Improved Outcomes in Colon and Rectal Surgery part 7 pps
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (262.56 KB, 10 trang )
improved outcomes in colon and rectal surgery
A more difficult or dense adhesion can be approached from differ-
ent angles to help define the appropriate plane. Oftentimes, simpler
adhesions can be taken down on either side or even behind the
dense adhesion to help delineate the proper path of dissection.
Placing one’s fingers on either side of the adhesion and palpating
can be of assistance to feel the plane and also sometimes stretch out
the adhesion for easier division. Of course, one of the biggest keys
to success is proper traction and counter-traction. If the traction is
too forceful though, tearing of the bowel may occur.
If an enterotomy does occur, it should be repaired immediately
with absorbable sutures to minimize contamination. If the case
is difficult and more injuries are predicted, temporary closure
can be employed until all adhesiolysis is complete. A segment of
bowel with extensive injuries may be best resected. Waiting until
all injuries have been identified and a plan made can save sig-
nificant time on unnecessary repairs. Sometimes dissection can
be performed in an extraperitoneal plane to avoid bowel injury,
leaving peritoneum adherent to the bowel wall. Other times a
small piece of bowel wall may be left behind, adherent to a more
critical structure, such as the ureter or iliac vessels, in order to
avoid morbid injury at these crucial sites. Leaving devascularized
bowel serosa or muscularis in-situ is not a problem. Any mucosa
left behind, however, should be desiccated with electrocautery to
prevent formation of mucoceles or malignancy.
Consideration should be given in each case to preventing adhe-
sions, which lowers the risk of bowel obstruction and makes any
future surgeries easier. Adhesion formation is a local response of the
peritoneum and pertonealized structures to ischemia, desiccation,
or trauma and may form as result of the primary disease process
or due to contact with surgical instruments, staples, suture, gloves,
sponges, and other irritants introduced at the time of surgery. It
is assumed that laparoscopy can minimize some of these insults
by limiting bowel manipulation and exposure of the peritoneal
surface to potential irritants.(28–30) Preliminary evidence in this
regard can be found by noting that laparoscopic assisted ileocolic
resection is associated with reduced rate of bowel obstruction
when compared to open surgery.(31, 32) Adhesions to the anterior
abdominal wall are minimal or absent.(33) (See Figures 6.3 and
6.4) (34) Additionally, the CO2 pneumoperitoneum is felt to be
protective of certain types of injury.(35, 36) Initial hope for elimi-
nation of adhesive disease with the advent of laparoscopy (37) has
been replaced by the realization that adhesions do indeed form and
reform after laparoscopy, primarily in the operative field, (38, 39)
but to a lesser extent than with open surgery.
Despite these advantages, bowel obstruction continues to
occur frequently in patients following laparoscopic surgery. The
mechanism, severity, and risk of obstruction have shifted how-
ever. In a report for the French Association for Surgical Research,
Duron (39) and colleagues noted that only 33% of postoperative
bowel obstructions following various laparoscopic surgeries were
due to multiple adhesions, while an additional 17% were due to
a single band. Intestinal incarceration (in abdominal wall defect
or port site) (See Figures 6.5 and 6.6) was responsible for another
46%. All told, 25% of patients required resection.
A report from the Western Pennsylvania Hospital describes
unique mechanisms of bowel obstruction, such as internal hernia,
are common after laparoscopic bariatric surgery.(40) The reason for
this is assumed to be the result of a laparoscopy-related decrease in
scar formation between newly apposed peritoneal surfaces which
leaves defects open.(41) One can imagine this same phenomenon
following laparoscopic colon resection. Obstructions due to inter-
nal hernias are associated with a high incidence of bowel threatening
ischemia and therefore require a high index of suspicion and prompt
surgical management. The authors’ experience is that relaparoscopy,
in this patient population, is an excellent technique for diagnosing
and managing these obstructions and other complications.(40)
General principles to minimize adhesions include gentle han-
dling of the tissue, hemostasis, and avoidance of infection and
ischemia. Products such as Seprafilm
TM
(Genzyme), a bioabsorb-
able membrane of sodium hyaluronate, and carboxymethylcellu-
lose, can be placed at the time of surgery to reduce the incidence
of adhesions.(42–44) It should be noted that these products
should not be placed adjacent to a fresh anastomosis.(45)
A qualification must be maintained in the case of adhesions
encountered when operating on a patient with a malignancy. If
the adhesions are between a cancer and another structure, they
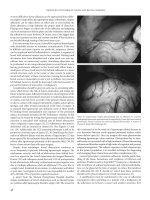
Figure 6.3 Laparoscopic images demonstrating lack of adhesions in a patient
undergoing laparoscopic appendectomy for appendicitis 2 years after hand-assisted
laparoscopic anterior resection for recurrent sigmoid diverticulitis. (Courtesy of
Thomas E. Read, MD, Pittsburgh, PA).
other intraoperative challenges
should be treated as an extension of the malignancy. In other
words, they should not be divided, but instead resected with the
specimen. This process might require partial resection of another
structure such as another limb of bowel or abdominal wall. Not all
adhesions encountered during surgery for malignancy are malig-
nant adhesions however. Attention should be paid to the extent of
the tumor such as growth through the full-thickness bowel wall
and its relationship to the adhesions as well as the characteristic
of the adhesion.
lesiOn lOcalizatiOn
Up to 22% of endoscopically unresectable colorectal neoplasms
with benign histology on initial biopsy harbor invasive adeno-
carcinoma. Adhering to oncologically sound principles for these
neoplasms is advised.(46) Many of these will not be easily palpable
during surgery and even more difficult to localize laparoscopically.
For operative planning, particularly when considering a laparo-
scopic approach, accurate localization of the tumor is imperative to
avoid removal of the wrong segment of intestine.(47) Colonoscopy
alone as a localizing technique is inaccurate (48) unless the tumor
is clearly noted to be in the direct proximity of to an unmistakable
landmark such as the rectum or cecum. As such, localization should
be more definitively accomplished preoperatively. Endoscopic
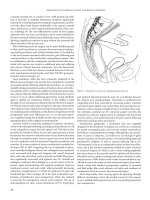
injection of India ink in three or four quadrants of bowel adjacent
to and distal to, but not through the tumor, is safe and reliable, and
preferred in most centers (49, 50) (See Figure 6.7).
Other adjuncts for localization include endoscopic placement of
clips and subsequent plain film of the abdomen (See Figure 6.8).
Alternatively, barium enema or CT colography can be employed.
(51) Though more costly than India ink injection and associated
with radiation exposure, these modalities offer the additional
advantage of preoperative planning for room set up and patient
positioning for left vs. transverse vs. right colectomy. One disad-
vantage of these approaches is they offer no direct intraoperative
evidence of the lesion localization. Therefore they may be most
effectively used in conjunction with India ink marking.
Some centers have reported success with preoperative endo-
scopic clip placement followed by intraoperative laparoscopic
Figure 6.4 Laparoscopic images demonstrating lack of adhesions in a patient
undergoing laparoscopic appendectomy for appendicitis 2 years after hand-assisted
laparoscopic anterior resection for recurrent sigmoid diverticulitis. (Courtesy of
Thomas E. Read, MD, Pittsburgh, PA).
Figure 6.6 A port site hernia causing a bowel obstruction and injury to the bowel.
Figure 6.5 A port site hernia causing a bowel obstruction and injury to the bowel.
improved outcomes in colon and rectal surgery
ultrasonography or intraoperative fluoroscopy.(52) These intra-
operative imaging modalities, though effective, tend to be cum-
bersome, resource intensive, and operator dependent.
Due to the flexible nature of the colonoscope, the distance of
the tumor from the anal verge cannot be accurately measured on
colonoscopy. When the tumor is obviously within the colon or is
palpable within the rectum, this limitation of the colonoscope is
not an issue. Unfortunately, not uncommonly, a tumor reported
to be in the sigmoid colon by colonoscopy is actually much lower
and represents rectal cancer. A rigid proctoscope is very useful to
accurately measure the distance of the tumor from the anal verge,
which not only helps in planning surgery but also determines if
the tumor is in a location that its stage might warrant preoperative
neoadjuvant therapy.(53)
Careful preoperative assessment and planning is the best way to
ensure that the appropriate segment of the intestine is removed.
The most notable preventable cause includes assumptions made
based on colonoscopic determination of a site that is not within
the direct proximity to an unmistakable landmark such as the
rectum or cecum. Occasionally, however, despite our best efforts,
localization attempts fail to identify lesions intraoperatively in
up to 12% of cases.(54) Failure to visualize a tattoo can result
from disappearance of the tattoo compound, particularly when
products other than India ink are used.(55) Additionally, failure
to inject the ink compound into the submucosal tissue plane can
result in dissemination of the ink and imprecise localization or
intraperitoneal injection. Although this presents little direct risk
to the patient, it does present a problem with definitive intra-
operative localization. Techniques that have been described to
minimize this occurrence include injecting saline to develop the
submucosal plane before injection of ink.(56)
The surgeon must be prepared to deal with the case where local-
ization efforts have failed. Blind resection is not advised unless
confidently guided by preoperative imaging. Mobilization of the
flexures and dissection of the omentum off the transverse colon
may reveal a hidden tattoo mark. During laparoscopy, palpation
cannot be performed well but a hand assist device can be used to
overcome this limitation. Still there are cases where the lesion is too
small to palpate and remains unfound. Under such circumstances,
intraoperative colonoscopy can permit localization.(57) Use of
CO
2
insufflation during the colonoscopy will minimize bowel dis-
tention. This is critical if laparoscopic assisted surgery is planned.
Requiring equipment, expertise, and time, this is best reserved as a
back-up rather than a primary localization modality. Regardless of
the technique of localization, opening the specimen after resection
to confirm the presence of the lesion is recommended.
abdOminal wall clOsure
Abdominal wall closure is required following laparotomy and at
the specimen retrieval site for laparoscopic colectomy. Wound-
related complications such as acute wound failure (dehiscence),
infection, and incisional hernia can result in significant morbid-
ity. Malnutrition, tobacco abuse, and/or requirement for systemic
corticosteroids or chemotherapy will increase risk. Ideally, these
factors should be modified preoperatively whenever possible.
Intraoperatively, proper technique minimizes the risk of wound
complication and will be the focus of this discussion.
Acute wound failure, defined as an early separation of the
abdominal musculoaponeurotic layers, occurs at an incidence of
approximately 1.2% (range 0–2.3%), (58–62) with the majority
occurring between the 6th and 9th postoperative days.(63, 64)
The most common cause is felt to be suture tearing through the
fascia but may also occur as a result of abdominal wall rupture
away from the incision or excessive suture interval. Suture break-
age and knot slippage are rare.(58, 6, 65–70)
An incisional hernia is failure of complete abdominal wall heal-
ing following abdominal surgery, resulting in a myofascial defect.
The reported incidence of incisional hernias in the literature varies
from 9–19%. They often require repair, with recurrence rates as
high as 45%, causing further complications. The ideal abdominal
wound closure should minimize this complication.
Figure 6.8 A plain film of the abdomen after endoscopic placement of clip
(arrow) can provide valuable information about the location of the lesion and aid
in preoperative planning.
Figure 6.7 India ink injected endoscopically before laparoscopy provides excellent
lesion localization.
other intraoperative challenges
technique
Numerous studies have demonstrated mass closure to be superior
to layered closure in clinical practice (71–73), since incorporating
large bites of tissue reduces the pressure per unit area caused by
the suture and decreases the risk of suture cut-through (74, 75).
Although a randomized trial of mass versus layered closure showed
no significant difference in wound rupture, (76) and most clini-
cal studies comparing mass closure to layered abdominal closure
have not revealed a difference in the incidence of incisional hernia
formation, (73, 77) mass closure of the abdominal wall is currently
favored because of its safety, efficacy, and speed. It is important to
note that peritoneum heals by regeneration of the layer over the
entire defect, and not in incremental advancement from the wound
edge.(78, 79) Randomized studies revealed no difference between
a one-layered closure (peritoneum not sutured) and a two-layered
closure (peritoneum sutured) in midline and paramedian inci-
sions.(73, 80) Peritoneal closure is therefore not vital in abdominal
closures and may contribute to adhesion formation.
Experimental models and cadaveric studies have shown that
continuous abdominal wall closure provides the greatest wound
security in terms of abdominal dehiscence.(81, 82) Continuous
suturing is thought to equalize the tension differences between
individual stitches and distributes the tension along the suture
line, thus reducing the risk of tissue strangulation and late cut-
through.(65, 81, 83, 84) The number of knots and therefore the
likelihood of knot slippage may be minimized. A meta-analysis
comparing six randomized controlled trials of continuous versus
interrupted closure (irrespective of suture type) found the inci-
dence of incisional hernias to be significantly less with continuous
closure.(85)
There is a zone of collagenolysis and matrix degradation that
extends out 0.75 cm from each wound edge.(86, 87) Further, fascia
strength near its cut edge decreases by 50% during the first 48 hours
after an operation.(88) Experimental models have demonstrated
a continuous closure while maintaining a 1 cm stitch interval and
a 1 cm tissue bite reduces dehiscence rate as compared to smaller
tissue bites by minimizing the risk of suture cut-through.(89)
suture material
Slowly resorbed monofilaments (polydioxanone: PDS
®
and polygly-
conate: Maxon
®
) are the strongest sutures in the fresh state, followed
by the nonresorbable monofilaments (nylon: Ethilon
®
and polypro-
pylene: Prolene
®
), and then the braided sutures (polyglactin: Vicryl
®
,
polyglycolic acid: Dexon
®
).(90) Silk and chromic catgut, are not
appropriate.(91, 66, 92, 93)
With regard to incisional hernia formation, it is known from
experimental studies that the abdominal fascia continues to gain
strength up to 3 months after surgery.(94) Nylon (Ethilon
®
)
loses approximately 20% strength per year while Polypropylene,
Surgilene
®
, Ethibond
®
, Tevdek
®
, and polybutester (Novafil
®
) seem
to retain their strength indefinitely.(95) Catgut, Dexon
®
, and
Vicryl
®
have tensile strength half-lives in the range of 1–4 weeks,
and are not suitable for fascial closure. Vicryl
®
,
compared with
nonresorbable sutures (Prolene
®
), is associated with an increased
rate of wound failure and incisional hernias (85). This is in con-
tradistinction to more slowly resorbed materials, such as poly-
dioxanone (PDS
®
), that do not appear to increase the rate of
incisional hernia (85). Multiple randomized trials have failed to
demonstrate a difference in dehiscence rates between resorbable
and nonresorbable sutures.(58, 96, 97) Additionally, persistent
sinus formation and chronic wound infection can be virtually
eliminated with the use of resorbable suture.(73, 98)
Multiple clinical studies implicate wound sepsis as the
most important factor associated with incisional herniation.
Multifilament sutures provide a better growth environment for
bacteria and are associated with a higher incidence of wound
infection compared to monofilament sutures.(77, 99, 100)
In summary, a continuous, mass closure using slowly-resorbable
monofilament suture with a 1 cm tissue bite and a 1 cm interval is
likely the best technique for primary abdominal wall closure.(101)
It is assumed that minimizing abdominal wall trauma vis-à-vis
laparoscopic approaches may minimize wound related morbidity.
retentiOn sutures
Retention sutures are thought to aid abdominal closure by prevent-
ing wound necrosis and avoiding evisceration. However, problems
associated with retention sutures are several and include exacer-
bation of the intraabdominal hypertension when the viscera are
forcibly contained, and abdominal wall ischemia when the sutures
become too tight. Furthermore, several studies have implicated
retention sutures in the development of enterocutaneous fistu-
lae even when they are placed extraperitoneally. With caution,
retention sutures may be considered in abdominal wall closure in
the patient with multiple risk factors for delayed wound healing;
they are not recommended for those at risk for development of
abdominal compartment syndrome.
If loss of abdominal domain does not permit a tension free
fascial closure, one can consider relaxing incisions to permit
medial mobilization of the rectus. This requires dissection above
the fascia laterally to the lateral edge of the anterior rectus sheath,
which is then incised in the sagittal plane, similar to the technique
for separation of parts. This technique should be used cautiously
in patients at risk for abdominal compartment syndrome and
those at above average risk for wound infection.
synthetic prOstheses
The use of synthetic mesh has been a popular technique in
abdominal wall closures and reconstructions for many years
and the most extensive experience is with polypropylene mesh
(Prolene
®
and Marlex
®
).(102) Multiple reports in the literature
cite the advantages of this permanent material, which include
availability, ease of use, high tensile strength and durability,
maintenance of abdominal wall compliance, potential avoid-
ance of future reconstruction, and permeability allowing for
peritoneal drainage. However, several investigators have pointed
out the many long-term complications related to polypropylene
mesh. Most notably, the mesh acts as a nidus of infection and
is associated with severe foreign body reactions leading to mesh
extrusion and enterocutaneous fistulae with an incidence on the
order of 23%.(103) The placement of omentum between the
mesh and the viscera has been shown to reduce the early fistula
rate to 1–4%.(104) Recently mesh with an adhesion preventative
film bonded to it (e.g., Sepramesh
®
(Genzyme)) has been intro-
duced. The adhesive reductive film may reduce the problem of
improved outcomes in colon and rectal surgery
small bowel adherence to the underside of the mesh, which may
reduce complications such as enterocutaneous fistulae and allow
for an easier re-exploration or mesh removal.
Use of polytetrafluoroethylene mesh (PTFE, Gore-Tex
®
) decreases
the incidence of fistulization and mesh extrusion. However, PTFE
impedes the free egress of abdominal fluid and may contribute to
abdominal compartment syndrome and seroma formation. After
appropriate consideration, polypropylene, or polytetrafluoroeth-
ylene mesh may be considered for abdominal wall reconstruction
when there is tissue loss.
Resorbable meshes, polyglactin acid (Vicryl
®
) and polyglycolic
acid (Dexon
®
), may provide a temporary solution in the man-
agement of the difficult abdominal wall. In experimental studies,
Dexon
®
is 50–70% absorbed and Vicryl
®
is almost fully absorbed by
10 weeks. Resorbable mesh offers the early advantages of permanent
mesh without the late complications and allows for egress of fluid
reducing the chances of intraabdominal hypertension. However,
not surprisingly, the reported incidence of hernia is unacceptably
high with the use of resorbable synthetic mesh.(105)
biOlOgic meshes
There are several commercially available meshes (either allografts
or xenografts) that are derived from naturally occurring sources
of collagen and related connective tissues. The most extensively
studied of these is Alloderm (Lifecell) (acellular dermal matrix
derived from donated human skin) (106–109) and Permacol
(Tissue Science Laboratories) (intact porcine dermal collagen)
(110). Other variations on the theme include Collamend (Bard)
(cross-linked acellular porcine dermal collagen and its constitu-
ent elastin fibers), Allomax
®
(Bard) (human dermal collagen), and
Strattice
®
(Lifecell) (acellular dermal matrix derived from porcine
skin). The purported advantage of all of these agents is a low risk of
mesh infectious complication in contaminated fields. In the case
of acellular dermal matrix, extracellular material provides a sig-
nal for fibroblast incorporation, collagen deposition, and matu-
ration resulting in tissue that cannot be differentiated from fascia.
(111–113) Long-term data are lacking.
cOnclusiOns
The colorectal surgeon can be faced with any number of potential
disasters. Proper preparation and maintenance of a diverse tool-
box of solutions can help avert or salvage even the most dramatic
of these.
references
1. Chee YL, Crawford JC, Watson HG, Greaves M. Guidelines
on the assessment of bleeding risk prior to surgery or invasive
procedures. British Committee for standards in haematology.
Br J Haematol 2008; 140(5): 496–504.
2. Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and
perioperative care. JAMA 2001; 286(2): 208–16.
3. Spell NO. Stopping and restarting medications in the peri-
operative period. Med Clin N Am 2001; 85(5): 1117–28.
4. Ansell J, Hirsh J, Poller L et al. The pharmacology and man-
agement of the Vitamin K antagonists: The seventh ACCP
conference on antithrombotic and thrombolytic therapy.
Chest 2004; 126(3 Suppl): 204S–33S.
5. Malek MM, Greenstein AJ, Chin EH et al. Comparison of
iatrogenic splenectomy during open and laparoscopic colon
resection. Surg Laparosc Endosc Percutan Tech 2007; 17(5):
385–7.
6. Recommended Adult Immunization Schedule United
States October 2004–September 2005. The Advisory
Committee on Immunizations Practices. Department of
Health and Human Services. Centers for Disease Control
and Prevention. Available at: www.cdc.gov/nip/recs/adult-
schedule.pdf. Accessed on July 6, 2006.
7. National Guideline Clearinghouse Surgical Treatment of
Disease and Injuries of the Spleen. Society for Surgery of
the Alimentary Tract (SSAT). 2004 Feb. Available at: www.
guideline.gov/summary/summary.aspx?view_id=1& doc_
id=5698. Accessed on July 6, 2006.
8. Davies JM, Barnes R, Milligan D. Update of guidelines for
the prevention and treatment of infection in patients with
an absent or dysfunctional spleen. Clin Med 2002; 2: 440–4.
9. Tribble CG, Joob AW, Barone GW, Rodgers BM. A new
technique for wrapping the injured spleen with polyglactin
mesh. Am Surg 1987; 53: 661–3.
10. Fuller J, Scott W, Ashar B, Corrado J. Laparoscopic Trocar
Injuries: A report from a US Food and Drug Administration
Center for Devices and Radiological Health Systematic
Technology. Assessment of Medical Products Committee.
FDA; 2003.
11. Stone HH, Strom PR, Mullins RJ et al. Management of the
major coagulopathy with onset during laparotomy. Ann
Surg 1983; 197: 532–5.
12. Burch JM, Ortiz VB, Richardson RJ et al. Abbreviated
laparotomy and planned reoperation for critically injured
patient. Ann Surg 1992; 215(5): 476–83.
13. Rotondo MF, Schwab CW, McGonigal MD et al. ‘Damage
control’: an approach for improved survival in exsanguinat-
ing penetrating abdominal injury. J Trauma 1993; 35(3):
375–82.
14. Morris JA Jr, Eddy VA, Blinman TA, Rutherford EJ, Sharp
KW. The staged celiotomy for trauma. Issues in unpacking
and reconstruction. Ann Surg 1993; 217(5): 576–84.
15. Lee JC, Peitzman AB. Damage-control laparotomy. Curr
Opin Crit Care 2006; 12(4): 346–50.
16. Finlay IG, Edwards TJ, Lambert AW. Damage control lapa-
rotomy. Br J Surg 2004; 91: 83–5.
17. Moore EE, Burch JM, Franciose RJ et al. Staged physiologic
restoration and damage control surgery. World J Surg 1998;
22: 1184–91.
18. Loveland JA, Boffard KD. Damage control in the abdomen
and beyond. Br J Surg 2004; 91(9): 1095–101.
19. Offner PJ, de Souza AL, Moore EE et al. Avoidance of
abdominal compartment syndrome in damage-control lap-
arotomy after trauma. Arch Surg 2001; 136: 676–81.
20. O’Mara MS, McCormick JT, Semins H, Caushaj PF.
Abdominal Compartment Syndrome as a Consequence of
Rectus Sheath Hematoma. Abdominal Surgery: Fall 2002/
Winter 2003, 5–7.
21. Schreiber MA. Damage control surgery. Crit Care Clin 2004;
20: 101–18.
other intraoperative challenges
22. Barker DE, Kaufman HJ, Smith LA et al. Vacuum pack
technique of temporary abdominal closure: a seven year
experience with 112 patients. J Trauma 2000; 48: 201–7.
23. Garner GB, Ware DN, Cocanour CS et al. Vacuum assisted
wound provides early fascial reapproximation in trauma
patients with open abdomens. Am J Surg 2001; 182: 630–8.
24. Abikhaled JA, Granchi TS, Wall MJ et al. Prolonged abdomi-
nal packing for trauma is associated with increased morbidity
and mortality. Am Surg 1997; 63: 1109–12.
25. Caushaj PF. In Atlas of laparoscopic surgery. Jacobs,
Plasescia and Caushaj. ed, Williams and Wilkins, Baltimore,
MD, 1996: 13–5.
26. Rohatgi A, Widdison AL. Left subcostal closed (Veress needle)
approach is a safe method for creating a pneumoperitoneum.
J Laparoendosc Adv Surg Tech A 2004; 14(5): 278–80.
27. Thomas ER, Javier S, David F, Richard F, Philip FC. “Peek
Port”: a novel approach to avoid conversion in laparoscopic
colectomy. Surg Endosc 2008; in press.
28. Hiki N, Shimizu N, Yamaguchi H et al. Manipulation of the
small intestine as a cause of the increased inflammatory
response after open compared with laparoscopic surgery. Br
J Surg 2006; 93(2): 195–204.
29. Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical
manipulation of the gut elicits an intestinal muscularis
inflammatory response resulting in postsurgical ileus. Ann
Surg 1998; 228: 652–63.
30. Schwarz NT, Kalff JC, Turler A et al. Selective jejunal manip-
ulation causes postoperative pan-enteric inflammation and
dysmotility. Gastroenterology 2004; 126: 159–69.
31. Alabaz O, Iroatulam A, Nessim A et al. Comparison of Lapa-
roscopically Assisted and Conventional Ileocolic Resection for
Crohn’s Disease. Eur J Surg 2000; 166(3): 213–7.
32. Bergamaschi R, Pessaux P, Arnaud J. Comparison of
conventional and laparoscopic ileocolic resection for crohn’s
disease. Dis Colon rectum 2003; 46: 1129–33.
33. Hasegawa H, Watanabe M, Nishibori H et al. Laparoscopic sur-
gery for recurrent Crohn’s disease. Br J Surg 2003; 90: 970–3.
34. McCormick JT, Simmang CL. Laparoscopic reoperation
after laparoscopic colorectal surgery: are the rules different?
Clin Colon Rectal Surg 2006; 19(4): 217–22.
35. Daphan CE, Agalar F, Hascelik G, Onat D, Sayek I. Effects
of laparotomy, and carbon dioxide and air pneumoperito-
neum, on cellular immunity and peritoneal host defenses in
rats. Eur J Surg 1999; 165: 253–8.
36. Hanly EJ, Mendoza-Sagaon M, Murata K et al. CO2
Pneumoperitoneum modifies the inflammatory response
to sepsis. Ann Surg 2003; 237: 343–50.
37. de Wilde RL. Goodbye to late bowel obstruction after
appendectomy. Lancet 1991; 338: 1012.
38. Parker JSN, Segars J, Godoy H, Winkle C, Stratton P. Adhesion
formation after laparoscopic excision of endometriosis and
lysis of adhesions. Fertil Steril 2005; 84: 1457–61.
39. Duron JJ, Hay JM, Msika S et al. Prevalence and mecha-
nisms of small intestinal obstruction following laparoscopic
abdominal surgery: a retrospective multicenter study.
French Association for Surgical Research. Arch Surg 2000;
135(2): 208–12.
40. Papasavas PK, Caushaj PF, McCormick JT et al. Laparoscopic
management of complications following laparoscopic Roux-
en-Y gastric bypass for morbid obesity. Surg Endosc 2003; 17:
610–4.
41. Higa K, Ho T, Boone K. Internal Hernias after laparoscopic
Roux-en-Y gastric bypass: incidence, treatment and preven-
tion. Obes Surg 2003; 13: 350–4.
42. Becker JM, Dayton MT, Fazio VW et al. Prevention of post-
operative abdominal adhesions by a sodium hyaluronate-
based bioresorbable membrane: a prospective, randomized,
double-blind multicenter study. J Am Coll Surg 1996; 183:
297–306.
43. Diamond MP. Reduction of adhesions after uterine
myomectomy by Seprafilm membrane (HAL-F): a blinded,
prospective, randomized, multicenter clinical study. Fert
Steril 1996; 66(6): 904–10.
44. Nordic Adhesion Prevention Study Group. The efficacy of
INTERCEED (TC7) for prevention of reformation of post-
operative adhesions on ovaries, fallopian tubes, and fim-
briae in microsurgical operations for fertility: a multicenter
study. Fertil Steril 1995; 63(4): 709–14.
45. Beck DE, Cohen Z, Fleshman JW et al. Adhesion Study
Group Steering Committee. A prospective, randomized,
multicenter, controlled study of the safety of seprafilm
adhesion barrier in abdominopelvic surgery of the intes-
tine. Dis Colon Rectum 2003; 46(10): 1310–9.
46. Brozovich M, Read TE, Salgado J et al. Laparoscopic
Colectomy for “Benign” Colorectal Neoplasia: A Word of
Caution. Surg Endosc 2007; [Epub ahead of print]
47. Larach SW, Patankar SK, Ferrara A et al. Complications of
laparoscopic colorectal surgery: analysis and comparison
of early vs. latter experience. Dis Colon Rectum 1997; 40:
592–6.
48. Piscatelli N, Hyman N, Osler T. Localizing colorectal cancer
by colonoscopy. Arch Surg 2005; 140(10): 932–5.
49. McArthur CS, Roayaie S, Waye JD. Safety of preopera-
tion endoscopic tattoo with india ink for identification of
colonic lesions. Surg Endosc 1999; 13(4): 397–400.
50. Nizam R, Siddiqi N, Landas SK, Kaplan DS, Holtzapple PG.
Colonic tattooing with India ink: benefits, risks, and alter-
natives. Am J Gastroenterol 1996; 91(9): 1804–8.
51. Cho YB, Lee WY, Yun HR et al. Tumor localization for
laparoscopic colorectal surgery. World J Surg 2007; 31(7):
1491–5.
52. Nagata K, Endo S, Tatsukawa K, Kudo SE. Intraoperative
fluoroscopy vs. intraoperative laparoscopic ultrasonogra-
phy for early colorectal cancer localization in laparoscopic
surgery. Surg Endosc 2008; 22(2): 379–85.
53. McCormick JT, Gregorcyk SG. Preoperative Evaluation
of Colorectal Cancer. Surgical Oncology Clinics of North
America. Surg Oncol Clin N Am 2006; 15(1): 39–49.
54. Feingold DL, Addona T, Forde KA et al. Safety and reliabil-
ity of tattooing colorectal neoplasms prior to laparoscopic
resection. J Gastrointest Surg 2004; 8(5): 543–6.
55. Price N, Gottfried MR, Clary E et al. Safety and efficacy of
India ink and indocyanine green as colonic tattooing agents.
Gastrointest Endosc 2000; 51: 438–42.
improved outcomes in colon and rectal surgery
56. Park JW, Sohn DK, Hong CW et al. The usefulness of preop-
erative colonoscopic tattooing using a saline test injection
method with prepackaged sterile India ink for localization
in laparoscopic colorectal surgery. Surg Endosc 2008; 22(2):
501–5.
57. Zmora O, Dinnewitzer AJ, Pikarsky AJ et al. Intraoperative
endoscopy in laparoscopic colectomy. Surg Endosc 2002;
16: 808–11.
58. Carlson M. Acute wound failure. Surg Clin North Am 1997;
77: 607–36.
59. Wissing J, van Vroonhoven TJMV, Schattenkerk ME et al.
Fascia closure after midline laparotomy: results of a random-
ized trial. Br J Surg 1987; 74: 738.
60. Trimbos JB, Smit IB, Holm JP et al. A randomized clinical
trial comparing two methods of fascia closure following
midline laparotomy. Arch Surg 1992; 127: 1232.
61. Niggebrugge AHP, Hansen BE, Trimbos JB et al. Mechanical
factors influencing the incidence of burst abdomen. Eur J Surg
1995; 161: 655.
62. Riou JO, Cohen JR, Johnson H Jr. Factors influencing wound
dehiscence. Am J Surg 1992; 163: 324.
63. Miles RM, Moore M, Fitzgerald D et al. The etiology and
prevention of abdominal wound disruption. Am Surg 1964;
30: 566–73.
64. Reitamo J, Moller C. Abdominal wound dehiscence. Acta
Chir Scand 1972; 138: 170–5.
65. Bowen A. Postoperative wound disruption and evisceration:
an analysis of thirty four cases with a review of the literature.
Am J Surg 1940; 47: 3.
66. Alexander HC, Prudden JF. The causes of abdominal wound
disruption. Surg Gynecol Obstet 1966; 122: 1223–9.
67. Gisalson H, Gronbech JE, Soreide O. Burst abdomen and inci-
sional hernia after major gastrointestinal operation—compar-
ison of three closure techniques. Eur J Surg 1995; 161: 349.
68. Greenburg AG, Saik RP, Peskin GW. Wound dehiscence:
Pathophysiology and prevention. Arch Surg 1979; 114: 143.
69. Lythgoe JP. Burst Abdomen. Postgrad Med J 1960; 36: 388.
70. Poole GV, Meredith JW, Kon ND et al. Suture technique and
wound bursting strength. Am Surg 1984; 50: 569–72.
71. Marsh RL, Coxe JW, Ross WL et al. Factors involving wound
dehiscence. JAMA 1954; 155: 1197–200.
72. Ellis H, Heddle R. Closure of the Abdominal Wound. J Royal
Soc Med 1979; 72: 17–8.
73. Humphries Al, Corley WS, Moretz WH. Massive closure vs.
layer closure for abdominal incisions. Am Surg 1964; 30:
700–5.
74. Dudley HAF. Layered and mass closure of the abdominal
wall. Brit J Surg 1970; 57: 664–7.
75. DeBruin ThR. Prevention of abdominal disruption and
postoperative hernia. Int Surg 1973; 58: 408–11.
76. Ausobsky JR, Evans M, Pollock AV. Does mass closure of
midline laparotomies stand the test of time? A random
control clinical trial. Ann R Coll Surg Engl 1985; 67: 159.
77. Bucknall TE, Cox PJ, Ellis H. Burst abdomen and incisional
hernia: a prospective study of 1129 major laparotomies.
Br Med J 1982; 284: 931.
78. Ellis H, Harrison W, Hugh TB. The healing of peritoneum
under normal and pathologic conditions. Br J Surg 1965;
52: 471.
79. Hubbard TB Jr, Khan MZ, Carag VR et al. The pathology of
peritoneal repair: its relation to the formation of adhesions.
Ann Surg 1967; 165: 908.
80. Hugh TB, Nankivell C, Meagher AP et al. Is closure of the
peritoneal layer necessary in the repair of midline surgical
abdominal wounds? World J Surg 1990; 14: 231.
81. Rodeheaver GT, Nesbit WS, Edlich RF. Novafil. A dynamic
suture for wound closure. Ann Surg 1986; 204: 193–9.
82. Haxton H. The influence of suture materials and methods on
the healing of abdominal wounds. Br J Surg 1965; 52: 372.
83. McNeill PM, Sugerman HJ. Continuous absorbable vs. inter-
rupted non-absorbable fascial closure. Arch Surg 1986; 121:
821–3.
84. Richards PC, Balch CM, Aldrete JS. Abdominal wound
closure. A randomized prospective study of 571 patients
comparing continuous vs. interrupted suture techniques.
Ann Surg 1983; 197: 238–43.
85. Hodgson NC. The search for an ideal method of abdomi-
nal fascial closure: a meta-analysis. Ann Surg 2000; 231(3):
436–42.
86. Adamsons RJ, Musco F, Enquist IF. The chemical dimensions
of a healing incision. Surg Gynecol Obstet 1966; 123: 515.
87. Hogstrom H, Haglund U. Neutropenia prevents decrease in
strength of rat intestinal anastomosis: partial effect of oxy-
gen free radical scavengers and allopurinol. Surgery 1986;
99: 716–20.
88. Hogstrom H, Haglund U, Zederfeldt B. Suture technique
and early breaking strength of intestinal anastomoses and
laparotomy wounds. Acta Chir Scand 1985; 151: 441.
89. Jenkins TPN. The burst abdominal wound: a mechanical
approach. Br J Surg 1976; 63: 873.
90. Greenwald D, Shumway S, Albear P et al. Mechanical com-
parison of 10 suture materials before and after in vivo incu-
bation. J Surg Res 1994; 56: 372.
91. Kronborg O. Polyglycolic acid (Dexon) vs. silk for fascial clo-
sure of abdominal incisions. Acta Chir Scand 1976; 143: 9.
92. Goligher JC, Irvin TT, Johnston D et al. A controlled trial of
three methods of closure of laparotomy wounds. Br J Surg
1975; 62: 823–92.
93. Postlethwait RW, Schauble JF, Dillon ML et al. Wound
healing II. An evaluation of surgical suture material. Surg
Gynecol Obstet 1959; 108: 555.
94. Douglas DM. The healing of aponeurotic incisions. Br J Surg
1952; 40: 79–84.
95. Mathes SJ, Abouljoud M. Wound Healing. In Davis JH,
Drucker WR, Foster RS Jr, et al. eds. Clinical Surgery. St. Louis,
CV Mosby Company, 1987: 493.
96. Lewis RT, Wiegand FM. Natural history of vertical abdomi-
nal parietal closure: Prolene versus Dexon. Can J Surg 1989;
32: 196.
97. Krukowski ZH, Cusick EL, Engeset J et al. Polydioxanone or
polypropylene for closure of midline abdominal incisions:
A prospective comparative trial. Br J Surg 1987; 74: 828.
other intraoperative challenges
98. Irvin TT, Kofman CG, Duthie HL. Layer closure of laparo-
tomy wounds with absorbable and non-absorbable suture
materials. Brit J Surg 1976; 63: 793–6.
99. Alexander JW, Kaplan JZ, Altemeier WA. Role of suture
materials in the development of wound infection. Ann Surg
1967; 165: 192–9.
100. Sharp WV, Belden TA, King PH et al. Suture resistance to
infection. Surgery 1982; 91: 61–3.
101. Ceydeli A, Rucinski J, Wise L. Finding the best abdominal
closure: an evidence-based review of the literature. Curr
Surg 2005; 62(2): 220–5.
102. Morris-Stiff GJ, Hughes LE. The outcomes of nonabsorbable
mesh placed within the abdominal cavity: literature review
and clinical experience. J Am Coll Surg 1998; 352–67.
103. Jones JW, Jurkovich GJ. Polypropylene mesh closure of
infected abdominal wounds. Am J Surg 1989; 55: 73–6.
104. Mayberry JC, Mullins RJ, Crass RC et al. Prevention of
abdominal compartment syndrome by absorbable mesh
prosthesis closure. Arch Surg 1997; 132: 957–62.
105. Dayton MT, Buchele BA, Shirazi SS et al. Use of an absorb-
able mesh to repair contaminated abdominal wall defects.
Arch Surg 1986; 121: 954–60.
106. Kim H, Bruen K, Vargo D. Acellular dermal matrix in the
management of high-risk abdominal wall defects. Am J Surg
2006; 192(6): 705–9.
107. Butler MD, Langstein MD, Kronowitz MD. Pelvic, abdomi-
nal, and chest wall reconstruction with alloderm in patients at
increased risk for mesh-related complications. Plast Reconstr
Surg 2005; 116(5): 1263–74.
108. Patton MD, Berry MD, Kralovich MD. Use of human
acellular dermal matrix in complex and contaminated
abdominal wall reconstructions. Am J Surg 2007; 193:
360–3.
109. Diaz JJ Jr, Guy J, Berkes MB, Guillamondegui O, Miller
RS. Acellular dermal allograft for ventral hernia repair in
the compromised surgical field. Am Surg 2006; 72(12):
1181–8.
110. Catena F, Ansaloni L, Gazzotti F et al. Use of porcine dermal
collagen graft (permacol) for hernia repair in contaminated
fields. Hernia 2007; 11: 57–60.
111. Silverman RP, Li EN, Holton LH 3rd et al. Ventral hernia
repair using allogenic acellular dermal matrix in a swine
model. Hernia 2004; 8: 336–42.
112. Sclafani AP, McCormick SA, Cocker R. Biophysical and
microscopic analysis of homologous dermal and fascial
materials for facial aesthetic and reconstructive uses. Arch
Facial Plast Surg 2002; 4: 164–71.
113. An G, Walter RJ, Nagy K. Closure of abdominal wall
defects using acellular dermal matrix. J Trauma 2004; 56:
1266–75.
7
Postoperative anastomotic complications
Daniel L Feingold
CHALLENGING CASE
A 64-year-old man is 10 days status postlow anterior resection. He
complains of pelvic pressure and pain. His abdominal exam dem-
onstrates mild suprapubic tenderness, but no peritoneal signs. He
has a low-grade fever and a white blood count of 15,000.
CASE MANAGEMENT
A CT scan with oral, rectal, and intravenous contrast demon-
strates a contained anastomotic leak. The patient is managed with
pecutaneous drainage and intravenous antibiotics.
INTRODUCTION
Surgical research over the past three decades has vastly enhanced
our technical abilities and knowledge with respect to creating col-
orectal anastomoses. The Miles operation, considered state of the
art for many years after its description in 1908, has been supplanted
by sphincter-saving operations which are now considered the gold
standard for the majority of patients with rectal cancer. The era of
anal sphincter salvage was ushered in with the commercialization of
mechanical staplers that permitted colorectal surgeons to resect can-
cers even in the distal rectum and maintain intestinal continuity.(1)
In 1979, Heald articulated the concept of total mesorectal exci-
sion for rectal cancer resection which was subsequently validated and
popularized adding a new dimension to our understanding of curative
rectal cancer surgery.(2) In addition, appreciation of the distal mural
spread of rectal cancer allowed for closer distal margins without com-
promising oncologic adequacy. Concomitantly, chemoradiation was
demonstrated to be an effective adjuvant therapy and became part of
the armamentarium routinely used to treat patients with rectal cancer.
With the ushering in of the era of low, stapled colorectal anastomo-
ses, and sphincter preservation, experience was gained diagnosing and
treating patients in whom complications of these operations arise.
The most common complications related to colorectal anasto-
mosis are dehiscence and stricture. The following chapter reviews
the relevant surgical literature with emphasis on diagnosis, treat-
ment, and prevention of these complications. Less-common
complications such as anastomotic cancer recurrence and anas-
tomotic hemorrhage and other forms of intestinal anastomoses
(ileorectal, ileal pouch anal) will not be reviewed.
Leaks and strictures are uncommon events and many of the
studies describing these complications present conflicting results,
are not definitive, or are statistically under-powered. For a more
thorough understanding of the literature, this chapter relies fre-
quently on meta-analysis that combines independent clinical tri-
als to come to a statistical consensus supporting evidence-based
practice. While meta-analysis is not an infallible tool, a well con-
ducted meta-analysis can allow for more objective appraisal of
the evidence, which may lead to resolution of uncertainty and
disagreement and may reduce the probability of false negative
results (i.e., lower the rate of a type II error).
ANASTOMOTIC DEHISCENCE
Anastomotic leak is the most serious complication of colorectal
operations as the clinical outcome due to anastomotic disruption
can be catastrophic. The risk of death within 30 days of colorectal
resection is significantly higher in patients who suffered a leak and
mortality has been reported as high as 36% in some series.(3, 4)
For patients who survive the acute physiologic trespass of an anas-
tomotic leak, there may be formidable, far-reaching implications in
terms of long-term survival, quality of life, and function.(5–7)
GENERAL CONSIDERATIONS
The incidence of anastomotic dehiscence is about 10% for col-
orectal anastomoses within 7 cm of the anal verge.(8) The lack
of a standardized definition of what actually constitutes a leak
makes it difficult to compare series and draw meaningful conclu-
sions.(9) Absence of a universal definition and the low frequency
of leak events may explain why the surgical literature has so many
similarly constructed trials with contradictory results supporting
conflicting conclusions with regard to leaks.
Common definitions include leaks identified by reoperation
for peritonitis, demonstration of extraluminal contrast during an
imaging study or observation of colonic contents through a pel-
vic drain or through the vagina. When reviewing the literature, it
is important to differentiate between patients with clinically rel-
evant leaks and asymptomatic patients who have only radiologic
evidence of leak as they have different clinical consequences and
are treated differently.
Due to the potentially devastating consequences of anasto-
motic leak, there has been significant research investigating the
causes of leaks as well as techniques to reduce the likelihood of
anastomotic failure. A number of technical factors considered to
contribute to the occurrence of anastomotic leak are subjective
assessments made at the time of surgery and are difficult, if not
impossible, to quantify objectively.
Adequate blood supply to the ends of the bowel to be anas-
tomosed is of critical importance. The mesentery and epiploic
appendages should be stripped only enough to allow adequate
visualization to permit anastomosis. Overzealous cleaning of the
bowel compromises the blood supply to the anastomosis and
must be avoided.
In terms of the blood supply to the colon proximal to the anas-
tomosis, preserving the left colic artery by transecting the main
sigmoidal artery versus ligating the actual inferior mesenteric
artery before the takeoff of the left colic (i.e., a high ligation) is
oncologically sound but has not been shown to decrease the risk
of leak.(8, 10) Rather than dogmatically coming across a specific
named blood vessel, the level of transection along the mesenteric
blood supply in a particular operation should be chosen to allow
a tension-free anastomosis.(11) In cases where a colostomy is cre-
ated for proximal diversion, care should be taken to preserve the
7
postoperative anastomotic complications
marginal artery blood supply to the distal colon; this is especially
important if the inferior mesenteric artery is transected. Although
a variety of methods can be used to assess the blood supply to
the anastomosis including Doppler ultrasound and intravenous
fluorescein visualized with a Wood’s lamp, in the vast majority of
cases, straightforward clinical assessment by inspection and pal-
pation is sufficient for this determination.
In an effort to improve the blood supply to the rectal side of
the anastomosis (and to potentially better protect the hypogastric
nerves), it is possible to spare the superior hemorrhoidal artery.
Preserving the inferior mesenteric arterial supply to the rectum
by transecting the individual sigmoidal branches mid-mesentery
may be useful in cases of diverticulitis but would be wholly inap-
propriate in cancer cases where mesenteric clearance and lymph
node harvest are paramount. While sparing the superior hemor-
rhoidal artery, there may be a tendency to avoid dissecting out
the proximal presacral space in order to prevent injury to the
artery. In operations for diverticulitis, the proximal rectum must
be mobilized in order to ensure complete resection of the sigmoid
colon and to facilitate passage of the trans-anal circular stapler
to the stapled end of the rectum. In theory, sparing the superior
hemorrhoidal artery may preserve blood supply to a colorectal
anastomosis but data regarding a potential reduction in the leak
rate is lacking.
Tension across the anastomosis can decrease blood supply and
physically disrupt the anastomosis. Care must be taken to suffi-
ciently mobilize the bowel to eliminate or minimize any tension
at the anastomosis. Technically, this may require division of the
inferior mesenteric vein at the level of the pancreas to adequately
release the descending colon mesentery to permit the colon to
reach to the low pelvis. Similarly, the inferior mesenteric artery
may be divided proximal to the takeoff of the left colic artery so
that the left colic does not tether the colon up in the abdomen.
In addition, splenic flexure release should be performed to afford
tension-free reach of the colon to the pelvis when required, as
is most commonly the case. Although not mandatory from an
oncologic perspective, splenic flexure takedown is only omitted
from curative resections when patient anatomy and tumor loca-
tion permit.(12)
To further reduce the chance of leak, the bowel to be anas-
tomosed should be healthy. Inflammation, edema, radiation
changes, and thickened bowel wall due to chronic obstruction
each influence the risk of leak. Under these suboptimal condi-
tions, the bowel should be resected to normal, healthy tissue to
allow safe anastomosis; otherwise, a primary anastomosis should
be avoided. If unhealthy tissue precludes safe stapled anastomo-
sis, then the anastomosis should not be handsewn; tissue unfit for
staples is unfit for sutures.
When preparing the colon for anastomosis, it is important to
note the presence of any diverticula as incorporating a divertic-
ulum into the staple line jeopardizes the anastomosis. To avoid
this, it is helpful to suture the diverticulum in toward the anvil of
the stapler so that the diverticulum ends up in the tissue donuts.
Alternatively, the diverticulum can be eliminated by resecting
additional colon.
When marrying the circular stapler, it is important to pre-
vent any extraneous tissue (i.e., vagina, adnexa, bladder, epiploic
appendages, etc.) from catching in the stapler. This tissue can
interfere with the firing mechanism of the stapler and increases
the risk of anastomotic failure. Once the anastomosis is created,
air testing with the pelvis under saline should be performed rou-
tinely to identify occult defects requiring repair. Once a defect
demonstrated by a leak test has been repaired, as evidenced by a
negative repeat on-table leak test, the risk of postoperative anas-
tomotic leak is not increased.(13) Similarly, in situations where
the anastomotic donuts are incomplete but the leak test is nega-
tive, the risk of anastomotic leak is not increased.(14)
PROXIMAL DIVERSION
Many surgeons divert patients undergoing low anterior resection
with total mesorectal excision in the hopes of influencing the
leak rate and/or the clinical consequences of a leak.(15, 16) Given
the low frequency of anastomotic leak, in order to determine
whether fecal diversion protects patients from leaking, large, well-
designed, multiinstitution trials with homogenous study popula-
tions are required. Nonrandomized studies testing the hypothesis
that diversion decreases anastomotic failure are inherently biased
because of patient selection as surgeons are more likely to divert
patients in whom complications are anticipated.
The Rectal Cancer Trial On Defunctioning Stoma in Sweden, a
large, prospective trial including 234 patients, randomly assigned
patients undergoing stapled colorectal anastomosis within 7 cm
of the anal verge to have proximal fecal diversion.(17) The clinical
leak rate in the diverted and nondiverted groups was 10.3% and
28%, respectively (p < 0.001). In addition, the need for urgent
re-operation in the diverted and nondiverted groups was 8.6%
and 25.4%, respectively (p < 0.0001). To further evaluate the pos-
sible utility of a proximal stoma, a meta-analysis was performed
evaluating the role of a defunctioning stoma in low rectal cancer
surgery including the Swedish trial and three other smaller ran-
domized, controlled trials.(18) The odds ratios for clinical leak
and for re-operation due to a leak in diverted patients were 0.32
and 0.27, respectively (p < 0.001). While this meta-analysis and
a few other studies demonstrate significant benefits in terms of
decreasing the occurrence of leak, much of the remaining litera-
ture only supports the concept that proximal diversion amelio-
rates the septic consequences of leak but does not influence the
actual rate of leak.(14, 19–22)
Temporary fecal diversion is not without its own ramifications.
It is difficult to predict which individual patients will develop a
leak and routine stoma creation will reduce the quality of life
in patients in whom no anastomotic complication would have
occurred. Moreover, a certain percentage of diverted patients will,
inevitably, never have intestinal continuity restored; although,
a “temporary” diversion is more likely to become permanent
in patients who have experienced a leak.(17, 23) Finally, stoma
creation carries its own morbidity rate (i.e., increased wound
infection rate at the original operation, stoma complications,
morbidity of the reversal operation, etc.) and consumes signifi-
cant healthcare resources.(20)
Although there is no consensus regarding which patients should
undergo proximal fecal diversion at the time of colorectal anasto-
mosis, many surgeons routinely consider diversion in the setting
of low pelvic anastomoses as these are more likely to leak.(5, 24)