Improved Outcomes in Colon and Rectal Surgery part 5 potx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (583.8 KB, 10 trang )
improved outcomes in colon and rectal surgery
oral antibiotic prophylaxis in colon surgery (8) compared three
oral doses to one oral dose to no oral antibiotics. Patients in all
three groups received intravenous cefoxitin before incision and
two doses after surgery ended. They found no benefit to the oral
antibiotics and indeed found that patients randomized to three
doses of oral medications had lower tolerance of the prep. They
concluded that there is no benefit to oral antibiotics assuming
that appropriate intravenous antibiotics are given.
The long-standing practice of mechanical bowel prepara-
tions before surgery to decrease the fecal load has also come
under closer scrutiny in the past decade. Multiple case series were
reported which led to randomized trials in the 1990s and early
2000s. A meta-analysis in 2004 (9) demonstrated in an evalua-
tion of five such randomized trials that mechanical bowel prepa-
ration did not improve outcome or decrease the risk of surgical
site infection. They concluded that mechanical bowel preparation
might be omitted, but that further studies should be performed.
A recently published Cochrane Database Review (10) further
concluded that there is not good evidence that mechanical bowel
preparation reduces the risk of anastomotic and infectious com-
plications. Furthermore, although the data does not support con-
clusively that mechanical bowel preparation can be deleterious,
there is some evidence to that point. All in all, the authors felt that
routine mechanical bowel preparation should be reconsidered.
A recent multiinstitutional randomized trial also showed similar
results, leading the authors to conclude that routine mechanical
bowel preparation is unnecessary.(11) Two large multiinstitu-
tional randomized trials recently published also showed simi-
lar outcomes between patients receiving bowel preparation and
those not receiving one.(12, 13) Close evaluation of the data
from these two trials does however raise concern that the lack
of a bowel preparation may be deleterious as the rate of abscess
and leak (when combined) was higher in the patient group who
did not undergo mechanical bowel prep.(14) Further details on
bowel preparation are discussed in chapter 2.
Some clinicians have advocated using incision protectors to pre-
vent or decrease the amount of contamination of the subcutane-
ous tissues from both the surrounding skin as well as from enteric
organisms during anastomosis formation. One study in the late
1960s gave some hope that draping of the incision with a plastic
barrier would decrease wound infection (2.4% vs. 15% without
the wound drape).(15) However, subsequent studies over the next
decades did not fully support these findings. Nystrom reported
a randomized, controlled trial comparing a plastic wound drape
to no drape in colorectal surgery patients.(16) They found no
improvement in infection rate (9% vs. 10%) by using the wound
drape. Indeed, they performed culture swabs of the subcutaneous
tissues at the time of surgery in most cases and did not notice any
difference in rates of contamination with enteric organisms.
Beside preoperative antibiotics and bowel preparation, body
temperature and oxygenation may be important in prevent-
ing surgical site infection. Kurz et al. showed that in a group of
patients undergoing colorectal surgery, there was a higher risk
of surgical site infection in patients with lower body tempera-
ture (34.7 degrees vs. 36.6 degrees C).(17) However, these find-
ings are somewhat controversial as another group found that
there was no relationship between hypothermia and surgical site
infection in patients undergoing colonic surgery.(18) In terms
of tissue oxygenation, many studies have demonstrated lower
rates of surgical site infection following colon and rectal sur-
gery in patients receiving inspired oxygen peri- and immediately
postoperatively.(19–21) Indeed, in a meta-analysis of random-
ized trials of immediate postoperative oxygen usage, Chura et al.
determined that postoperative oxygen did lower rates of surgical
site infection.(22)
Despite our best techniques and proper antibiotic prophylaxis,
wound infections do still occur. Their exact incidence is hard
to gauge given that studies on wound infection incidence have
a wide range of results.(6, 23–27) Managing a wound infection
properly is important to prevent a relatively small problem from
becoming a large, life-threatening problem.
Laparoscopic surgery has been postulated by some to result in
a lower risk of surgical site infection than does the corresponding
open procedure. This has not been shown to be true of all types
of surgery. However, it does seem to hold true at least for chole-
cystectomy and for colonic surgery; laparoscopic colectomy has a
lower risk of surgical site infection than does open colectomy.(3)
Case selection bias may have affected this result however, as this
was an observational study without randomization.
Erythema at the surgical incision is often the first sign of a sur-
gical site infection. The incision site should be carefully examined
to see if there is any evidence of abscess deep to the skin (e.g.,
fluctuance). In cases of suspected abscess, the incision should
be opened over the area of concern, draining any infection. The
rest of the superficial incision should be examined to make sure
that all areas of abscess are adequately drained. Routine wound
care is then employed, often amounting to “wet-to-dry” dressing
changes or negative-pressure dressing device placement. In cases
of surrounding skin erythema and in immunocompromised
patients, appropriate antibiotics should be employed.
The management of open skin and subcutaneous tissue is
important. Once nonviable tissue has been debrided and any
abscesses drained, the tissue needs to be cared for to promote
healing. This often occurs with “wet-to-dry” dressings consist-
ing of gauze dampened with water or saline. Dressing changes
occur once or twice daily and the wound closes by secondary
intention over time. Another option for closure of a re-opened
abdominal incision is with a negative-pressure dressing, for
example, the Wound-Vac (KCI). Even in complex wounds, the
negative-pressure dressing is a good adjunct for subcutaneous
tissue closure.(28)
Though uncommon in the postoperative setting, soft tis-
sue necrotizing infections can occur at a surgical site and can
be disastrous if not treated aggressively. Wide debridement to
healthy, bleeding tissue should be performed urgently and is the
mainstay of treatment, along with appropriate antibiotic therapy.
The surgeon should have a low threshold to return to the operat-
ing room for re-evaluation of the surgical site. Adjunctive treat-
ments of necrotizing soft tissue infections (including Fournier’s
gangrene and postoperative soft tissue necrotizing infections)
include hyperbaric oxygen (29, 30) and intravenous immuno-
globulin administration (31). However, their application is con-
troversial and adherence to surgical treatment at this point is
standard of care.
sepsis
intRaabDOminal infeCtiOnS
Intraperitoneal anastomoses have a relatively low risk of disrup-
tion; nevertheless, abdominal abscess is an all too common com-
plication of abdominal surgery. An abdominal abscess can lead
to sepsis and needs to be dealt with in a timely fashion. Again,
a spectrum of severity of sepsis and physiologic effect exists.
Abscess associated with anastomotic failure can be devastating.
Studies have shown mortality rates >20% (32) as well as worse
cancer survival (33, 34).
In most cases, the intraabdominal abscess is discovered on a
CT scan ordered for fever, persistent ileus, leukocytosis, abdom-
inal pain, or other complaint. The surgeon must now decide
what steps to take in managing the abscess. One retrospective
review showed that many intraabdominal abscesses can be
managed with antibiotics alone.(35) Kumar et al. showed a high
level of success (55%) with antibiotic treatment alone. There
were only two patients who initially succeeded but ultimately
required intervention. Most of the abscesses in this study were
diverticular or periappendicular, although 11% were postopera-
tive. All patients diagnosed with an abscess were started on par-
enteral antibiotics. They showed that patients with an abscess
>6.5 cm in greatest diameter or in patients presenting with a
temperature more than 101.2 were likely to require percutane-
ous drainage. These authors treated initially with appropriate
intravenous antibiotics, and 54% of patients improved with that
treatment alone. 44% of patients required percutaneous drain-
age after 48–72 hours of intravenous antibiotic treatment for
failure to improve significantly.
Percutaneous drainage of intraabdominal abscesses has
become the treatment of choice for intraabdominal abscesses
(see Figures 4.1 and 4.2). However, percutaneous drainage is
not successful in 100% of patients.(35–37) A patient who is
not stable for an attempt of nonoperative management, or one
who fails to improve without operation will need to go to the
operating room for therapy. Of important note, some studies
have indicated a higher mortality for surgical management of
intraabdominal abscess after failed percutaneous drainage.(38,
39) Therefore, it is imperative that a surgeon knows the fac-
tors which predict failure with nonoperative management. One
group looked at 73 patients with abscess in whom attempted
percutaneous drainage was performed. They experienced a 19%
failure of nonoperative treatment. Using multivariate analysis,
they showed that only abscess diameter <5 cm or failure to start
the patient on antibiotic therapy before drainage were predictive
of failure of percutaneous drainage.(36)
Another study examined factors that would predict which
patients would fail versus succeed with percutaneous manage-
ment of intraabdominal abscesses.(40) Of 96 patients pro-
spectively evaluated, they found that 70% of patients were
successfully managed with a single percutaneous drainage and
that an additional 12% were successful with a second attempt.
Further attempts were not often successful. Only 16% of the
patients ultimately required surgery. In evaluating their results,
success was predicted in postoperative abscess, whereas failure
was predicted with pancreatic source of abscess and when yeast
was present in the abscess cavity.
The following algorithm can be used as a guideline: (Figure 4.3)
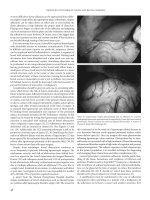
Figure 4.1 Computerized tomography of the pelvis showing a large presacral
space abscess. Note that staple-line is visible anterior to the large collection.
Figure 4.2 Computerized tomography of pelvic abscess resolved after percutaneous
drainage. Note the drain remains in place just anterior and left of the coccyx.
PelviC SePSiS POStOPeRatively
A surgeon who operates on the rectum needs to know how to
deal with pelvic sepsis as the result of an anastomotic leak associ-
ated with rectal resection, regardless of the level of anastomosis.
One comprehensive study from 2004 looking at which factors
related to rectal resection were associated with an increased risk
of anastomotic leak showed interesting results.(41) Multivariate
analysis of 432 rectal resection patients showed that anastomo-
sis <6 cm from the anal verge, history of preoperative radiation
improved outcomes in colon and rectal surgery
therapy, the presence of adverse intraoperative events, and male
sex were independent risk factors for anastomotic leak. Their
overall symptomatic leak rate was 12%.
In a hemodynamically stable patient with a pelvic abscess
(obviously, hemodynamically unstable patients need to be dealt
with urgently, often surgically), broad-spectrum antibiotics
should be started and a decision about drainage of the abscess
needs to be made. In general, the guidelines above for intraab-
dominal abscesses can be extrapolated to pelvic abscesses. The
algorithm above (Figure 4.3) can likewise be used as a guide in
management.
One common CT-guided approach for pelvic abscesses is
via the transgluteal approach. Harisinghani performed a study
examining 154 cases of pelvic abscess treated in this manner (42),
showing a 96% success rate in completely resolving the abscess via
this approach. Complications were uncommon, but did include
hemorrhage in 2% (though all had transpiriformis approaches to
their abscesses). Two of these patients required angio-emboliza-
tion for pseudoaneurysm of the inferior gluteal artery while the
third patient resolved spontaneously.
Endoscopic ultrasound guidance with aspiration and drainage
has also been described in treating deep pelvic abscess.(43) They
described their first 12 patients in this fashion. 25% of patients
required surgical drainage, though eight of nine patients who
were able to have a stent placed into the cavity were completely
treated via this approach.
Duration of antibiotic therapy for treating intraabdominal
infections is controversial. There is a paucity of good data on
length of treatment, and much of the decision is based upon
“because that’s how we always do it” logic. Current data and
trends are leaning toward shorter duration of treatment. In a
review of antibiotic treatment for intraabdominal infections,
Mazuski et al. (44) state that two current approaches to dura-
tion exist: making decisions based upon intraoperative findings
versus tailoring treatment based upon clinical condition and
improvement of the patient. One prospective randomized trial
looked at minimum length of antibiotic treatment after compli-
cated appendicitis.(45) Appropriate patients were divided into
two groups and given IV antibiotics. One group had a minimum
of 5 days of antibiotics whereas the other group did not have a
minimum number of treatment days. Antibiotic treatment was
terminated based upon clinical findings: resolution of fever,
improved physical examination, and return of GI function. The
group with no minimum number of days of treatment received
less doses of antibiotics overall. The authors also determined that
stopping antibiotic treatment based upon clinical indices resulted
in the same amount of recurrent infections as having a minimum
number of days of treatment (i.e., 5 days).
Figure 4.3 Treatment algorithm for dealing with intraabdominal and pelvic abscesses.
sepsis
The decision that nonoperative management has been unsuc-
cessful can be difficult to make. Any hemodynamically unstable
patient should return to the operating room. Further, patients who
fail to respond to nonoperative management may need surgery.
When operating for sepsis secondary to a failed anastomosis, there
is some controversy as to whether diversion and washout is ade-
quate or if the anastomosis needs to be resected. One retrospective
study of 27 leaks showed that proximal diversion with drainage is
adequate and results in a high chance of anastomotic salvage.(46)
Knowledge of appropriate treatment of the septic patient follow-
ing colon and rectal surgery is mandatory for any surgeon operating
on these organs. Some patients can be successfully managed with
nonoperative techniques. Timely identification of which patients
will require intervention, whether it is percutaneous or surgical, is
essential as is choosing the most effective treatment plan. Finally,
the surgeon must appropriately carry out that plan and know when
to switch to another course if failure occurs.
RefeRenCeS
1. Bone RC, Balk RA, Cerra FB et al. Definitions for sepsis
and organ failure and guidelines for the use of innovative
therapies in sepsis. The ACCP/SCCM consensus conference
committee. American college of chest physicians/society of
critical care medicine. Chest 1992; 101(6): 1644–55.
2. Haley RW, Culver DH, Morgan WM et al. Identifying patients
at high risk of surgical wound infection. A simple multivari-
ate index of patient susceptibility and wound contamination.
Am J Epidemiol 1985; 121(2): 206–15.
3. Gaynes RP, Culver DH, Horan TC et al. Surgical site infec-
tion (SSI) rates in the United States, 1992–1998: the National
nosocomial infections surveillance system basic SSI risk
index. Clin Infect Dis 2001; 33(Suppl 2): S69–77.
4. Nichols RL, Condon RE, Gorbach SL et al. Efficacy of pre-
operative antimicrobial preparation of the bowel. Ann Surg
1972; 176(2): 227–32.
5. Nichols RL, Broido P, Condon RE et al. Effect of preoperative
neomycin-erythromycin intestinal preparation on the inci-
dence of infectious complications following colon surgery.
Ann Surg 1973; 178(4): 453–9.
6. Clarke JS, Condon RE, Bartlett JG et al. Preoperative oral
antibiotics reduce septic complications of colon operations:
results of prospective, randomized, double-blind clinical
study. Ann Surg 1977; 186(3): 251–9.
7. Zmora O, Wexner SD, Hajjar L et al. Trends in preparation for
colorectal surgery: survey of the members of the American
Society of colon and rectal surgeons. Am Surg 2003; 69(2),
150–4.
8. Espin-Basany E, Sanchez-Garcia JL, Lopez-Cano M et al.
Prospective, randomized study on antibiotic prophylaxis in
colorectal surgery. Is it really necessary to use oral antibiot-
ics? Int J Colorectal Dis 2005; 20(6): 542–6.
9. Bucher P, Gervaz P, Soravia C et al. Randomized clinical
trial of mechanical bowel preparation versus no preparation
before elective left-sided colorectal surgery. Br J Surg 2005;
92(4): 409–14.
10. Guenaga K, Atallah AN, Castro AA et al. Mechanical bowel
preparation for elective colorectal surgery. Cochrane
Database Syst Rev 2005; 1: CD001544.
11. Fa-Si-Oen P, Roumen R, Buitenweg J et al. Mechanical bowel
preparation or not? Outcome of a multicenter, randomized
trial in elective open colon surgery. Dis Colon Rectum 2005,
48(8), 1509–16.
12. Contant CM, Hop WC, van’t Sant HP et al. Mechanical bowel
preparation for elective colorectal surgery: a multicentre ran-
domised trial. Lancet 2007; 370(9605): 2112–7.
13. Jung B, Pahlman L, Nystrom PO et al. Multicentre random-
ized clinical trial of mechanical bowel preparation in elective
colonic resection. Br J Surg 2007; 94(6): 689–95.
14. Platell C, Hall J. Mechanical bowel preparation before col-
orectal surgery? Lancet 2007; 370(9605): 2073–5.
15. Harrower HW. Isolation of incisions into body cavities. Am J
Surg 1968; 116(6): 824–6.
16. Nystrom PO, Broome A, Hojer H et al. A controlled trial of
a plastic wound ring drape to prevent contamination and
infection in colorectal surgery. Dis Colon Rectum 1984;
27(7): 451–3.
17. Kurz A, Sessler DI, Lenhardt R. Perioperative normo-
thermia to reduce the incidence of surgical-wound infec-
tion and shorten hospitalization. Study of wound infection
and temperature group. N Engl J Med 1996; 334(19):
1209–15.
18. Barone JE, Tucker JB, Cecere J et al. Hypothermia does not
result in more complications after colon surgery. Am Surg
1999; 65(4): 356–9.
19. Greif R, Akca O, Horn EP et al. Supplemental perioperative
oxygen to reduce the incidence of surgical wound infec-
tion. Outcomes Research Group. N Engl J Med 2000; 342(3):
161–7.
20. Belda FJ, Aguilera L, Garica de la Asuncion J et al. Spanish
Reduccion de la Tasa de Infeccion Qirirgica Group.
Supplemental perioperative oxygen and the risk of surgical
wound infection: a randomized controlled trial. JAMA 2005;
294(16): 2035–42.
21. Brasel K, McRitchie D, Dellinger P. EBRS Group. Canadian
association of general surgeons and American college of sur-
geons evidence based reviews in surgery. 21: the risk of surgi-
cal site infection is reduced with perioperative oxygen. Can J
Surg 2007; 50(3): 214–6.
22. Chura JC, Boyd A, Argenta PA. Surgical site infections and
supplemental perioperative oxygen in colorectal surgery
patients: a systematic review. Surg Infect (Larchmt) 2007;
8(4): 455–61.
23. Stone HH, Hooper CA, Kolb LD et al. Antibiotic prophylaxis
in gastric, biliary and colonic surgery. Ann Surg 1976; 184(4):
443–52.
24. Coppa GF, Eng K, Gouge TH et al. Parenteral and oral antibi-
otics in elective colon and rectal surgery. A prospective, ran-
domized trial. Am J Surg 1983; 145(1): 62–5.
25. Kaiser AB, Herrington JL Jr, Jacobs JK et al. Cefoxitin versus
erythromycin, neomycin, and cefazolin in colorectal opera-
tions. Importance of the duration of the surgical procedure.
Ann Surg 1983; 198(4): 525–30.
26. Schoetz DJ Jr, Roberts PL, Murray JJ et al. Addition of par-
enteral cefoxitin to regimen of oral antibiotics for elective
colorectal operations. A randomized prospective study. Ann
Surg 1990; 212(2): 209–12.
improved outcomes in colon and rectal surgery
27. Itani KM, Wilson SE, Awad SS et al. Ertapenem versus cefo-
tetan prophylaxis in elective colorectal surgery. N Engl J Med
2006; 355(25): 2640–51.
28. Heller L, Levin SL, Butler CE. Management of abdominal
wound dehiscence using vacuum assisted closure in patients
with compromised healing. Am J Surg 2006; 191(2): 165–72.
29. Riseman JA, Zamboni WA, Curtis A et al. Hyperbaric oxygen
therapy for necrotizing fasciitis reduces mortality and the
need for debridements. Surgery 1990; 108(5): 847–50.
30. Jallali N, Withey S, Butler PE. Hyperbaric oxygen as adju-
vant therapy in the management of necrotizing fasciitis. Am
J Surg 2005; 189(4): 462–6.
31. Norrby-Teglund A, Muller MP, Mcgeer A et al. Successful man-
agement of Sever Group A streptococcal soft tissue infections
using an aggressive medical regimen including intravenous
polyspecific immunoglobulin together with a conservative
surgical approach. Scan J Infect Dis 2005; 37(3): 166–72.
32. Kanellos I, Blouhos K, Demetriades H et al. The failed
intaperitoneal colon anastomosis after colon surgery. Tech
Coloproctol 2004; 8(Suppl 1): s53–5.
33. Walker KG, Bell SW, Rickard MJ et al. Anastomotic leakage
is predictive of diminished survival after potentially curative
resection for colorectal cancer. Ann Surg 2004; 240(2): 255–9.
34. Law WL, Choi HK, Lee YM et al. Anastomotic leakage is
associated with poor long-term outcome in patients after
curative colorectal resection for malignancy. J Gastrointest
Surg 2007; 11(1): 8–15.
35. Kumar RR, Kim JT, Haukoos JS et al. Factors affecting the
successful management of intra-abdominal abscesses with
antibiotics and the need for percutaneous drainage. Dis
Colon Rectum 2006; 49(2): 183–9.
36. Benoist S, Panis Y, Pannegeon V et al. Can failure of percu-
taneous drainage of postoperative abdominal abscesses be
predicted? Am J Surg 2002; 184(2): 148–53.
37. Shuler FW, Newman CN, Angood PB et al. Nonoperative
management for intra-abdominal abscesses. Am Surg 1996;
62(3): 218–22.
38. Brolin RE, Flancbaum L, Ercoli FR et al. Limitations of per-
cutaneous catheter drainage of abdominal abscesses. Surg
Gynecol Obstet 1991; 173(3): 203–10.
39. McLean TR, Simmons K, Svensson LG. Management of
postoperative intra-abdominal abscesses by routine per-
cutaneous drainage. Surg Gynecol Obstet 1993; 176(2):
167–71.
40. Cinat ME, Wilson SE, Din AM. Determinants for success-
ful percutaneous image-guided drainage of intra-abdominal
abscess. Arch Surg 2002; 137(7): 845–9.
41. Matthiessen P, Hallbook O, Andersson M et al. Risk factors
for anastomotic leakage after anterior resection of the rec-
tum. Colorectal Dis 2004; 6(6): 462–9.
42. Harisinghani MG, Gervai DA, Maher MM et al. Transgluteal
approach for percutaneous drainage of deep pelvic abscesses:
154 cases. Radiology 2003; 228(3): 701–5.
43. Giovannini M, Bories E, Moutardier V et al. Drainage of
deep pelvic abscesses using therapeutic echo endoscopy.
Endoscopy 2003; 35(6): 511–4.
44. Mazuki JE, Sawyer RG, Nathens AB et al. Therapeutic agents
committee of the surgical infections society. The surgi-
cal infection society guidelines on antimicrobial therapy
for inta-abdominal infections: an executive summary. Surg
Infect (Larchmt) 2002; 3(3): 161–73.
45. Taylor E, Dev V, Shah D et al. Complicated appendicitis:
is there a minimus intravenous antibiotic requirement?
A prospective randomized trial. Am Surg 2000; 66(9):
887–90.
46. Hedrick TL, Sawyer RG, Foley EF et al. Anastomotic leak and
the loop ileostomy: friend or foe? Dis Colon Rectum 2006;
49(8): 1167–76.
5
Intraoperative anastomotic challenges
David E Beck
CHALLENGING CASE
A 28-year-old man is undergoing a restorative proctocolectomy
using a double-stapled technique for the ileoanal anastomosis.
During insertion of the stapler into the anus, the anal canal distal
linear staple line is disrupted. What are your options?
CASE MANAGEMENT
Initial action is to visualize the distal staple line using retractors. If
the ends of the partially closed distal bowel can be visualized and
grasped with clamps or traction sutures, the amount of residual
bowel can be assessed. If adequate length is present, one option is
to reclose the bowel with a linear stapler placed below the disrupted
staple line. After the stapler is fired, the residual bowel end can be
resected with scissors or a scalpel. A second option is to reclose the
disrupted staple line with sutures placed from the abdominal side
or placed intralumenally via a retractor (lighted Chelsea-Eaton,
Hill-Ferguson, etc.,) placed into the anal canal. If the defect and
bowel are successfully closed, the anastomosis can proceed.
If the distal segment of bowel is impossible to visualize or close,
a musectomy can be performed via the anus and a hand sewn ileo
anal anastomosis can be performed, as described in chapter 31.
Most surgeons will create a diverting loop ileostomy when the
anastomosis has been this challenging.
Colon and rectal Surgery is a technique-oriented specialty with
many procedures requiring an anastomosis to reestablish bowel
continuity. Achievement of a successful anastomosis is related to
a number of surgical principles, which can be divided into patient
factors and surgeon factors.(1–3) Patient-related factors, such as the
patient’s nutritional status and associated medical conditions or
medications, are not under the operating surgeon’s control and are
discussed in other chapters. This chapter focuses on anastomotic
principles and problems (e.g., leakage, ischemia, stenosis, and hem-
orrhage) that can be identified and managed during the procedure.
PREANASTOMOTIC CONSIDERATIONS
Preoperative Discussion and Planning
Before surgery the surgeon should have a plan which includes
the expected operative findings or pathology and restoration of
intestinal continuity if possible. If the preoperative findings are
confirmed, the operation should proceed along an organized
pathway. Unexpected findings will obviously require modifica-
tions. Before the procedure, the surgeon should also have a dis-
cussion with the patient, which includes these considerations with
special emphasis on aspects of the anastomosis and the possible
need for a temporary or permanent stoma should restoration of
intestinal continuity be impossible or ill advised.(4) Proximal
diversion will reduce the clinical sequalae from an anastomotic
dehiscence. This is more likely in patients receiving preoperative
chemotherapy and/or radiation, with poor nutrition, associated
infection, or comorbid conditions (steroid use, hypotension,
etc.,). The appropriate site for a potential stoma should be cho-
sen preoperatively, with the assistance of an enterostomal thera-
pist. The selection and marking of a stoma site provides another
opportunity for dialogue between the surgeon and patient.
Operative Principles
The key to uncomplicated healing of an intestinal anastomosis
depends on adherence to well-established principles as well as the
specifics of the technique. The principles of intestinal anastomo-
sis include: (1) appropriate access and exposure to the two ends
of bowel, (2) healthy bowel to be joined, (3) good blood supply,
(4) gentle handling of the bowel, and (5) good apposition of ends
with no tension on the anastomosis (5). Any compromise of these
principles places the anastomosis at risk for complications.
Exposure and access to bowel ends can be maximized by tak-
ing the time to set up and position retractors and intraabdominal
packs. Headlights and lighted retractors minimize the frustration
of inadequate overhead lights. Deep pelvic retractors such as the
St. Marks retractor or Wiley vein retractors assist the visualization
of the distal rectum prior to anastomosis. Extending the mid-line
incision to the symphysis pubis likewise, allows maximum expo-
sure of the distal rectum. Operating with poor lighting and inad-
equate exposure not only jeopardizes the anastomosis but also
increases operating room time.
Techniques of intestinal anastomosis should also be performed
following the principle of gentle bowel handling. Clamps should be
used only when absolutely necessary with the least amount of clo-
sure required to occlude the lumen. Care should be taken to exclude
mesenteric blood vessels within the intestinal clamp. Gingerly
inserting appropriately sized intraluminal staplers prevents inad-
vertent splitting and tearing of bowel ends to be anastomosed.
Excessive use of electrocautery at the anastomosis can cause unap-
preciated tissue necrosis with potential for disruption. Mobilization
of intestinal ends is required for exposure, access, and freedom of
tension on the anastomosis. However, during mobilization it is
important to preserve those blood vessels required for adequate
anastomotic healing. For example, excessive skeletonization of the
cut intestinal ends may compromise their blood supply.
Having two healthy ends of bowel to anastomose is ideal. In
some cases (bowel obstruction, diverticular disease, radiation
enteritis, Crohn’s disease), this situation may not be possible and
the plan to anastomose may be questioned. Optimizing patient
nutrition, treating infection, and minimizing inflammation in
the preoperative period may improve the bowel status. At opera-
tion, all diseased bowel is resected whenever possible to provide
soft, pliable bowel ends for anastomosis.
Bowel Preparation
As described in chapter 2, bowel preparation has undergone major
changes over the past 70 years. Until the past decade, mechanical
improved outcomes in colon and rectal surgery
bowel preparation was a standard feature of elective bowel surgery
and the lack of a bowel preparation or poor results with a mechani-
cal preparation was in many surgeons view a contraindication to
a primary anastomosis. Recent studies have failed to support the
accepted view that bowel cleansing, in the presence of appropri-
ate antibiotics, reduced the risk of anastomotic leak or wound
infection.(6) Case series and reports from the trauma literature
suggested that good or better outcomes could be achieved in unpre-
pared bowel with an anastomosis.(7, 8) A Cochrane review of five
randomized trials showed equal or better morbidity or mortality in
576 patients with a mechanical bowel preparation and 583 patients
without a mechanical preparation.(9) An additional meta-analysis
of seven randomized trials containing 1,454 patients showed no
significant differences for wound infection and septic and nonsep-
tic conditions.(10) Certain situations, such as laparoscopic proce-
dures, potential need for intraoperative colonoscopy, or avoidance
of spillage from proximal stool loading after a low colorectal anas-
tomosis, still require adequate mechanical bowel preparation. For
other situations, many surgeons are minimizing or eliminating a
mechanical bowel preparation in elective situations. The current
evidence suggests that intralumal contents should not be the pri-
mary factor in deciding if an anastomosis should be performed.
Other options to consider with unprepareed bowel are to perform a
subtotal colectomy with ileocolonic or ileorectal anastomosis. This
option has been shown to be a safe option for avoiding a stoma
in left colon obstruction.(11) Alternatively, intraoperative colonic
lavage in some hands offers the ability to construct an anastomosis
in patients with this condition.(11–13)
Whatever the bowel preparation used, it is critical that spillage
of intralumenal contents be avoided to minimize complications
and neoplastic dissemination. Most surgeons agree that a clean,
empty colon has less potential for spillage, but cannot compen-
sate for poor technique. An additional protection against spillage
of residual intestinal contents is provided by controlling the ends
of bowel used in the anastomosis. This can be accomplished by
elevating of the ends (with traction sutures) or by occluding the
bowel proximal and distal to the anastomosis with tapes or non-
crushing clamps.(14)
Bowel Status
When intestinal surgery is being performed for urgent situa-
tions or even during certain elective operations, the first decision
is whether or not an anastomosis is appropriate. Healing of an
anastomosis is at risk in certain clinical situations. Traditionally,
intestine that is unprepared, obstructed, irradiated, inflamed, or
ischemic may not be suitable for anastomosis.(15–17) However,
other than ischemia, current evidence suggests that constructing
an anastomosis is safe in selected cases of obstruction, irradia-
tion, inflammation, and without bowel preparation.(5, 11, 18, 19)
In addition to these local factors, patient factors such as malnu-
trition, diabetes, renal failure, chronic hepatic disease, anemia,
shock, steroid use, and other immunocomprised states may place
an anastomosis at risk for failure.(4, 5, 6)
The safety of intestinal anastomosis in any particular clinical
scenario thus depends upon patient and intestinal factors that
must be carefully weighed by the operating surgeon.(5) Optimally,
the bowel will have a good blood supply, (documented by pink
color, parastalysis and pulsitile bleeding from the cut edge), lack
edema, be free of tension (see later section), and have adequate
lumen for the type of anastomosis. The ultimate decision to per-
form an intestinal anastomosis ultimately depends on surgical
judgment derived from an understanding of documented risks as
well as knowledge of one’s own ability and experience.
Exposure
The importance of adequate exposure cannot be overempha-
sized. Exposure is facilitated by patient position, adequate length
of incision, appropriate choice of retractors, and lighting. If the
possibility of a left-sided anastamosis exists, the patient should be
placed in lithotomy position, using either Lloyd-Davies, Allen or
yellow-fin stirrups, after anesthesia is induced. (Figure 5.1) Great
care is taken to avoid pressure on the peroneal nerves and hips.
(20) The perineum should extend slightly over the end of the
operating table to allow easy access for transanal stapled anasta-
mosis, upward pressure on the perineum for exposure of the dis-
tal rectum, or a two-surgeon combined approach to hand sewn
coloanal anastamosis or abdominoperineal resection. Once the
patient is correctly positioned, irrigation of the rectum should be
performed to ensure the quality of the bowel preparation and to
evacuate any remaining fecal residue. Leaving a large mushroom-
shaped or Foley catheter in the rectum alerts the surgeon to the
level of dissection in the low pelvis, prevents rectal distension and
possible enterotomy during mobilization, and allows drainage of
rectal contents which minimizes luminal spillage.
The trend toward smaller incisions should be critically evalu-
ated when planning a colorectal anastamosis.(4) Pelvic expo-
sure is greatly facilitated by incisions that extend to the pubic
bone. The incision may require proximal extension if mobiliza-
tion of the splenic flexure is required. The extent of this exten-
sion will depend on factors such as the patient’s body habitus,
disease process, and surgical technique. Adequate exposure with
less generous incisions is often possible in thin patients or those
with low splenic flexures, mobile colons, or left-sided Crohn’s
disease (the splenic flexure that is contracted down into the
abdominal cavity). Placement of the operating surgeon between
the patient’s legs often improves visualization during splenic
flexure mobilization. Adequate visualization is imperative for
safe splenic flexure mobilization, and additional retraction or
incision extension should be one of the first considerations if
mobilization is difficult.
Different retractors are available to improve exposure. Those
that are fixed to the bed, such as the Bookwalter, “upper hand,”
omnitract, or polytract, make life easier for surgical assistants and
provide a consistent view throughout the operation.(4) When
placing retractor attachments, the surgeon must be aware of the
relation of the retractor to the femoral vessels, nerves, and iliac
crests. Prolonged constant traction on the bowel may also be a
problem, and consideration of relieving the pressure intermit-
tently during long cases may be appropriate.
Finally, adequate lighting is extremely important during pelvic
dissection as well as to provide a view of the anastomosis, deep
in the pelvis. Equipment available to enhance vision includes
headlights, lighted retractors, cautery instruments, and suction
devices.
5
intraoperative anastomotic challenges
Obtaining Adequate Length
After the appropriate resectional procedure is completed, suf-
ficient proximal and distal mobilization provides tension-free
bowel ends for a secure anastomosis. Tension is rarely a problem
for small bowel or ileocoioc anastomosis. The small bowel mes-
entery has an avascular plane anterior to the aorta to the takeoff
of the superior mesenteric artery. The right and left colon have a
posteriolateral fusion plane anterior to Geroda’a fascia. This avas-
cular plane can be opened using a lateral or medial approach.
Difficulty in obtaining tension-free bowel occurs more com-
monly with a left-sided (e.g., colorectal) anastomosis. Additional
left colon length is obtained using the following maneuvers in
this order: division of the lateral colonic attachments, division of
the splenic flexure, division of the inferior mesenteric artery at its
aortic takeoff, and division of the inferior mesenteric vein at the
inferior border of the pancreas (Figure 5.2).(4) If these maneu-
vers do not provide adequate bowel length, branches of the distal
middle colic artery and veins may need division. Unfortunately
this last action may compromise the blood supply to the remain-
ing colonic end. If this occurs, the ischemic bowel must be
resected and additional vessels will need to be divided to provide
the required bowel length. In some cases the middle colic vessels
will have to be divided proximally and the blood supply of the
residual colon will need to be based on the right and/or ileocolic
artery. In most patients these vessels will provide adequate blood
supply to the proximal transverse colon or hepatic flexure which
can be made to reach the rectum with one of two techniques.
One method is to open a window in the ileal mesentery medial
to the ileocolic artery and vein. The proximal transverse colon
is brought through this window to reach the pelvis (Figure 5.3).
(21) Another option is to completely mobilize the right colon and
then derotate it to the right. This rotates the cecal tip to the right
middle abdomen (pointed toward the liver), reverses the direc-
tion of the colon, and provides enough length for the hepatic
flexure to reach the pelvis (Figure 5.4). As this maneuver moves
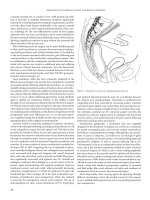
Figure 5.1 Stirrups for modified lithotomy position.
Figure 5.2 Operative techniques to obtain left colon length. (1) division of lateral colonic
attachments. (2) division of the splenic flexure. (3) division of the inferior mesenteric
artery at its aortic takeoff and the inferior mesenteric vein. (4) second division of the
inferior mesenteric vein at the inferior border of the pancreas. (5) incision of splenic
flexure mesentary. (From Rafferty JF. Obtaining adequate bowel length for colorectal
anastomosis. Clin Colon Rectal Surg 2001; 14: 25–31. With permission.)
improved outcomes in colon and rectal surgery
Figure 5.3 (A) Window in mesentary is created medial
to the ileocolic atrery and vein. (B) Transverse is brought
through ileal mesenteric window to reach the pelvis.
(a)
(b)
Figure 5.4 (A) Right colon is mobilized, right colic
vessels are divided, and appendix is removed. (B) The
right colon is derotated to allow the hepatic flexure to
reach the pelvis.
the cecum to an abnormal position, it is important to remove the
appendix. Development of appendicitis would produce confus-
ing signs and symptoms.
In addition to a lack of tension, it is also critically impor-
tant that the anastomotic site have a good blood supply. Before
the anastomosis is constructed, the bowel should be routinely
checked for viability (normal color and peristalsis and a pulsa-
tile blood supply). Well-perfused bowel and its appendages (e.g.,
appendices epiploicae) will bleed when cut. Dividing an appendi-
ces epiploicae at the proximal bowel end intended for the anasto-
mosis is frequently used by the author to objectively confirm an
adequate blood supply.
In addition to the factors described previously, bowel used for
an anastomosis must not be edematous, radiated, or ischemic. An
appropriate resection should remove ischemic or radiated bowel,
while edema of the residual bowel (e.g., associated with peritonitis)
may mandate forgoing an anastomosis in favor of a diverting stoma.
ANASTOMOTIC TECHNIQUE
In performing an anastomosis, surgeons have multiple options.
Each technique has associated advantages and disadvantages, and
the specific one favored by an individual surgeon depends more
on training, personal experience, and perhaps blind faith than on
the results of randomized, prospective studies. There has been no
(a) (b)
intraoperative anastomotic challenges
STAPLES VERSUS SUTURES
Suturing has been used since the beginning of intestinal surgery.
Different suture materials have shown some experimental differ-
ences, but the clinical difference is arguable. In general a stapled
anastomosis usually takes less time but is more expensive.(22, 23)
Blood flow may be higher with a stapled anastomosis, and in cer-
tain situations, such as a low colorectal anastomosis, the use of
staples is technically easier.(24) A final consideration is that any
device can malfunction and lead to the need for use of additional
staplers or conversion to a sutured anastomosis.(5)
A meta-analysis of 13 trials comparing hand-sewn with stapled
anstomosis showed similar mortality, leak rates, local cancer recur-
rences, and wound infections.(25) This review did reveal a higher
rate of postoperative stricture with the stapled anastomosis, most
of which were asymptomatic and easily managed with diltation.
Suture techniques such as the number of layers or use of inter-
rupted versus running sutures, have shown some clinical differ-
ences. An inverting anastomosis is superior to an everting technique.
A number of investigators advocate a single layer anastomosis
because they believe it causes less narrowing of the lumen since
a smaller amount of tissue is strangulated.(26, 27) A single layer
anastomosis is also felt to cause less devascularization, infection,
and necrosis, while the continuous suture distributes tension more
evenly around the lumen.(27, 28) In clinical practice, however, tech-
nical factors such as the correct placement of sutures, correct ten-
sion of the suture, and secure knots appear to be more important
than the experimental findings discussed previously. Experience,
training, clinical judgment, and ability are major factors in a sur-
geon’s choice of anastomotic technique; however, some of the
reported experience with suturing merits additional comment.
An extensive experience using a running monofilament tech-
nique has been described by Max and colleagues.(28) In a ret-
rospective report of 1,000 single layer continuous polypropylene
intestinal anastomoses, the authors believed that this technique
was quick, simple, economical, and safe. Although an intraopera-
tive leak rate was not reported, their postoperative leak rate of 1%
with the technique compares very favorably with other that in
reports using alternate techniques.(28)
A Cochrane Database Systemic reviewed six trials with 955
ileocolic participants.(29) The three largest prospective random-
ized trials comparing stapled versus hand-sewn methods for ile-
ocolic anastomoses conducted between 1970 and 2005 showed
fewer leaks with stapled anastomosis. All other outcomes: stric-
ture, anastomotic hemorrhage, anastomotic time, re-operation,
mortality, intraabdominal abscess, wound infection, and length
of stay, showed no significant difference.
End-To-End
The use of surgical staplers has advantages in certain situations
(e.g., the very low colorectal anastomosis), and they have enjoyed
widespread clinical usage. These mechanical devices, however, do
not compensate for improper or poor technique.
In an early survey of stapler complications by the American
Society of Colon and Rectal Surgeons (ASCRS) published in
1981, 243 surgeons responded that they had performed 3,594
end-to-end anastomoses (EEA).(30) Intraoperative complica-
tions were reported in 15.1% of patients. These complications
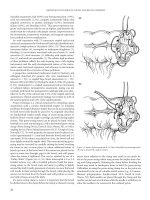
Figure 5.5 Types of anastomoses. (A) End-to-end anastomosis. (B) Side-to-side
functional end-to-end anastomosis. (C) End-to-side anastomosis. (From Beck
DE. Malignant lesions. In Beck DE, ed. Handbook of Colorectal Surgery. Quality
Medical Publishing, St Louis, MO. 1997, pp 400–430. With permission.).
consistent scientific proof that one intestinal anastomotic technique
is superior. Options range from the physical configuration of the
anastomosis (end-to-end, side-to-side, end-to-side, side-to-end, etc.
Figure 5.5) and the method used to construct it: sutures, staples, a
combination of these, and experimental methods such as compres-
sion devices or adhesives. Several of these merit discussion.
(a)
(b)
(c)