an origin in the appendix.
AmJSurgPathol.1991;15:415-429.
6. Lewis MR, Euscher ED, Deavers MT, Silva EG, Malpica A. Metastatic
colorectaladenocarcinomainvolvingtheovarywithelevatedserumCA125:a
potentialdiagnosticpitfall.GynecolOncol.2007;105:395-398.
7.YoungRH,HartWR.Metastasesfromcarcinomasofthepancreassimulating
primary mucinous tumors of the ovary: a report of seven cases. Am J Surg
Pathol.1989;13:748-756.
8. Khunamornpong S, Lerwill MF, Siriaunkgul S, et al. Carcinoma of the
extrahepaticbileductsandgallbladdermetastatictotheovary:areportof16
cases.IntJGynecolPathol.2008;3:366-379.
9. Kwon JS, Gutierrez-Barrera AM, Young D, et al. Expanding the criteria for
BRCA mutation testing in breast cancer survivors. J Clin Oncol.
2010;28(27):4214-4220.
10.Simpkins F,Zahurak M,ArmstrongD,etal.Ovarian malignancy inbreast
cancerpatientswithanadnexalmass.ObstetGynecol.2005;105:507-513.
11.YoungRH.FromKrukenbergtotoday:theeverpresentproblemsposedby
metastatictumorsintheovary.PartII.AdvAnatPathol.2007;14:149-177.
12.QuanML,FeyJ,EitanR,etal.Roleoflaparoscopyintheevaluationofthe
adnexa in patients with stage IV breast cancer. Gynecol Oncol.
2004;92(1):327-330.
13. Kumar NB, Hart WR. Metastases to the uterine corpus from extragenital
cancers:aclinicopathologicstudyof6cases.Cancer.1982;50:2163-2169.
14. Hara F, Kiyoto S, Takabatake D et al. Endometrial metastasis from breast
cancerduringadjuvantendocrinetherapy.CaseRepOncol.2010;3:137-141.
15.YookJH,OhST,KimBS.Clinicalprognosticfactorsforovarianmetastasis
inwomenwithgastriccancer.Hepatogastroenterology.2007;54:955-959.
16. Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary. A
clinicopathologic analysis of 120 cases with emphasis on their variable
pathologicmanifestations.AmJSurgPathol.2006;30:277-299.
17. Moore RG, Chung M, Granai CO et al. Incidence of metastases to the
ovariesfromnongenitaltractprimarytumors.GynecolOncol.2004;93:87-91.
18. Lewis MR, Deavers MT, Silva EG, Malpica A. Ovarian involvement by
metastaticcolorectaladenocarcinoma:stilladiagnosticchallenge.Am J Surg
Path.2006;30:177-184.
19.YedemaCA,KenemansP,WobbesT,etal.Useofserumtumormarkersin
the differential diagnosis between ovarian and colorectal adenocarcinomas.
TumourBiol.1992;13:18-26.
20. Kitajima K, Suzuki K, Senda M, et al. FDG PET/CT features of ovarian
metastasis.ClinRadiol.2011;66:264-268.
21.SerovSF,ScullyRE.Histologictypingofovariantumours,vol.9.Geneva,
Switzerland:WorldHealthOrganization;1973:17-18.
22. Lee KR, Young RH. The distinction between primary and metastatic
mucinouscarcinomasoftheovary:grossandhistologicfindingsin50cases.
AmJSurgPathol.2003;27:281-292.
23.FalchookGS,Wolff RA, VaradhacharyGR.Clinicopathologic featuresand
treatment strategies for patients with pancreatic adenocarcinoma and ovarian
metastases.GynecolOncol.2008;108:515-519.
24. Misdraji J. Appendiceal mucinous neoplasms. Controversial issues. Arch
PatholLabMed.2010;134:864-870.
25.VangR,GownAM,WuLSF.ImmunohistochemicalexpressionofCDX2in
primary ovarian mucinous tumors and metastatic mucinous carcinomas
involving the ovary: comparison with CK20 and correlation with coordinate
expressionofCK7.ModernPathol.2006;19:1421-1428.
26.TornosC,SoslowR,ChenS,etal.ExpressionofWT1,CA125andGCDFP15 as useful markers in the differential diagnosis of primary ovarian
carcinomas versus metastatic breast cancer to the ovary. Am J Surg Pathol.
2005;29:1482-1289.
27.NonakaD,ChiribogaL,SoslowRA.ExpressionofPax8asausefulmarker
in distinguishing ovarian carcinomas from mammary carcinomas. Am JSurg
Pathol.2008;32;10:1566-1571.
28.McCluggageWG,WlkinsonN.Metastaticneoplasmsinvolvingtheovary:a
review with emphasis on morphological and immunohistochemical features.
Histopathology.2005;47:231-247.
29. Joang R, Tng J, Cheng X, Zang RY. Surgical treatment for patients with
differentoriginsofKrukenbergtumors:outcomesandprognosticfactors.Eur
JSurgOncol.2009;35:92-97.
30.GoereD,DaveauC,EliasD,etal.Thedifferentialresponsetochemotherapy
of ovarian metastases from colorectal carcinoma. Eur J Surg Oncol.
2008;34:1335-1339.
31. Kim HK, Heo DS, Bang YJ, Kim NK. Prognostic factors of Krukenberg’s
tumor.GynecolOncol.2001;82:105-109.
32. Garg R, Zahurak ML, Trimble ET, et al. Abdominal carcinoma-tosis in
womenwithahistoryofbreastcancer.GynecolOncol.2005;99:65-70.
33. Bigorie V, Morice P, Duvillard P, et al. Ovarian metastases from breast
cancer.Cancer.2010;799-804.
34. Prat J. Ovarian carcinomas, including secondary tumors: diagnostically
challengingareas.ModPathol.2005;18:S99-S111.
PartIIIClinicalManagementTopics
PerioperativeandCriticalCare
PrinciplesofRadiationTherapy
PrinciplesofChemotherapy
TargetedTherapyandImmunotherapy
IntegrativeOncology,QualityofLife,andSupportiveCare
SurgicalInstrumentationandSutures
PerioperativeandCriticalCare
RenataUrbanandLee-mayChen
PREOPERATIVERISKEVALUATION
A thorough preoperative historyandphysicalshouldbetakenfromallpatients
undergoing surgery. Preoperative testing may include a complete blood count
and chemistries with additional testing being based on the findings of the
preoperative history, physical examination, indication for surgery, and planned
procedures. Special attention needs to be paid to the preoperative and
intraoperative issues that arise in the care of obese patients, cardiac patients,
respiratory-compromised patients, and any other patients with significant
medical comorbidities including patients with venous thromboembolism or
malnutrition. Counseling and postoperative management of patients and their
familieswillbeinfluencedbytheuniquesurgeriesandconditionsencounteredin
gynecologiconcology.
In general, preoperative evaluation and testing are stratified based on a
patient’s comorbidities.Allpatientsundergoingsurgeryforgynecologiccancer
shouldundergoathoroughevaluationofothermedicalissues.Suchevaluations
will provide an individualized preoperative assessment. In addition, the
identification of preoperative medical issues will allow these conditions to be
medicallyoptimized.
PreoperativeTesting
Manypatientswithgynecologicmalignancieswillbeofolderage.Asaresult,
theyoftenhaveothermedicalcomorbidities.In2007,theleadingcausesofdeath
intheUnitedStateswereheartdisease,cancer,stroke,chroniclowerrespiratory
disease,andaccidents.Giventheprevalenceofcoronaryarterydisease,diabetes,
peripheral vascular occlusive disease, and obesity in our population, many
patients will require some preoperative testing to assess their cardiopulmonary