Improved Outcomes in Colon and Rectal Surgery part 6 potx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (406.03 KB, 10 trang )
improved outcomes in colon and rectal surgery
included anastomotic leak (9.8%), tear during extraction (1.9%),
anvil not extractable (1.2%), complete anastomotic failure that
required conversion to another technique (0.9%), instrument
failure (0.8%), and bleeding (0.5%). This report represents sur-
geons’ early experience with the use of staplers, and therefore the
results must be evaluated in the proper context. Improvements in
the instruments, anastomotic technique, and surgeon experience
have resulted in fewer complications.
An early experience with 73 consecutive stapled end-to-end
colorectal anastomoses by Gordon and Vasilevsky identified intra-
operative complications in 19 patients (26%).(31) These included
instrument failure (4), incomplete or inadequate doughnuts (5),
bleeding (3), bowel injury associated with use of sizers (1), anvil
extraction (1), anvil insertion (3), difficulty with stapler extrac-
tion (1), and anvil not extractable (1). The relative high incidence
of these problems reflects the early learning curve with stapling
instruments and the early developmental nature of the instru-
ments used. Increased experience and advances in instruments
have minimized the occurrence of these problems.
A prospective randomized multicenter study by Dochetry and
colleagues described 652 patients who were randomized to a
sutured (n = 321) or stapled large bowel anastomosis (n = 331)
between 1985 and 1989.(32) During the study, 5 of the 331 patients
(1.5%) randomized to a stapled anastomosis had an instrument
or technical failure. Intraoperative anastomotic testing was not
routinely performed, but postoperative radiologic leaks were iden-
tified in 14.4% of the sutured and 5.2% of the stapled colorectal
anastomoses. Clinical anastomotic leakage was evident in 4.4% of
the sutured patients and 4.5% of the stapled patients.
Proper technique is a critical component to obtaining a good
anastomosis with a circular intraluminal stapler. To minimize
problems, the largest diameter stapler that can be accommodated
by both bowel ends should be used.(33) As originally described,
an intraluminal stapler entails usage of purse-string sutures to
hold the bowel over the stapler cartridge and anvil during stapler
closure. This purse-string suture can be placed by hand (with a
baseball or in-and-out technique), with a fenestrated purse-string
clamp (Purse String Device, Davis & Geck, Wayne, NJ), or with a
stapling device (Purse String Instrument-65, U. S Surgical Corp.,
Norwalk, CT). To work properly, the sutures must be placed cor-
rectly (approximately 1–2 mm back from the bowel ends and 2–3
mm apart). If the sutures are placed too close, the bowel will not
close tightly around the stapler shaft. This nonconstricting purse-
string may be corrected by carefully cutting the bowel overlying
the suture in two or more places to release additional suture to
bunch up more of the bowel end. If the sutures are placed too far
apart or some tear through, gaps in the bowel ends will appear
when the suture is tightened. This can be repaired by use of a
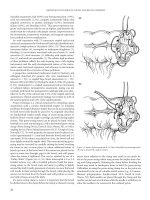
“Pulley Stitch” (Figure 5.6).(1, 34) These interrupted 4-0 or 3-0
braided sutures (e.g., silk or braided polyester) hold the purse-
string suture to the bowel ends and assist in pulling it tightly
around the shaft. Finally, placement of sutures, too near the bowel
end results in their tearing through the bowel, while placing the
sutures too far back from the bowel ends will produce an exces-
sive bulk of tissue around the shaft.
If a purse-string clamp is used, it is important that the bowel
be divided close to the clamp before the clamp is released. Leaving
excess tissue adjacent to the clamp may result in too much tis-
sue at the purse-string which may prevent the stapler from clos-
ing and firing properly. Releasing the clamp before dividing the
bowel may result in inadequate tissue to hold the purse-string.
Difficulties in using the purse-string clamp low in the pelvis are
minimized by the use of a double armed suture (e.g., 2–0 mono-
filament polypropylene, double-armed TS-9, David & Geck,
Wayne, NJ). Both needles are placed through the clamp and the
needles can be bent several times while the needle is withdrawn to
allow the needles to be removed in the confined pelvis.
Figure 5.6 Repair of pursestring stitch. (A) Gap is identified in pursestring suture.
(B) Gap is closed with «pulley» sutures.
(a)
(b)
intraoperative anastomotic challenges
Many surgeons use clamps to hold the bowel ends while plac-
ing the purse-string or to hold the bowel open to assist place-
ment of the anvil or stapler. Several problems can occur with use
of these clamps. If the clamps are placed too far back from the
bowel end and placed too tightly, an injury to the bowel wall can
occur which can produce a leak despite a secure anastomosis. If
open ended clamps (e.g., Babcock clamps) are used, it is possible
for the purse-string to go through the end of the clamp. If this
occurs, the clamp or the purse-string suture will need to be cut.
Use of solid ended clamps eliminates the chance of this happen-
ing. Large clamps increase the difficulty in inserting an anvil in
bowel diameter close to the diameter of the anvol.
Double Staple
Another end-to-end stapling option involves a double staple
technique.(35, 36) With this method a linear staple line is placed
across the distal bowel and a circular stapler is inserted into this
bowel (via the anus for a left-sided anastomosis). To avoid creat-
ing an ischemic area, the trocar of the circular stapler should exit
adjacent or as close as possible to the linear staples. The anvil is
placed in the proximal bowel and secured with a purse-string as
described previously. When closed and fired, the circular stapler
removes a portion of the crossed linear staple line to create the
anastomosis. Concern was initially expressed about these cross-
ing staple lines. However, subsequent experimental and clinical
evidence has confirmed the relative safety of this method.(37, 38)
The double staple technique is helpful in anastomosing bowel
ends of dissimilar size and in ultralow colorectal or coloanal
anastomoses. Outside of these situations, the extra cost of using
a stapler rather than a sutured purse-string argues more for the
use of a purse-string.
With low distal staple lines, it can be challenging to insert the
stapler into the anus and not disrupt the staple line. Distal staple
line disruption can occur if the distal bowel is tenous or under
too much traction. It has anecdotically seemed to occur more fre-
quently with the use of a contour stapler (Ethicon). If this occurs,
several options are available. Initial action is to visualize the distal
staple line. If the ends of the partially closed bowel can be grasped
with clamps or traction sutures, the amount of residual bowel can
be assessed. If adequate length is present the bowel can be closed
with a linear stapler placed below the disrupted staple line. After
the stapler is fired, the residual bowel end can be resected with
scissors or a scalpel. A second option is to recluse the disrupted
staple line with sutures placed from the abdominal side or placed
intralumenally via a retractor placed into the anal canal.(39) If the
bowel is successfully closed, the anastomosis can proceed. If the
distal segment of bowel is impossible to close, a musectomy can
be performed via the anus and a hand-sewn coloanal or ileoanal
anastomosis can be performed.
A serious problem associated with double stapling of the low
rectum is the inadvertent creation of a recto-vaginal fistula. This
unfortunate complication results from incorporating the poste-
rior wall of the vagina into the staple lines. Maneuvers to reduce
this occurrence include an adequate dissection of the rectum off
the posterior vagina, careful visualization of the bowel ends dur-
ing closure of the stapler, and intravaginal palpation of the poste-
rior vaginal wall before firing the stapler.(1)
A variation of double stapling is triple stapling. In this anasto-
motic method, an extra linear stapler is used to close the bowel
end after placement of the anvil into the proximal bowel.
The anvil trocar is then advanced through the closed bowel.
This technique has been suggested for intracorporeal laparoscopic
techniques; however, it is costly and produces another linear
suture line that must be incorporated into the final anastomotic
staple line. The technique has not gained widespread acceptance
due to the relative ease in placing the proximal purse-string.
Difficulty with anvil insertion in the proximal bowel lumen
usually occurs when the stapler is too large for the diameter
of the bowel. Experience or the use of scissors allows accurate
selection of the correct size of circular stapler. Additional helpful
techniques include the use of dilators to overcome bowel spasm,
lubrication of the anvil head (with betadine, saline, or blood),
and distraction of the bowel ends with three small-ended forceps
or clamps. Use of a recently developed low profile anvil (CDH
Ethicon-Endosurgery, Inc. Cincinnati, Ohio) has diminished this
occurrence.
Detachable Staplers
For colorectal anastomosis, the circular stapler is usually placed
through the anus. With currently available detachable head sta-
plers, the flat stapler shaft may be difficult to pass atraumatically
through the anal sphincter muscles. Khoury and Opelka, in 1995,
described a technique to facilitate this maneuver.(40) A Faensler
or Chelsey-Eaton anoscope allows a gradual controlled dilation of
the sphincters. After removal of the obturator, the stapler shaft can
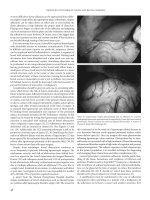
easily be passed through the anoscope (Figure 5.7). Once through
the sphincter, the stapler must be inserted up to the resected end of
the rectum. Knowledge of rectal anatomy, adequate mobilization
of the posterior rectum, and selection of an appropriate size of sta-
pler assist in accomplishing this advancement. Incorrect insertion
can tear or split the rectum. Such an injury to the rectum man-
dates a very low or coloanal anastomosis to reestablish intestinal
continuity. A proctoscopic examination of the rectum insures an
adequate lumen, confirms an adequate preparation and mobiliza-
tion, and assists in identifying the apex of a Hartman’s pouch.
Once the stapler is closed and fired it must be removed. Stapler
extraction from the anastomotic area may be aided with a trac-
tion stitch. Bowel spasm or a stapler misfire may cause extrac-
tion difficulty. Gentle traction and careful stapler manipulation
usually allow it to be removed. If a misfire results in inability to
remove the stapler, it may be necessary to excise and reaccomplish
the anastomosis.
End-To-Side and Side-To-Side (Functional End-To-End)
An end-to-side or side-to-end (the proximal bowel is usually listed
first) is useful for joining bowel of different diameter. The size of
the anastomosis is not limited by the bowel diameter. This con-
figuration is often used for ileocolic or ileorectal anastomoses.
A side-to-side anastomosis is frequently used to join bowel with
a linear cutting stapler. Use of the bowel ends for a side-to-side
anastomosis, serves as a functional end-to-end anastomosis. A
surgical atlas should be consulted for additional technical details.
A meta-analysis of studies published between 1992 and
2005 of end-to-end versus other anastomotic configurations in
improved outcomes in colon and rectal surgery
Crohn’s disease used eight studies including 661 patients.(41)
The authors conclude that a side-to-side anastomosis led to fewer
anastomotic leaks and overall complications, a shorter hospital
stay, and a perianastomotic recurrence rate comparable to end-
to-end anastomoses.
anastomotIC testIng
All surgeons test their anastomoses in some way. At a minimum,
the anastomotic site is inspected and in some cases palpated.
A visual inspection of a side-to-side anastomosis may be per-
formed before closing the ends of the bowel. Gentle constriction
of the bowel proximal or distal to the anastomosis will confirm
a patent lumen and the absence of a gross leak. A more sensitive
test can easily be performed in the colorectal anastomosis (which
is at higher risk for a leak).(42–45)
The author prefers to test low colorectal anastomosis with
intraluminal instillation of a dilute solution of povidine-iodine
(Betadine, Purdue Frederick Co, Norwalk, CT). After the bowel
is occluded above the anastomosis with finger pressure, the
testing solution is instilled gently with a bulb syringe inserted
into the anus. Any leak is readily apparent. Irrigation with this
dilute providine-iodine solution also provides antimicrobial and
tumorcidal activity. Others have suggested testing with a dilute
solution of methylene blue.(46) Larger volumes are infused via
a rectal tube, and with care even ileocolic anastomosis can be
tested for leaks with this technique. The optimal pressure recom-
mended for detecting intraopeartive leaks with air/water testing is
25–30 cm H
2
O.(47, 48) If an infusion system is used, the pressure
can be controlled by the height of the infusion bag.
Some surgeons prefer to test their anastomosis with air.(45) The
pelvis is first filled with saline and the distal bowel (containing the
anastomosis) is distended with air (instilled transanally). Any anas-
tomotic defect will produce air bubbles. Unfortunately, with this
method it is often difficult to accurately identify the location of the
leak if any blood has mixed with the saline. The saline must also be
removed before any identified leak can be repaired. Testing with
air may be preferable for higher colorectal anastomosis as infused
intralumenal fluid may not reach a higher anastomosis.
A proctoscope can also be used to inspect the colorectal anas-
tomosis. Sufficient lumen size is usually confirmed by the lack
of stenosis, hemostasis is confirmed, and the bowel can easily be
distended with air.
Finally, some surgeons inspect the intraluminal stapler “dough-
nuts.” The author has not found this to be helpful as complete
“dough-nuts” do not ensure the absence of a leak at the anas-
tomotic site (e.g., due to a tear of the bowel or staple lines dur-
ing stapler removal). Also, an incomplete “dough-nut” may be
produced with an intact anastomosis. Intraoperative testing as
described above is more sensitive and specific.
Whatever method is used to inspect or test an anastomosis, it is
important to act on any defect or leak identified. Options include
suture reinforcement, reconstruction, or proximal diversion.
Challenges
Inadequate Anastomotic Lumen
Adequate lumenal patency is important for several reasons. Bowel
edema occurs in the perioperative period, and a marginal lumen
Figure 5.7 Anoscopic assisted stapler insertion. (A) Faensler anoscope is inserted
after gentle anal dilation. (B) The anoscope obturator is removed and the circular
stapler is inserted through the anoscope. (C) The anoscope is withdrawn and
taken off the shaft of the stapler.
(a)
(b)
(c)
intraoperative anastomotic challenges
may lead to a partial obstruction. The anastomotic lumen can be
sized by palpation or visually inspected. The ability to remove the
anvil of a circular stapler confirms a lumen corresponding to the
size of the stapler, while distal rectal anastomosis can be evaluated
by a proctoscope. An alternative technique for colorectral anasto-
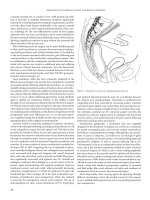
mosis is an isoparastaltic side-to-side anastomosis (Figure 5.8).
Leakage
An accurate incidence of anastomotic leakage is difficult to deter-
mine. Few studies have reported the incidence of intraoperatively
identified anastomotic problems. The incidence of leaks identi-
fied in the postoperative period is described in chapter 6.
If a defective anastomosis is identified, it may be repaired in
several ways. Additional sutures can approximate a small gap, or
the anastomosis can be resected and completely redone using
a stapler or hand-sewn technique. Another option is to replace
purse-string sutures around the defective anastomosis and rein-
sert a new stapler through the lumen. The purse-string sutures are
tightened, which should close the defect and hold the previously
placed staples toward the stapler shaft. After closure and firing of
the new stapler, the new donuts (which should also contain the
old staples) are removed with the stapler.(49) If the anastomosis
is very low, the defect may also be repaired transanally.
Anastomotic Hemorrhage
Hemorrhage can occur at both a staple and a suture line. Proper
size staple height and correct tension of sutures minimize the
occurrence of this problem. Techniques to stop hemorrhage
include cautery of the bleeding vessels or placement of a suture at
the site of bleeding. Excessive cautery is to be avoided as the staple
line has the potential to transfer the electrical energy to adjacent
portions of the bowel. Reduction or stoppage of the bleeding may
also be helped by digital compression or intraluminal instillation
of an epinephrine solution (1 to 100,000 or 1 to 200,000 u/mL).
Another option is submucosal injection of an epinephrine solu-
tion.(50)
Proximal Protection (Stomas)
For high-risk anastomosis, a proximal diverting stoma is often
used. A diverting stoma will not prevent an anastomotic leak but
will reduce the septic morbidity and mortality associated with
the leakage. A properly constructed loop stoma is almost totally
diverting.(51) However, if absolute total diversion is desired, a
Prasad type of end loop stoma may be constructed.(52)
If diversion is needed, the author and editors prefer an ileos-
tomy over a colostomy. A diverting colostomy following a colonic
resection has several problems. A colostomy includes a larger
stoma, and due to its proximal location, the ostomy output is
loose or liquid and very odorous. If a significant colonic resection
has been performed, the remaining colon length is often insuf-
ficient to easily reach the abdominal wall at a preferred stomal
location.
A loop ileostomy has several advantages.(53) First, it is easy
to construct and close. As it is usually created in bowel removed
from the anastomotic site, tension and blood supply are rarely a
problem. Ileostomy output is liquid, has little odor, and unless
the mesentery is abnormally shortened, an ileostomy will reach
almost any site on the abdominal wall.
Adjuvants and Drains
Due to the morbidity associated with leaks, several adjuvants have
been used in high risk of potential compromised anastomoses.
Wrapping the anastomosis with omentum is a popular adjunct
that is felt by many surgeons to prevent disruption. Unfortunately,
there is no evidence to support this practice in humans.(54, 55)
The use of foreign materials around the anastomosis has been
shown to be harmful.(56, 57) Reinforcing sutures positioned
around a stapled anastomosis, while not routinely necessary, may
provide security especially for low rectal anastomoses.
Controversy continues regarding the use of drains as an
adjunct to intestinal anastomosis. The abdominal cavity cannot
be adequately drained, but in cavities like the low pelvis it is pos-
sible. Proponents believe that the drain removes contaminated
fluid and blood and, should a leak occur, it would be controlled.
Opponents argue that the drain is dangerous as it allows bacteria
a portal of entry and it may erode the anastomosis. Trials have
clearly shown no benefit from drainage of intestinal anastomoses.
(58, 59) Despite evidence to the contrary, the practice of closed
suction drainage for low pelvic anastomoses the first few days
postoperatively continues due to individual surgeon’s beliefs.(5)
summary
Adherence to established surgical principles and techniques
should minimize anastomotic problems. Mechanical devices
cannot overcome limitations in experience, skill, or judgment.
Intraoperative identification of problems that occur permits cor-
rection with minimal morbidity.
Figure 5.8 Isoparastaltic side-to-side functional end-to-end anastomotic technique.
improved outcomes in colon and rectal surgery
referenCes
1. Beck DE. Intraoperative anastomotic complications. In Hicks
TC, Beck DE, Opelka FG. Timmcke AE, eds. Complications
of Colon and Rectal Surgery. Williams & Wilkins, Baltimore,
1996: 70–81.
2. Goligher JC. Surgery of the Anus, Rectum, and Colon. 5th
ed. London: Balliere Tindall; 1984.
3. Steichen FM, Ravitch MM. Contemporary stapling instru-
ments and basic mechanical suture techniques. Surg Clin
North Am 1984; 64: 425–40.
4. Rafferty JF. Obtaining adequate bowel length for colorectal
anastomosis. Clin Colon Rectal Surg 2001; 14: 25–31.
5. Sweeney WB. Intra-abdominal anastomotic techniques. Clin
Colon Rectal Surg 2001; 14: 15–23.
6. Delaney CP, Mackeiggan JM. Preoperative management –
risk assessment, medical evaluation, and bowel preparation.
In Wolff BG, Fleshman JW, Beck DE, Pemberton JH, Wexner
SD, eds. ASCRS Textbook of Colorectal Surgery. Springer-
Verlag, New York, 2007: 116–29.
7. Demetriades D, Murray JA, Chan L et al. Penetrating colon
injuries requiring resection: diversion or primary anastomo-
sis? An AAST prospective multicenter study. J Traum 2001;
50: 765–75.
8. van Geldere D, Fa-Si-Oen P, Noach LA et al. Complicatons
after colorectal surgery without mechanical bowel prepara-
tion. J Am Coll Surg 2002; 194: 40–7.
9. Guenga KF, Matos D, Castro AA, Atallah AN, Wille-Jorgensen
P. Mechanical bowel preparation for elective colorectal sur-
gery. Cochrane Database Syst Rev 2003; 2: CD001544.
10. Slim K, Vicaut E, Panis Y et al. Meta-analysis of randomized
clinical trials of colorectal surgery with or without mechani-
cal bowel preparation. Br J Surg 2004; 91: 1125–30.
11. Torralba JA, Robles R, Parrilla P et al. Subtotal colectomy
vs. intraoperative colonic irrigation in the management of
obstructed left colon carcinoma. Dis Colon Rectum 1998; 41:
18–22.
12. Kressner U, Antonsson J, Ejerblad S et al. Intraoperative
colonic lavage and primary anastomosis – an alternative to
Hartmann procedure in emergency surgery of the left colon.
Eur J Surg 1994; 160: 287–92.
13. Forloni B, Reduzzi R, Paludetti A et al. Intraoperative colonic
lavage in emergency surgical treatment of left-sided colonic
obstruction. Dis Colon Rectum 1998; 41: 23–7.
14. Wexner SD, Beck DE. Sepsis prevention in colorectal surgery.
In Fielding LP, Goldnerg SM, eds. Operative Surgery 5th Ed.
Butterworth-Heineman, Ltd. London, 1993: 41–6.
15. Irvin TT, Goliger JC. Etiology of disruption of intestinal
anastamosis Br J Surg 1973; 60: 461–4.
16. Schrock TR, Deveney CW, Dunphy JE. Factors contribut-
ing to leakage of colonic anastomoses. Ann Surg 1973; 177:
513–8.
17. Khoury GA, Waxman BP. Large bowel anastomosis. The
healing process and sutured anastomosis: a review. Br J Surg
1983; 70: 61–3.
18. Hsu T-C. One-stage resection and anastomosis for acute
obstruction of the left colon. Dis Colon Rectum 1998; 41:
28–32.
19. Weiber S, Jiborn H, Zederfeldt B. Preoperative irradiation
and colonic healing. Eur J Surg 1994; 160: 47–51.
20. Karulf R. Anesthesia and intraoperative positioning. In Hicks
TC, Beck DE, Opelka FG, Timmcke AE. eds, Complications
of colorectal surgery. Baltimore: Williams & Wilkins, 1996:
34–9.
21. Le TH, Gathright JB, Jr. Reconsitution of intestinal continu-
ity after extended left colectomy. Dis Colon Rectum 1993; 36:
197–8.
22. Graffner H, Andersson L, Lowenhielm P, Walther B. The
healing process of anastomoses of the colon: a comparative
study using single, double-layer or stapled anastomosis. Dis
Colon Rectum 1984; 27: 767–71.
23. Fingerhut A, Hay JM, Elhadad A et al. Supraperitoneal
colorectal anastomoses: hand-sewn versus circular staples - a
controlled clinical trial. Surgery 1995; 118: 479–85.
24. Wheeless CR Jr, Smith JJ. A comparison of the flow of iodine
125 through three different intestinal anastomoses: standard,
Gambee, and stapler. Obstet Gynecol 1983; 62: 513–8.
25. MacRae HM, McLeod RS. Handsewn vs. stapled anastomo-
ses in colon and rectal surgery: a meta-analysis. Dis Colon
Rectum 1998; 41: 180–9.
26. Gambee LP, Garnjobst W, Hardwisk CE. Ten years’ experi-
ence with a single layer anastomosis in colon surgery. Am J
Surg 1956; 92: 222–7.
27. Templeton JL, McKelvey ST. Low colorectal anastomoses:
an experimental assessment of two sutured and two stapled
techniques. Dis Colon Rectum 1985; 28: 38–41.
28. Max E, Sweeney WB, Baily HR et al. Results of 1,000 single-
layer continuous polypropylene intestinal anastomoses. Am
J Surg 1991; 162: 461–7.
29. Choy PY, Bissett IP, Docherty JG, Parry BR, Merrie AE.
Stapld versus handsewn methods for ileocolic anastomoses.
Cochrane Database Syst Rev 2007; 3: CD004320.
30. Smith LE. Anastomosis with EEA stapler after colonic resec-
tion. Dis Colon Rectum 1981; 24: 236–42.
31. Gordon PH, Vasilevsky CA. Experience with stapling in
rectal surgery. Surg Clin North Am 1984; 64: 555–66.
32. Dochetry JG, McGregor JR, Akyol AM, Murray GD, Galloway
DJ. Comparison of manually constructed and stapled anas-
tomoses in colorectal surgery. Ann Surg 1995; 221: 176–84.
33. Fazio VW. Cancer of the rectum-sphincter-saving operation.
Stapling techniques. Surg Clin North Am 1988; 68: 1367–82.
34. Last MD, Fazio VW. The rational use of the purse-string
device in constructing anastomoses with the circular stapler.
Dis Colon Rectum 1985; 28: 979–80.
35. Cohen Z, Myers E, Langer B et al. Double stapling tech-
nique for low anterior resection. Dis Colon Rectum 1983; 26:
231–5.
36. Griffen FD, Knight CD. Stapling technique for primary and
secondary rectal anastomoses. Surg Clin North Am 1984; 64:
579–90.
37. Julian TB, Ravitch MM. Evaluation of the safety of end-
to-end (EEA) stapling anastomoses across linear stapled
closures. Surg Clin North Am 1984; 64: 567–78.
38. Ravitch MM. Varieties of stapled anastomosis in rectal resec-
tion. Surg Clin North Am 1984; 64: 543–54.
intraoperative anastomotic challenges
39. Tan WS, Ng KH, Eu KW. Salvaging a linear staple line defect
in ultra-low anterior resection. Tech Coloproctol 2007; 11:
266–7.
40. Khoury DA, Opelka FG. Anoscopic-assisted insertion of ene-
to-end anastomosing staplers. Dis Colon Rectum 1995; 38:
553–4.
41. Simillis C, Purkayastha S, Yamato T et al. A meta-analysis
comparing conventional end-to-end anastomosis vs. other
aastomotic configurations after resection in Crohn’s disease.
Dis Colon Rectum 2007; 50: 1674–87.
42. Beard JD, Nicholson ML, Sayers RD, Lloyd D, Everson NW.
Intraoperative air testing of colorectal anastomoses: a pro-
spective tandomized trial. Br J Surg 1990; 77: 1095–7.
43. Griffith JM, Trapnell JE. Intraoperative testing of anasto-
motic integrity after stapled anterior resection for cancer. J R
Coll Surg Edinb 1990; 35: 35–6.
44. Yalin R, Aktan AO, Yegen C, Döslüog lu H, Okboy N.
Importance of testing stapled rectal anastomoses with air.
Eur J Surg 1993; 159: 49–51.
45. Davies AH, Bartolo DC, Richards AE, Johnson CD, McC
Mortensen NJ. Intra-operative air testing: an audit on rectal
anastomosis. Ann R Coll Surg Engl 1988; 70: 345–7.
46. Smith S, McGeehin W, Kozol RA, Giles D. The efficacy of
intraoperative methylene blue enemas to assess the integrity
of a colonic anastomosis. BMC Surg 2007; 7: 15.
47. Gilbert JM, Trapnell JE. Intraoperative testng of the integrity
of left-sided colorectal anastomosis: a technique of value to the
surgeon in training. Ann R Coll Surg Engl 1988; 70: 158–60.
48. Wheeler JM, Gilbert JM. Controlled intraoperative water
testing of left-sided colorectal anastomosis: are ileostomies
avoidable. Ann R Coll Surg Engl 1999; 81: 105–818.
49. Makabeli G, Williams LG. Repair of defective EEA anasto-
mosis. Dis Colon Rectum 1984; 27: 490–1.
50. Perez RO, Sousa A Jr, Bresciani C et al. Endoscopic manage-
ment of postoperative stapled colorectal anastomosis hem-
orrhage. Tech Coloproctol 2007; 11: 64–6.
51. Pearl RK, Abcarian H. Diverting stomas. In MacKeigan JM,
Cataldo PA. Intestinal Stomas: Principles, Techniques, and
Management. St. Louis, MO: Quality Medical Publishing,
Inc 1993: 107–26.
52. Prasad ML, Pearl RK, Orsay CP. End-loop ileocolostomy
for massive trauma to the right side of the colon. Arch Surg
1984; 119: 975–6.
53. Williams NS, Nasmyth DG, Jones D et al. De-functioning
stomas: A prospective controlled trial comparing loop
ileostomy with transverse colostomy. Br J Surg 1986: 73:
566–70.
54. Carter DC, Jenkins DHR, Whitfield HN. Omental reinforce-
ment of intestinal anastomoses. Br J Surg 1972; 129–33.
55. McLachlin AD, Denton DW. Omental protection of intesti-
nal anastomoses. Am J Surg 1973; 125: 134–40.
56. Trowbridge PR, Howes EL. Reinforcement of colon anasto-
moses using polyurethane foam treated with neomycin: an
experiemental study. Am J Surg 1967; 113: 236–40.
57. Laufman H, Method H. Effects of absorbable foreign
substance on bowel anastomosis. Surg Gynecol Obstet 1948;
86: 669.
58. Hoffmann J, Shokouh-Amiri MH, Damm P, Jensen R.
A prospective, controlled study of prophylactic drainage after
colonic anastomoses. Dis Colon Rectum 1987; 30: 449–52.
59. Sagar PM, Couse N, Kerin M, et al. Randomized trial of drain-
age of colorectal anastomoses. Br J Surg 1993; 80: 769–71.
6
Other intraoperative challenges
James T McCormick and Sharon G Gregorcyk
CHALLENGING CASE
You are operating on 64-year-old man with a locally advanced
rectal cancer that has been treated with neoadjuvant chemora-
diation. The mass is large, the dissection is difficult and the tissue
is edematous and friable. As you are dissecting along the right
pelvic sidewall your assistant adjusts the retractor and there is an
immediate, brisk rush of blood into the field. You attempt to pack
the area but the blood soaks the lap immediately. Your patient
is bleeding. You also note significant edema and are already
concerned about closing the abdomen.
CASE MANAGEMENT
You alert the anesthesia personnel of the occurrence of ongoing
blood loss. Various intraoperative challenges exist and can occur
on any given case. Some challenges may be predictable while
others may not. One must be prepared to address any number
of events during any given case. All of these challenges should be
approached with calm reason and sound surgical principles to
optimize the outcome.
PREOPERATIVE EVALUATION
The preoperative evaluation is useful to anticipate and in some
cases minimize intraoperative challenges. As always, one should
start with a thorough history and physical examination. The surgi-
cal history including indications and complications is important
in predicting intraabdominal adhesive disease. Multiple abdomi-
nal surgeries, intraabdominal abscess, perforation, hernia repairs
with mesh, and enterocutaneous fistulae are all concerning for sig-
nificant adhesive disease. On examination, a stiff, noncompliant,
scarred abdominal wall adds to the concern.
With regards to bleeding risk, you should question the patient
about any prior bleeding problems with surgery, easy bruising,
bleeding gums when brushing teeth, heavy menses, or a family
history of hemorrhagic complications. Any of these may indi-
cate an underlying coagulation disorder. Medical problems such
as renal failure, hepatic failure, and portal hypertension should
likewise raise a red flag with regards to bleeding risk. If there is no
indication by history of a bleeding problem, routine blood work
to further assess this issue is not indicated as the yield is very low.
(1) When further evaluation is needed, a bleeding time is the
most effective single test covering all aspects of the coagulation
system. If it is prolonged, then further testing is necessary.
Another important component of the history is the patient’s
medication list. Easy to identify medications that increase the risk
for bleeding are warfarin, aspirin, non-steroidal anti-inflammatory
drugs (NSAIDs), clopidogrel bisulfate, and ticlopidine hydrochlo-
ride. More subtle, often missed and increasingly popular are herbs,
vitamins, and dietary supplements. Some of the more common
agents that can prolong bleeding time include garlic, ginkgo, ginseng,
capsaicin, fish oil, ginger, and vitamin E.(2) In general, these agents
should be stopped 1 week before surgery. The same is true for aspi-
rin, NSAIDs, and clopidogrel bisulfate, while warfarin is held 3 to
5 days before surgery.(3) For patients at a higher risk for a throm-
boembolic event, such as those with recent coronary stent, a recent
(<3 mo) history of venous thromboembolism, or mechanical
cardiac valve in the mitral position, a discussion with the patients
treating physician or cardiologist is advised and the risks and
benefits weighed.(4) In the emergency setting, platelets and fresh
frozen plasma may be necessary to immediately address bleeding
disorders, iatrogenic or otherwise.
INTRAOPERATIVE HEMORRHAGE
Intraoperative bleeding ranges from a small amount of oozing
to major hemorrhage. Even small, pesky bleeding can be an issue
laparoscopically by absorbing light and obscuring the view. Using
cautery or devices such as the Harmonic scalpel™ (Johnson and
Johnson) or Ligasure™ (Covidien) may help minimize this obsta-
cle. Dividing major vessels can always result in significant bleeding
if the vessel is not adequately ligated. For the open case, simply
regrasping the vessel and tying it off takes care of the problem.
Laparoscopically, one can quickly lose visualization and lose
track of the vessel. Being prepared can help avert this problem.
One technique is to hold on to the proximal portion of the ves-
sel being divided so it can be quickly occluded. A surgical clip
or Endoloop™ (Johnson and Johnson) can then be applied if
needed. Having those supplies in the room where they can be eas-
ily and quickly accessed is advantageous. Most important is wisely
choosing the device with which to divide the vessel. Older patients
may have atherosclerotic disease in their vessels with calcification,
which may cause devices such as the Ligasure™ (Covidien) to be
less effective. In these cases, stapling, clipping, or tying the vessels
may be more prudent.
The spleen can be a source of profound bleeding. Its anatomic
relationship to the colon and omentum makes it vulnerable
to injury, especially during mobilization of the splenic flexure.
Dividing these attachments without retracting too vigorously is
key to avoid a splenic capsular tear/avulsion. This can be achieved
by approaching the splenic flexure from different angles—medially
through the lesser sac, inferiorly coming over top of Gerota’s fascia,
and laterally by dissecting along the white line of Toldt.
If an injury does occur to the spleen, it is most commonly a cap-
sular tear that can be controlled by electrocautery. If the bleeding
is brisk, the spleen should be packed off and preparations made
to address the bleeding. The anesthesiologist should be alerted
to the potential ensuing blood loss. Once the anesthesiologist is
prepared, having given adequate fluids, and with blood products
available, the packs can be removed. Topical hemostatic agents,
such as microfibular collagen, methylcellulose, or fibrin glue may
be necessary and should be available. An argon beam coagulator
can also be beneficial in this setting. Other key preparatory points
other intraoperative challenges
are adequate exposure, working suction, and appropriate length
instruments. Laparoscopic splenic injuries may require conversion
to an open procedure to control the bleeding. However, the risk
of splenic injury is actually reported to be lower in laparoscopic
versus open cases with one series revealing no splenic injuries in
almost 2,000 laparoscopic colectomies compared to 0.24% in over
5,000 open colectomies.(5)
Once the packs are removed, if the bleeding cannot swiftly be
stopped by simple means, the spleen should be mobilized into the
operative field dividing its avascular ligaments with electrocautery.
While mobilizing the spleen, pressure is held directly on the spleen
or the splenic hilum to slow down the bleeding. The decision now
must be made as to whether splenic salvage or splenectomy should
be performed.
While one should be aggressive in attempting to save the spleen,
these attempts should not continue in the face of ongoing bleed-
ing or if the patient is unstable. A splenectomy may be necessary
and is very affective in stopping the bleeding. Splenectomy carries
a 5% lifetime risk of postsplenectomy sepsis syndrome, primar-
ily from encapsulated bacteria such as Streptococcus pneumoniae,
Haemophilus influenzae, and Neisseria meningitidis. Vaccinations for
pneumococcal, meningococcal and H. influenza are recommended
following splenectomy to curtail this incidence.(6–8) Additionally,
antibiotic prophylaxis and aggressive treatment of infections may
be advocated. Early complications associated with a splenectomy
include pneumonia, pancreatitis, and subphrenic abscess.
Attempts to preserve the spleen include partial splenectomy,
mattress suture repair, and mesh wrap. The mesh wrap is per-
formed with a polyglycolic mesh. A keyhole is cut in the mesh and
the spleen is passed through the defect such that the hole is encircl-
ing the splenic hilum. The mesh is then wrapped around the spleen
and sutured to itself resulting in compression on the spleen.(9)
Hemorrhage in the pelvis is a particularly difficult challenge.
In addition to the vessels themselves being difficult to control, the
confines of the pelvis limit the exposure and space in which to work.
A bulky tumor, inflammation, or radiation changes can magnify
the complexity. Portal hypertension which results in enlargement
of numerous pelvic collaterals can complicate matters further.
While bleeding associated with the posterior vaginal wall or
prostate can be frustrating, it does not compare to the potentially
exsanguinating hemorrhage that can occur from the pelvic side
wall or presacral region. Once again, immediate packing of the
region should be performed in an attempt to slow bleeding while
preparing to definitively control it. Long instruments, an extra
suction device, and any necessary equipment should be gathered.
Anesthesiologists should adequately resuscitate the patient and
be ready to give blood products.
The walls and floor of the pelvis are lined by the endopelvic
fascia. If this fascia is not violated, then bleeding is not typically
an issue. Deep to this endopelvic fascia on the side walls are the
internal iliac veins. Injury to one of these veins results in profuse
bleeding. The vessels are large and thin walled which can make
suturing difficult. Suture ligation is the best option. Compressing
the iliac artery to decrease the inflow may be of benefit but will
not stop the bleeding. If a vascular surgeon is readily available, this
expertise can prove very helpful. They routinely suture vessels and
are less likely to tear thin-walled vessel while attempting repair.
They will need assistance with exposure and help keeping the
blood suctioned out. This double-team approach with two skilled
surgeons is most advantageous. Other tools that may be useful are
clip appliers. The laparoscopic instruments, even in an open case,
can give extra length that may be necessary deep in the pelvis.
Presacral hemorrhage can also result from violation of the
endopelvic fascia over the sacrum and injuring the underlying pre-
sacral vein. These avalvular veins communicate with the internal
vertebral venous system through the basivertebral vein. This system
can attain high pressures and result in profuse bleeding. The veins
retract into the sacral foramen, which is problematic. In contrast
to injury to the iliac vein, packing the pelvis in the case of presacral
vein injury may be sufficient to stop the bleeding. When possible,
the specimen should be resected to optimize access. The packing
may need to be left in place for 10 minutes or more to be effec-
tive and patience is needed. Once again, having resources ready
to control the bleeding once the packs are removed is paramount.
The electrocautery should be turned up to high levels (up to 60–80
watts) and at times it alone can control the bleeding. Clips or suture
ligation can work, but are limited secondary to the retraction of the
vessels and the lack of mobility of the presacral tissue. Titanium
thumb tacks are commercially available and can be placed directly
into the sacrum to occlude the vessels. Multiple thumb tacks may
have to be placed. If the bleeding does not stop but is sufficiently
retarded, then topical agents or repacking the pelvis may achieve
complete hemostasis.
In severe cases of pelvic hemorrhage, when all else fails, the pelvis
should be tightly packed and the patient taken to the ICU for resus-
citation and correction of any coagulopathies. In the rare case of an
arterial injury, angiographic embolization may be useful. Typically,
the patient is taken back to the operating room in 24–36 hours
after having been optimized. At this time, most bleeding will have
stopped or at least become manageable. With any massive hemor-
rhage, consideration should be given to using a cell-saver to allow
autotransfusion. In addition to transfusing blood, FFP and plate-
lets may be necessary. The patient should be warmed to further
improve their clotting ability. Another issue to be addressed, in the
pelvis especially, is to whether to proceed with an anastomosis. The
patient’s hemodynamic status dictates this decision. Poor perfu-
sion to an anastomosis would result in a high risk of a leak, while a
pelvis full of blood clots would increase the risk of infection. Both
are deterrent to a successful anastomosis. Additionally, the actual
time to do the anastomosis may be a consideration, as this may
contribute to hypothermia and blood loss.
With laparoscopic surgery, an additional bleeding risk occurs at
each trocar site. A vessel can be injured during placement of the port
within the abdominal wall. The most commonly injured abdominal
wall vessels during laparoscopy are the inferior epigastric vessels,
with an average incidence of approximately 0.1%.(10) As the ports
themselves will often tamponade a vessel injured during insertion,
it is wise to remove as many ports as possible under direct laparo-
scopic visualization. Recognizing and dealing with the injury at this
point will prevent an untimely return the operating room and/or the
morbidity related to hematoma. The majority of time this is a small
vessel and can be controlled with pressure or cautery alone. In the
case of continued bleeding, the incisions may need to be extended
and suture ligation of the vessel performed. Alternatively, bleeding
6
improved outcomes in colon and rectal surgery
from the abdominal wall can be addressed by placing a stitch across
the port site defect. This can be accomplished with use of a Keith
needle or an Endo Close™ (Covidien) device. The stitch is passed
externally through full thickness abdominal wall into the peritoneal
cavity, grasped within the peritoneum, and laparoscopically passed
back out and tied. This can repeated as necessary.
DAMAGE CONTROL
The term damage-control laparotomy refers to a management
strategy first described for use in the unstable multiple organ
trauma patient.(11–14) The goal is to stop hemorrhage, cur-
tail contamination, and remove or debride any frankly necrotic
tissue. Reconstruction, definitive therapy, and abdominal wall
closure are deferred in favor of correction of metabolic derange-
ments, hypothermia, and coagulopathy, with the plan, ultimately,
to return to the operating room for completion of surgical ther-
apy and abdominal wall closure. This usually involves some sort
of temporary containment of the viscera and packing of the
open wound. It is not hard to imagine that these basic concepts
and principles may be applied to any patient who may benefit
from an abbreviated initial operation followed by stabilization
and optimization before definitive management.(15, 16)
As there is potential morbidity associated with leaving the
abdominal wall open and multiple trips to the operating room, indi-
cations must be carefully considered and proper patient selection
is critical. Patients with poorly controlled metabolic derangements
and acidosis, significant hypothermia, and clinical evidence of
coagulopathy may be considered appropriate candidates. Selection
criteria have been summarized as follows: inability to achieve
hemostasis due to coagulopathy, time-consuming procedure in an
appropriate patient (>90 min), inaccessible major venous injury,
associated life-threatening injury in a second anatomical location,
planned reassessment (in 24–72 hours) of abdominal contents (as
in a patient with questionable bowel viability), inability to close fas-
cia due to visceral edema, or concern for development of abdomi-
nal compartment syndrome.(17, 18)
The most common indication is related to hemorrhage and
massive resuscitation. This may be accompanied by hemodynamic
instability, coagulopathy, cardiac ischemia and often, massive
bowel edema. In the nontrauma venue this may be the patient who
has received large volume resuscitation for lower gastrointestinal
or intraoperative hemorrhage or who has returned to the operat-
ing room for postoperative hemorrhage. During the course of the
operation previously hemostatic sites may begin to bleed signal-
ing coagulopathy—dilutional, consumptive, and/or hypothermia
related. In this bleak scenario, it may be reasonable to pack the
abdomen, apply an occlusive dressing, and take the patient to ICU
for aggressive rewarming, ongoing resuscitation, and optimiza-
tion, followed by a return to the operating room in 24–72 hours
when these variables have been minimized. Likewise, a patient may
escape the coagulopathic and hypothermic effects of large volume
resuscitation but massive edema may manifest as increased pul-
monary pressures or hemodynamic compromise from abdominal
compartment syndrome when the fascia is closed.(19)
Occasionally, massive bowel edema can preclude closure of
the fascia and forcing the issue can lead to abdominal compart-
ment syndrome or at the very least compromise pulmonary
function. This can be seen when operating for bowel obstruction
but can be worsened further when septic complications accompany
the obstruction. Even after the obstruction is relieved, the bowel
remains edematous from both the obstruction and from the resus-
citation. Serosal compromise is not uncommon, in sometimes-
dramatic fashion, when longitudinal tears occur secondary to
massive dilation. The integrity of the bowel is in question but resec-
tion would be too extensive and anastomosis dubious. Protecting
the bowel and applying a suction dressing would allow for resolu-
tion of the systemic inflammatory response and diuresis, followed
by reoperation, reassessment, and definitive abdominal closure.
Abdominal compartment syndrome can be caused by increased
retroperitoneal volume, increased intraabdominal volume, and/
or restriction of abdominal wall expansion. When intraabdominal
pressure (IAP) increases rapidly, physiologic derangement can be
seen. This pressure can be measured directly by intraabdominal
catheter or indirectly by gastric, urinary, or inferior vena cava cath-
eterization, but urinary bladder pressure has been shown to best
correlate with IAP. Physiologic derangements seen in the course
of abdominal compartment syndrome occur in multiple systems.
Pulmonary changes are usually the most prominent with diaphrag-
matic elevation leading to decreased pulmonary compliance with
decreased lung capacity, decreased residual capacity, and decreased
volumes. Cardiovascular changes include decreased filling second-
ary to venous compression, decreased ventricular end-diastolic
volumes, increased afterload, decreased contractility, and loss of
cardiac output. Prerenal azotemia unresponsive to volume is a char-
acteristic finding, with oliguria leading to anuria due to decreased
renal perfusion, decreased glomerular filtration rate, and increased
retention of sodium and water with renin production. Compression
of splanchnic vasculature leads to ischemia and translocation of
bacteria. Hepatic insufficiency can also result. Intracranial pressure
is seen to increase with decreased cerebral perfusion and decreased
venous outflow.(20) Abdominal compartment syndrome is gener-
ally noted in patients with a urinary bladder pressure of more than
20 mmHg. Patients with high pressure will require decompression
if any of the aforementioned signs are noted. If when attempting
to close the abdomen pressure becomes unacceptably high, as evi-
denced by impairment of respiratory mechanics and an increase in
the peak airway pressures, the diagnosis should be considered and
routine closure should be avoided.
The objectives of the temporary closure are containment of vis-
cera, control of abdominal secretions, maintenance of tamponade,
and facilitation of future closure.(21)
A polyethylene sheet (or a large occlusive dressing folded in half
on itself) is perforated multiple times and placed over the perito-
neal viscera but beneath the abdominal wall peritoneum. Then,
sterile surgical towels are placed atop the protective sheet and the
edges tucked below the skin, fascia, and peritoneum. Jackson-Pratt
or similar suction drains are positioned on the towels and tunneled
beneath the skin to exit away from the wound edge. The skin is
prepared with tincture of benzoin and covered with a plastic drape
backed with iodophor-impregnated adhesive.(22) The drains are
kept to continuous wall suction. Alternatively, a vacuum-assisted
closure device, such as V.A.C.
®
(KCI) may be applied over the poly-
ethylene sheet and may be associated with a higher rate of primary
delayed fascial closure (23) (See Figures 6.1 and 6.2).
other intraoperative challenges
Careful planning, technique, and patient selection should mini-
mize the colorectal surgeon’s encounters with damage control
situations. However, when confronted by a scenario with suspect
options and dubious outcome, a damage-control laparotomy
can turn an uncontrolled situation into a controlled second-look
operation with potentially more desirable options and outcomes.
ADHESIVE DISEASE
Adhesions result from prior abdominal surgeries or infections. One
would expect adhesions to be worse in a patient with multiple prior
abdominal surgeries or a history of a bowel perforation. Particularly
concerning for adhesions are those patients with enterocutaneous
fistulas and a history of intraperitoneally placed mesh.
Sometimes the adhesions encountered are much less than antic-
ipated and other times they are, without warning, much worse
than anticipated. Adhesions can be categorized as demonstrated
in the grading system in Table 6.1. With surgery, one of the first
objectives is to enter the peritoneal cavity without causing a bowel
injury. With heightened concern about adhesions, more caution
is exercised and, if possible, the abdomen is entered in virgin ter-
ritory. With laparoscopic surgery, adhesions can be prohibitive.
Some patients are obviously not laparoscopic candidates, such as
the patient with a stiff abdominal wall with extensive scarring and/
or multiple enterocutaneous fistulas. Other patients may be bor-
derline candidates for laparoscopy. In these cases, an attempt can
be made to look in the abdomen with the laparoscope and then
make a decision whether to proceed laparoscopically or not. A lim-
ited number of laparoscopic instruments can initially be opened
to save resources until this decision is made. One may access the
peritoneal cavity using the Hasson technique. Alternatively, pneu-
moperitoneum can be established with a veress needle at a site
remote from previous surgery and the insufflated peritoneal cavity
accessed using a Visiport™ (USS/TYCO) or a clear optic tip port
(Ethicon). The author prefers the later technique most commonly
at a left upper quadrant site.(25, 26) Others have advocated the use
of a “peek-port” where an approximately 7 cm incision is made
and the abdomen assessed. If the abdomen appears hostile, the
incision is lengthened and a laparotomy preformed. If favorable,
hand-assisted laparoscopic surgery (HALS) can be employed.(27)
For open cases, again, entering the abdomen in virgin terri-
tory is advantageous. Exposure and visualization are important
to avoid bowel injury, so frequent suctioning or dabbing with a
laparotomy sponge is used. Different techniques for dividing adhe-
sions exist, but most will be taken sharply with scissors or scalpel.
Electrocautery is employed cautiously and judiciously, as collateral
damage may occur to adjacent bowel and go unrecognized until
the patient becomes sick postoperatively. A scalpel is especially
useful in the very dense adhesions of bowel to the abdominal wall.
Figure 6.2 Example of a damage control laparotomy: after placement of temporary
closure device, with application of the V.A.C.® (KCI) system. (Courtesy of Richard
Fortunato, DO, Pittsburgh, PA).
Figures 6.1 Example of a damage control laparotomy: before placement of
temporary closure device.
Optimal timing of return to the OR is poorly defined but it is felt
that patients who are returned to the OR after more than 72 hours
experience greater morbidity and mortality.(24)
Table 6.1 Grading system for bowel adhesions.
Grade Description
1 Thin filmy adhesions.
2 Adhesions that can be divided by blunt dissection.
3 Dense adhesions that require sharp division.
4 Dense adhesions, the division of which results in bowel injury.
Source: Adapted from Fazio VW. Personal communication, 1998.