Improved Outcomes in Colon and Rectal Surgery part 9 ppt
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (253.6 KB, 10 trang )
8
improved outcomes in colon and rectal surgery
a narcotic-sparing role. In a study of over 1,000 patients, the addi-
tion of ketorolac to standard intravenous morphine significantly
reduced the overall postoperative morphine requirements, and low-
ered side effects both directly attributable to the narcotic (mental
status, pulmonary) as well as gastrointestinal function (ileus, nau-
sea, vomiting).(4) The anti-inflammatory action of these agents,
especially when used on a set schedule, may be most beneficial for
the pain associated with the musculoskeletal trauma of the incision,
allowing a significant reduction in pain without the untoward side
effects associated with narcotic use.
Pain following anorectal surgery can be quite debilitating and
is often cited by patients as a primary deterrent toward undergo-
ing needed procedures such as hemorrhoidectomy.(5) In addition
to the local trauma associated with resection, pain following anal
canal procedures is often attributed to anal sphincter spasm. This,
in combination with the constipation and hard stools often asso-
ciated with narcotic use, results in additional pain and suffering
once return of bowel function commences. As in the laparotomy
literature, recent trials have shown a marked decrease in the nar-
cotic requirements using ketorolac and other NSAIDs periopera-
tively for anorectal surgery.(6)
Local anesthesia, which has been commonly employed in the
field of anorectal surgery, has recently been expanded to continued
use postoperatively following abdominal procedures. As a primary
modality during anorectal procedures, it has been shown to be effec-
tive and safe, with or without the addition of deep intravenous seda-
tion, and provides the additional benefit of less time in the recovery
room.(7, 8) As an adjunct following laparotomy, a local anesthetic
agent is applied via continuous infusion to the midline wound
through a set of subcutaneous catheters placed at the time of surgery.
(9) Despite mixed results, some prospective data does demonstrate
a decreased narcotic requirement and improved perioperative recov-
ery, including earlier ambulation, in the absence of significant overall
postoperative pain score differences.(10, 11) As increased experi-
ence is gathered using this modality, further data may determine its
appropriate place in the analgesia armamentarium.
Another method commonly employed is epidural anesthesia,
which works through inhibiting ascending neural pathways as well
as the sympathetic output from the spinal cord. This dual action
provides the beneficial effects of not only improved pain control,
but also has been shown to aid with earlier return of bowel function
through its sympathectomy. Various agents have been described
for use in epidurals, with the mainstays being local anesthetics and
narcotics. In a meta-analysis of sixteen randomized controlled tri-
als from 1987 to 2005 comparing the use of epidurals to paren-
teral controlled analgesia, Marret and colleagues found epidurals
were associated with improved analgesia and overall decreased
ileus, with only side effects such as pruritis and labile blood pres-
sure significantly associated with epidural use.(12) Gendall and
colleagues confirmed these findings in a recent review of the lit-
erature, again demonstrating that epidural anesthesia improves
functional recovery and pain relief, while potentially decreasing
pulmonary complications.(13) While the epidural is in place, the
anesthesiologist often manages all of the pain medications, per-
forming comprehensive pain management. When the epidural
is removed, the management returns to the surgeon. The use of
anticoagulation for deep venous thrombosis (DVT) prophylaxis
and epidural placement/removal must be coordinated between
the surgeon and anesthesiologist. Downsides to epidurals have
consistently been their potential for increasing urinary retention
and hypotension, higher costs, and perhaps decreased patient sat-
isfaction, with no change in length of stay.(14) Despite these nega-
tive attributes, epidural use for colorectal surgery provides the
potential for outcome improvement in analgesia and functional
recovery, and is a very useful alternative for pain control following
abdominal and large pelvic procedures.
Furthermore, gabapentin, a medication that was originally
designed for the treatment of epilepsy, has evolved as a treatment
for mainly neuropathic pain, and has been studied extensively in
both the pre- and postoperative settings. Although the exact mecha-
nism of action is unknown, it is believed to act through N-gated
calcium channels, and focus has been on both providing improved
perioperative analgesia as well as narcotic-reducing effects.(15)
Unfortunately, its use as an independent entity has been less effica-
cious, and it has not been extensively studied for either colorectal
or anorectal surgery.(16) Both it, and a newer analog pregabalin,
have been shown to decrease the need for opioids and thus reduce
side effects such as nausea, vomiting, and urinary retention in other
surgical arenas. While further study awaits recommendations spe-
cifically for use in the realm of colon and rectal surgery, these medi-
cations, along with standard postoperative regimen of increased
oral fluid intake, fiber, stool softeners, sitz baths, and avoidance of
constipation all aid in recovery from anorectal surgery.
Most importantly, these varying agents all operating through
different mechanisms convey the needed concept for the surgeon
to use a multimodality approach to ensure successful perioperative
pain management.
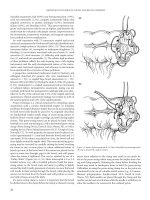
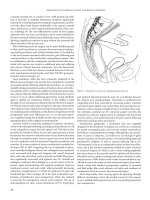
Figure 8.1 Thumbtack occlusion of a bleeding basivertebral vein.
general postoperative complications
bleedinG
Bleeding complications with any operation can be categorized
by many different methods including intraoperative, postopera-
tive, anastomotic bleeding, and gastrointestinal bleeding, such
as stress-related ulcers. One of the most important factors for
the surgeon is to preoperatively assess and determine the risk
of bleeding. A thorough history and physical examination with
emphasis on a personal or family history of bleeding tendency
is crucial to identification and subsequent evaluation of those
patients at risk, and should be completed before embarking on
surgery. Questions should focus on any predisposition for easy
bleeding or bruising, inability to clot even with mild cuts, or his-
tory of prior transfusions following surgeries, to identify certain
patients that require further evaluation. Preoperative laboratory
evaluation should include a complete blood count, coagulation
panel (PT, PTT, INR), and platelet count. For those patients at
increased risk, a more detailed analysis of platelet and clotting
cascade function to include bleeding times, mixing studies, or
evaluation by a hematologist may be appropriate.
Perioperative bleeding with colorectal surgery depends in many
aspects on the surgical procedure performed. Whereas bleeding
rates following hemorrhoidectomy range from 2% to 6%, those fol-
lowing major abdominal operations such as total mesorectal exci-
sion (TME) for rectal cancer have been shown to have much higher
blood loss estimates, with transfusion requirements reported in
up to 43–73% of patients.(17–20) The most common causes for
postoperative hemorrhoidal bleeding are technical failure (failed
knot) within the first 24 hours, and infection with erosion at
7 days. Despite this, bleeding following hemorrhoidectomy is
often able to be controlled either without surgical intervention or
with simple suture ligation in the outpatient setting.
In contrast, bleeding following major abdominal or pelvic
procedures can mandate return to the operating room with
corresponding physiological changes that may lead to cardio-
pulmonary complications. Yet, with the emergence of emphasis
on decreased mandatory transfusion requirements, and techno-
logical advancements such as improved minimally invasive tech-
niques, transfusion rates are decreasing and blood loss has also
decreased. In a study of 147 patients in a case-matched compara-
tive analysis between open and laparoscopic colectomies, Kiran
and colleagues found that both estimated blood loss and peri-
operative transfusion rates were significantly higher in the open
group.(21) Timing of the onset of bleeding also provides some
insight as to its etiology. Early postoperative bleeding is typically
from a technical error at the time of operation.(22) Late bleed-
ing, which tends to present days to weeks after surgery, (though
not outside the realm of technical problems) is more commonly
secondary to patient factors such as an underlying bleeding ten-
dency, concomitant coagulopathy, or spontaneous rupture or
hemorrhage. It is worth noting that the risk of severe bleeding
such as after a two or three quadrant hemorrhoidectomy, despite
being small (2–6%), can be catastrophic.(5, 19) Therefore, it is
imperative that the possibility of bleeding is discussed with all
patients preoperatively, no matter how minor of a surgical proce-
dure the patient is undergoing.
Although uncommon, massive presacral bleeding during pelvic
dissection can result in hemodynamic instability and even death.
Injury is secondary to dissection outside the avascular plane. Initial
management includes packing and leveling the patient on the oper-
ating room table, along with continued resuscitation. This is effective
for most patients within 20–30 minutes. Multiple other methods have
described including electrocautery, suture ligation, sacral thumbtacks
(Figure 8-1), muscle fragment welding, placement of tissue expand-
ers or cyanoacrylate adhesives, topical hemostatic agents, and endo-
scopic tacking devices.(23–28) Although the presacral veins may be
injured, continued bleeding nonresponsive to initial management
is most commonly from the basivertebral veins through the sacral
foramina.(24, 29, 30) When all else fails, pelvic packing, peritoneal
closure, warming, and resuscitation in the ICU with return to the
operating room 24–48 hours later may be required. Thus, emphasis
on proper technique, knowledge of the pertinent anatomy, and com-
prehension of options to immediately consider when things do go
awry are all important to decrease perioperative bleeding complica-
tions and subsequent clinical complications.
infection
Surgical-Site Infection (SSI)
Surgical site infections continue to be a major source of cost and
morbidity despite a strong emphasis on proper selection, timing
and duration of perioperative antibiotics. Infections can be clas-
sified as surgical site infections, general postoperative infections
such as pneumonia and urinary tract (which will be covered in
separate sections), and infectious processes that deal specifi-
cally with the operation itself (i.e., anastomotic leaks, abscesses).
Although multiple different patient and surgical factors contrib-
ute to the development of postoperative infections, development
of any infectious complication results in increased patient suf-
fering, length of stay, and delayed recovery. Additionally, hospital
costs encompassing antibiotics, interventional procedures, nurs-
ing support, and surgical intervention contribute to driving up
overall healthcare system costs.
In simple terms, our skin and mucosal lining remain the
primary defense mechanisms against infectious sources. With
surgery, the breach in these protective layers, along with manipu-
lation of the bowel and potential spillage of stool from various
colorectal procedures lead to increased rate of infections. Those
patients with an extensive component of cellulitis, characterized
by leukocytic infiltration of the dermis, bacterial presence, and
localized inflammatory response, typically require the addition of
antibiotics, especially in patients with immunosuppresion, diabe-
tes mellitus and the elderly. With the emergence of methicillin-
resistant Staphylococcus aureus (MRSA) and other multiresistant
bacteria, it is imperative for the surgeon to help control the emer-
gence of these more virulent pathogens by avoiding prolonged
usage of antibiotics and changing antibiotics once the pathogens
are cultured and appropriate sensitivity to antibiotics is known.
With such a drastic rise in the incidence of MRSA, some hospitals
are performing preadmission screening cultures for this patho-
gen. Thus, if there is an emergence of an active MRSA infection
in the postoperative period, the patient most likely brought the
infection into the hospital with them. Its presence then is not a
result of failure of the surgeon or hospital personnel to follow
protocol prevention. From a surgical perspective, proper wound
improved outcomes in colon and rectal surgery
care, drainage of abscesses, and debridement of any necrotic tis-
sue, where appropriate, remain important adjuncts to the medi-
cal management of infections. Aspiration and drainage under
imaging can often be used to convert an urgent reexploration to
either an elective procedure or provide the ability to avoid a reop-
eration altogether.
In addition to proper surgical technique that avoids con-
verting a clean contaminated to a dirty case, identification of
at-risk patients can aid in early identification and treatment of
infectious complications. In a review of 428 patients specifi-
cally undergoing colorectal operations, surgical site infections
were independently associated with increased body mass index
(BMI) (odds ratio [OR] 1.07), and those in which a revision/
creation/or takedown of a stoma was involved (OR = 2.2).(31)
In addition, with the emerging pandemic of obesity through-
out the world, increased BMI has been found to be associated
with not only higher rate of surgical site infections, but also is
an independent predictor of wound dehiscence, herniation, and
anastomotic leak.(32)
Different methods have been employed to attempt to decrease
the incidence of surgical site infections. There is some debate in
the literature regarding the duration of antibiotic use for elective
colon and rectal surgery. Although preoperative use of intrave-
nous antibiotics to ensure adequate tissue concentrations at the
time of incision has become standard of care, there is some con-
troversy regarding the use of a single dose versus multiple doses.
Fujita and colleagues performed a study including almost 400
patients undergoing elective resection of colorectal cancer and
found that the three dose regimen of an every 8 hour, second
generation cephalosporin (i.e., 24-hour perioperative coverage)
significantly decreased the incidence of surgical site infections
over a single preoperative dose (4.3% vs. 14.2%, p = 0.009).(33)
However, organ or space SSI and other postoperative infectious
complications did not differ between the two groups, and has
similarly been not significantly different in many other studies.
The practice of adding oral antibiotics has similarly contradic-
tory evidence, with large prospective randomized trials demon-
strating no decrease in infectious complications, while increasing
the rates of nausea, pain, and noncompliance.(34, 35) Yet, other
authors including a large prospective randomized trial and meta-
analysis of 13 studies demonstrated the addition of oral antibiot-
ics to systemic antibiotics was associated with a higher rate of
prevention of surgical site infections.(36, 37) Proponents cite
the ability of the oral antibiotics to decrease the bacterial load
in the colon, as well as the marked increase of colonic bacterial
isolates from the infected surgical wounds as evidence and ratio-
nale for its use. With such varying opinions, it is up to the indi-
vidual surgeon to evaluate the literature and determine the best
approach as it applies to their patient population. Finally, besides
the proper use and timing of preoperative antibiotics, supple-
mental postoperative high dose oxygen (80%) has been shown
to reduce surgical site infections by approximately 6–40%.(38, 39)
Through suggested mechanisms, including more efficient electron-
transport chain off-loading and improved neutrophil function,
postoperative high-flow oxygen in the immediate recovery period
has become part of a standardized postoperative pathway for many
institutions. Finally, fewer complications may be associated with
maintaining perioperative normothermia in patients undergo-
ing colorectal surgery. Employing methods such as preoperative
warming with bear-hugger devices, use of warm blankets and
fluids, and avoiding prolonged or unnecessary exposure, may all
result in less SSI.
urinary tract infection and retention
Urinary tract infections are the leading cause of nosocomial
infections, accounting for ~40% of all infections, of which 80%
are associated with transurethral catheter placement.(40) The
dilemma remains how to significantly reduce this rate, especially
in light of the chronic use of urinary catheters during colorectal
procedures. A recent Cochrane review evaluating the use of anti-
biotics during short-term catheter use demonstrated there was
a paucity of evidence that antibiotic prophylaxis was any better
than treating patients when clinically symptomatic. While it did
show some weaker evidence that bacteriuria, pyuria, and gram-
negative bacteria are all reduced following antibiotic use over 24
hours or until catheter removal, none of these studies specifically
focused on the colon and rectal patient. In addition, there was
limited data on cost or subsequent development of multiresis-
tant organisms, and most patients undergoing colorectal sur-
gery receive perioperative antibiotics to cover bowel flora. Thus,
caution needs to be taken when determining the applicability of
these results to colorectal surgical patients. Following rectal sur-
gery, urinary tract infection in part depends on the clinical prac-
tice of the surgeon regarding length of time of bladder catheter
drainage. In a study comparing catheter removal at 1 and 5 days
following rectal resection, Benoist and colleagues found urinary
tract infections to be increased in those patients with catheters in
for 5 days versus those who removed after 1 day (42% vs. 20%, p
< 0.01).(41) Increases in urinary tract infection with prolonged
drainage must be balanced with voiding dysfunction with early
catheter removal.
Unfortunately, urinary retention remains a well-known com-
plication of colorectal and anorectal surgery, as well as a result of
the spinal anesthesia commonly used during these operations.(42)
In the Benoist study, urinary retention was significantly increased
in those with the catheter present for only 1 day of postoperative
drainage over the 5-day cohort (25% vs. 10%, p < 0.05), especially
amongst those with tumors of the low rectum.(41) Following pel-
vic surgery, this may be, in part, secondary to third spacing and
edema around the urethra following disruption of these tissue
planes. Thus, in abdominal surgery, where the dissection does not
proceed below the peritoneal reflection, this may lower the rate
of dysfunction. The authors concluded that 1 day of drainage is
adequate for most patients, although for patients undergoing lower
resections, longer periods of drainage may be optimal. Changchien
and associates in a review of 2,355 patients with colorectal cancer
found urinary retention to be significantly associated with mul-
tiple factors to include older age, history of lung disease, rectal
cancer, longer operations, and additional pelvic procedures such
as hysterectomy or cystectomy.(43) Additionally, male gender,
American Society of Anesthesiologists’ (ASA) score of 2 or 3, rectal
cancer, use of a pelvic drain, and pelvic infection were indepen-
dently associated with prolonged urinary dysfunction, defined as
continued problems over one month postoperatively. To lessen
general postoperative complications
the higher rates associated with transurethral catheter placement,
some authors have also advocated suprapubic catheter drainage. In
patients undergoing pelvic surgery, this has been associated with
similar voiding dysfunction rates, but fewer infectious complica-
tions than the traditional transurethral route.(44, 45)
Following anorectal surgery, urinary retention rates have
been reported to be up to 50%.(46) Multiple methods that have
attempted to decrease this rate have limited perioperative fluid, use
of local versus spinal anesthesia, and even use of alpha-adrenergic
blockade preemptively.(46) Although the latter did not seem to sig-
nificantly affect rates of voiding dysfunction, both fluid restriction
and adequate pain control have consistently been shown to have a
positive effect at decreasing this difficult problem.(47, 48) Toyonaga
and associates, in a prospective study of over 2000, patients found
independent predictors significantly associated with the develop-
ment of urinary retention following anorectal surgery were female
sex, prior urinary difficulties, diabetes mellitus, intraoperative flu-
ids over 1 L, and prolonged need for postoperative analgesics.(48)
Although urinary retention is known to increase overall length of
hospital stay, (42) careful patient education and strict fluid control
have allowed most anorectal surgeries to be performed in an ambu-
latory setting with a low rate of return for urinary catheterization.
(49) Additional use of agents such as NSAIDs and Ketorolac may
minimize narcotic use and increase the success rate by avoiding this
complication in an outpatient setting.(6) Surgeons therefore need
to be in constant communication with their anesthesia counter-
parts to discuss excessive fluids and proper analgesia, as many are
unaware of the potential downfall of these common practices.
atelectasis
Prevention
Basic principles of airway clearance, avoidance of splinting and
alveolar collapse, while preserving functional residual capacity
and pulmonary reserve remain important components of proper
postoperative pulmonary toilet. As a part of the “5 W’s” of the
postoperative fever, “wind” as it relates to atelectasis reminds phy-
sicians that optimizing ventilation and oxygenation are keys to
successful recovery, and are subsequently passed down to succes-
sive generations of training surgeons (as much as absolute truth
as ancient lore). The fundamental principle behind avoidance of
atelectasis has been shown to be successful in pulmonary processes
ranging from cystic fibrosis and acute spinal cord injury to post-
operative esophagectomy.(50–52) As such, factors such as head of
bed elevation, early ambulation, and the ever-present incentive
spirometer have become the mainstays of postoperative inpatient
care. However, a recent Cochrane review of incentive spirometry
use in the postcoronary artery bypass graft population with 443
participants in 4 trials found no difference in pulmonary compli-
cations amongst incentive spirometry, positive pressure use con-
tinuous positive airway pressure (CPAP), bilevel positive airway
pressure (BIPAP), or simple preoperative patient education.(53)
Furthermore, a meta-analysis with 14 trials over a 26-year period
evaluating the use of incentive spirometry, positive pressure, and
deep breathing following upper abdominal surgery to prevent
postoperative pulmonary complications, also demonstrated no
statistically significant difference between these modalities and no
therapy alone.(54) Pasquina and colleagues performed a review
of 35 trials evaluating the use of respiratory physiotherapy after
abdominal surgery and found that only in one study was the
incidence of pneumonia decreased. In another study atelectasis
decreased from 77% to 59% using pulmonary toilet methods of
deep breathing, cough, and postural drainage.(55) They concluded
that there are only a few trials that support its use, and the routine
use of respiratory physiotherapy does not seem warranted based
on data alone. Despite these large reviews, atelectasis is known
to be present in anesthetized patients in the dependent portions
of the lungs and has been shown to contribute to decreased lung
compliance, worse oxygenation, increased pulmonary vascular
resistance, and shunting.(56) It seems at worst that the practice of
employing methods to decrease atelectasis is not harmful, and at
best, may help out to a small degree with avoidance of pulmonary
complications, and therefore, it is our continued practice.
Pneumonia
There is little data in the literature that directly addresses the
development of pneumonia following colorectal or anorectal sur-
gery. As stated above, the degree to which atelectasis and proper
pulmonary toilet corresponds to the development of pneumo-
nia is debatable. One thing that is clear is that development of
postoperative pneumonia is independently associated with worse
outcomes. Therefore, both prevention and early recognition and
treatment, are key components to ensuring optimal outcomes.
Johnson and colleagues found in a study of 180,359 patients that
postoperative respiratory failure (defined as mechanical ventila-
tion for longer than 48 hours after initial surgery or unantici-
pated reintubation) was found in 5,389 (3.0%) of patients and
was associated with an increased in hospital morbidity, cost,
and late mortality.(57) Additionally, factors that were found to
be independently associated with the development of this was
higher ASA classification, emergency operations, more complex
surgery, sepsis, older age, congestive heart failure (CHF), chronic
obstructive pulmonary disease (COPD), and smoking. These,
along with a history of obesity and obstructive sleep apnea, man-
date a need for careful postoperative monitoring and aggressive
pulmonary toilet. Many of the patients, especially with underly-
ing malignancy, have these comorbidities, and speak to the com-
plexity of operations and need for close surveillance to avoid this
feared complication.
deep venous thrombosis
Deep venous thrombosis (DVT) and its embolic corollary, pul-
monary embolism (PE), are a significant source of morbidity and
mortality in the perioperative period. Due to the predominance of
abdominal and pelvic surgery, colorectal surgery carries a higher risk
of these postoperative complications than other general surgical pro-
cedures. Yet, despite so much emphasis, DVT and PE continue to be
the most common cause of preventable deaths during in-hospital
admission, accounting for 1 out of every 4 hospitalized patients
deaths.(58, 59) More concerning, over 50% of all DVTs are asymp-
tomatic, while the vast majority of PEs are detected only after death
(58). Since Virchow’s original description of stasis, hypercoaguability,
and endothelial damage as risk factors, large epidemiological studies
have found an increase in the development of symptomatic venous
improved outcomes in colon and rectal surgery
thromboembolism in the perioperative period to be associated with
male gender, malignancy, trauma, immobility, COPD, sepsis, low
hematocrit, low albumin, and major surgery.(60)
One of the major problems with development of a DVT is the lack
of initial clinical signs. Patient complaints of pain, swelling, edema,
warmth, and tenderness of the affected limb are often absent.(61)
Those patients that progress onto pulmonary embolism present
many times in the late stages with cardiopulmonary shock and col-
lapse, though often heralded by symptoms such as acute shortness
of breath, dyspnea, pleuritic chest pain, along with tachycardia and
an increasing oxygen requirement. Thus, emphasis has been placed
on both prevention and screening. Despite its very high sensitiv-
ity and specificity of over 95%, screening with duplex and color
Doppler sonography, even in high risk patients, in the absence of
symptoms, has been questioned as to its cost-effectiveness.(62, 63)
Part of this may be that although the lower extremities are the most
common site of origin, approximately one-third of patients have
proximal (above popliteal) veins as the site of origin, which are not
visualized well by duplex.(59) Venous ultrasonography remains the
mainstay for diagnosis of deep venous thrombosis, especially when
combined with elevated d-dimer levels. The hallmarks of DVT via
ultrasound are both visualization of the clot and more commonly
the inability to compress the venous system under direct pressure.
(64) Similarly with the advent of multidetector row helical CT scan-
ners, this has essentially supplanted the pulmonary angiogram as
the procedure of choice for diagnosis of pulmonary embolism, with
sensitivity, specificity, and negative predictive value over 90%, even
for subsegmental pulmonary emboli.(65)
Prophylaxis of venous thrombotic events centers on both
mechanical and medical means. The current mainstays for chemi-
cal thromboprophylaxis are unfractionated and low- molecular
weight heparin. Unfractionated heparin works through anti-
thrombin III to deactivate thrombin and other factors in the
clotting cascade. Concerns about increased bleeding events as well
as its dose-effect relationship have led many to be wary of its use.
Low-molecular weight heparin has enhanced antifactor Xa activ-
ity and more predictable dose-effect relationships.(66) In a recent
Cochrane review addressing the prevention of thromboembolic
complications, the combined use of mechanical graduated stock-
ings with either unfractionated or low molecular heparin was
identified as the optimal prophylaxis.(67) Interestingly, despite the
extensive search, only 3 studies meeting inclusion criteria focused
specifically on colon and rectal surgery. That same group evalu-
ated 558 studies, of which 19 met the inclusion criteria, and again
found that unfractionated and fractionated heparin were equally
effective, and the addition of either to compression stockings was
superior to either alone.(68)
As pointed out in the opening challenging case, risk stratifi-
cation continues to be a mainstay for determining the extent of
prophylaxis in these patients. Young, healthy patients undergoing
routine anorectal surgery, with minimal patient-specific risk fac-
tors, do not require any therapy other than mechanical means
via graduated compression stockings and/or intermittent pneu-
matic compression boots and early ambulation. Those patients
with multiple risk factors and undergoing high risk surgery, such
as pelvic operations, warrant more aggressive means like unfrac-
tionated or low-molecular weight heparin, in addition to the
mechanical devices. Timing has been somewhat controversial
with some studies demonstrating higher bleeding without undue
increase in thrombotic events when given after the surgery and
others stating that dosing should begin preoperatively. Although
this question has yet to be definitively answered based on cur-
rent literature, it is well accepted that some form of perioperative,
including intraoperative means, has become the standard of care.
The risk of bleeding with thromboprophylaxis dosing is small,
with the majority revolving around injection site ecchymoses or
hematoma in up to 7% of cases.(69) More clinically significant
bleeding, such as gastrointestinal or intraabdominal bleeding,
occurs in <0.5%, and is rarely the cause for cessation of therapy.
One potential concern that arises frequently in the realm of
colorectal surgery is how to treat the patient receiving anticoagu-
lation for colonoscopy. Recent guidelines have shown that aspirin
and other NSAIDs do not need to be withheld, with the rate of
postpolypectomy bleeding around 2%.(70) On the other hand,
coumadin and other more potent antiplatelet medications (i.e.,
clopidogrel) are commonly held for 5 to 7 days before the pro-
cedure, especially when it is known that a polypectomy or other
procedure is likely. There is some evidence that the application
of endoclips with polypectomy in anticoagulated patients is safe;
however, small sample sizes hinder ability to make broad recom-
mendations.(71) Thus, most of the practice is based on guide-
lines and less on an abundance of available evidence supporting
or dissuading this practice.(72, 73)
nausea and vomitinG
Though often not deemed as significant or crucial to overall success
of an operation by surgeons, postoperative nausea and vomiting
(PONV) can be extremely bothersome for the patient. Clearly, the
etiology is multifactorial—with surgical, anesthetic, medication,
and patient-related factors all contributing significantly. Head of
bed elevation and early ambulation are minor modifications that
may be somewhat helpful. More useful, anesthesia providers have
found increasing success through prophylaxis for this phenom-
enon. As a part of that process, identification of those patients at
risk is imperative, as universal prophylaxis has not been shown
to be cost-effective.(74) A thorough review of prior surgeries and
response to anesthetics may help in identification of these individ-
uals. Intravenous use of ondansetron, a selective serotonin 5HT3
receptor antagonist, has been shown in multiple randomized trials
to be effective in complete prevention of postoperative emesis in up
to 60–85%, when given before the induction of general anesthesia.
(75–77) Finally, routine decompression with nasogastric tubes has
demonstrated no impact on PONV and has fallen out of favor.(78)
prolonGed ileus
In general, postoperative obstruction can be divided into two broad
categories—early and late. Early postoperative bowel obstruction is
defined as onset of symptoms within thirty days of surgery. The
majority of early postoperative bowel obstructions are due to para-
lytic ileus or adhesions—up to 90 percent in some series, with the
remaining possible etiologies including phlegmon, intraabdomi-
nal abscess, Crohn’s disease, hernia, volvulus, intussusceptions,
and malignancy.(79, 80) Late obstructions are those presenting at
any point >30 days following surgery. The management of bowel
general postoperative complications
obstruction including ileus remains a significant burden to health-
care costs. In 1994, according to Beck and colleagues, there were
303,836 hospitalizations during which adhesiolysis was performed,
accounting for 846,415 inpatient days and an estimated $1.3 billion
in expenditures.(81) In addition, reoperative surgery in the setting
of early bowel obstruction can prove to be significantly challeng-
ing, as abdominal inflammation and early adhesions create a hostile
environment marked by densely adhered bowel and friable tissues.
In order to safely and effectively manage these patients, one must
have an extensive understanding of the various conditions which
may result in prolonged ileus.
Like many of the complications discussed in this chapter,
the development of a prolonged ileus has multiple potential
causative factors including hormones, medications and sur-
gical stress. Postoperative ileus clinically manifests itself with
abdominal distension, bloating, failure to pass stool or gas, nau-
sea, emesis, and pain. Even more concerning, Senagore found
that in addition to the symptoms experienced as a result of the
ileus, delayed surgical wound healing and ambulation, atelecta-
sis, pneumonia, and deep vein thrombosis are all potentially
increased by the development of a postoperative ileus, which
increases hospitalization length of stay and overall costs.(82) The
definition of what constitutes a prolonged ileus widely varies in
the literature and contributes to discrepancies between different
studies. In general, when the symptom complex continues for over
7 days following abdominal surgery, most consider this prolonged
and should raise concern for more extensive evaluation.
Return of bowel function has multiple parameters that can be
controlled by the provider in the perioperative period. For exam-
ple, limiting the amount of intraoperative and postoperative fluid
and sodium has been shown to improve time to passage of flatus
and stool, and result in earlier hospital discharge.(83) In addition,
clinical pathways that include the use of restricted perioperative
intravenous fluids, early oral intake, early ambulation, and epidu-
ral anesthesia, have been shown to significantly decrease length of
stay and perioperative cardiopulmonary complications, although
readmissions are slightly higher.(84) Thus, working through
optimization of all components of postoperative care may con-
tribute more to a successful recovery than primary emphasis on
one factor alone. Through entry into a standardized program,
the avoidance of certain variables that negatively affect recovery
for both the intraoperative and postoperative settings can pro-
vide improved reproducible results.(85) A bonus of implemen-
tation of pathways is the ability to help all healthcare providers,
including nursing personnel, to become accustomed to a routine
postbowel resection course. Therefore, any deviations from this
can be recognized more readily, allowing intervention before the
patient enters a more severe or septic state.
A thorough history and physical examination help distinguish
some of the benign causes of obstruction and aid in differentiating
this from a prolonged ileus. For example, the history in a patient
with Crohn’s disease or prior radiation therapy can provide just
as many clues as to the etiology of the obstruction, such as pos-
sible stricture, or an obvious hernia detected on physical exami-
nation. As patients often present with concomitant dehydration
and electrolyte abnormalities, placement of a nasogastric tube,
with appropriate fluid resuscitation, and correction of electrolyte
abnormalities should occur while the work-up is in progress. Plain
film radiographs may confirm dilated small bowel loops with
stair-stepping air-fluid levels, but usually do not assist in defin-
ing the underlying etiology. Despite their frequent use, numerous
studies quote a poor sensitivity for plain abdominal radiographs
in diagnosis of small bowel obstruction, ranging from 13% for
low grade obstruction to 50–60% for high grade obstructions.
(86) CT may give anatomical information outside of the bowel
wall itself that may help with accurate diagnosis. Caution should
be used in giving oral contrast for the patient with high grade
ileus or obstruction, and in general, should be avoided.
Newer pharmacotherapeutic endeavors, such as the peripher-
ally acting mu-opioid receptor antagonist, alvimopan, have been
shown to reduce the incidence of postoperative ileus, nasogas-
tric tube insertion, time to gastrointestinal recovery, and overall
hospital length of stay.(87–89) Further studies are still ongoing
to evaluate whether its safety profile is acceptable for wide-scale
clinical use. Other methods that have been studied in attempt to
shorten bowel function return include prokinetic agents such as
erythromycin and cisapride, although the results have been mixed.
Erythromycin, a motilin agonist, has been shown in the past to be
effective for upper gastric and pancreatic surgery, especially with
regard to promotion of gastric emptying. In a randomized double-
blind placebo study of 134 patients, erythromycin was not shown
to affect clinically relevant outcomes such as time to intake of solid
foods, nausea rate, or length of stay.(90, 91) Similarly, cisapride,
before its removal from the market secondary to cardiac toxicity,
did show some, albeit limited, clinically significant improvements.
(92–94) Thus, for hindgut surgery, prokinetic agents have not yet
been shown to make a clinically relevant difference.
Probably the most important factor that has been shown to
make a difference in reducing ileus is postoperative clinical
pathways that include early oral feeding.(95) A recent Cochrane
review by Andersen et al. including 13 randomized controlled
trials and over 1,100 patients, evaluated the use of early feeding
and the development of complications and found early feeding
is safe, may reduce postsurgical complications, and concluded
there is no advantage to withholding oral intake.(96) Opponents
of this practice cite a lack of a consistent definition of what early
feeding encompasses. As such, although many surgeons prefer to
advance the postoperative diet slowly, it does seem clear that the
recovery of gastrointestinal function as evidenced by first bowel
movement or flatus and tolerance of an oral diet in the early post-
operative setting are independent of each other, and the practice
of early resumption of diet is safe.(97)
retained foreiGn bodies
In any complex surgical procedure there exists a potential for items
to be unknowingly left in body cavities.(98) To minimize this risk,
current standards require all sponges, needles, surgical instru-
ments, equipment, and items small enough to be misplaced be
counted before the procedure and one or two times after the com-
pletion of the procedure to confirm that all items are accounted
for. These activities are usually performed and documented by the
operating room nurses and technicians; however, the surgeon is
ultimately responsible and should conduct each operation so as to
minimize the risk of misplaced foreign bodies. In accordance with
improved outcomes in colon and rectal surgery
this goal, most surgeons, avoid using small Ray-tec sponges in the
abdomen and avoid placing laparotomy sponges in areas that are
hard to visualize. If sponges must be used to pack areas, the sponge
marker should be left in an obvious area, or a ring or clamp may
be attached to the sponge. Most important is a through explo-
ration of the entire operative field, which should be performed
routinely before closing the incision. Items used during the opera-
tion that have the potential to be easily lost should be radiopaque
or contain a radiopaque marker (e.g., Ray-tec sponges and Silastic
drains). Figures 8.2 and 8.3 demonstrate the radiologic view of
several common surgical items. Plain radiographs are often the
best for identifying the radiopaque markers incorporated into
these items; The markers may be much less obvious on studies
such as CT scans (Figures 8.4a and b).
If an instrument, sponge, or needle count is not correct, several
actions are indicated. All the sponge wrappers and suture pack-
ages should be counted to confirm the accuracy of the original
count. The entire operating room, and especially the trash bags
and floor under the operating table, should be searched for the
misplaced item. Simultaneously, the surgeon should inspect the
operative field thoroughly for the missing item. If the missing
item cannot be located, a radiograph of the entire operating field
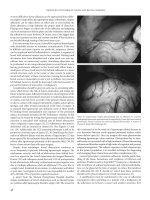
Figure 8.2 Radiograph demonstrating radoopaque markers. Left to right.
Laparotomy sponge, Ray-tec sponge. The upper image is flattened, whereas the
lower image demonstrates the radiologic view when the item is crumpled.
Figure 8.3 Radiograph of (left to right) Jackson-Pratt drain, Penrose drain,
nasogastric tube.
Figure 8.4 (A) CT scan of patient with a retained Ray-tec sponge. Image is the
inferior cut of the study. The upper edge of the Ray-tec marker is demonstrated
as white dots lines between the bladder (filled with contrast) and the sacrum. (B)
Pelvic radiograph of the same patient demonstrating Ray-tec marker in pelvis.
(A)
(B)
general postoperative complications
should be obtained before closure of the body cavity to identify
any radiopaque object and minimize the morbidity of locating
the missing item. Because of the potential for human error, a
“correct” instrument, needle, or sponge count does not absolutely
exclude the presence of a foreign body. Therefore, each member
of the team must maintain a high index of suspicion.
A sponge or other foreign body left in a body cavity can pres-
ent or be identified in a number of ways. The foreign body may
be seen on a radiograph obtained for other reasons, or the patient
may develop symptoms that lead to the need for radiographs or
an exploratory procedure. Symptoms may be infectious (fever,
elevated white cell count, wound infection, or abscess) or inflam-
matory (ileus, tenderness, mass effect). For any postoperative
patient with unusual or unexplained symptoms, radiographs
should be included in the evaluation.
Finding a retained foreign body is unusually an indication for
an urgent exploratory procedure. The exception may be asymp-
tomatic patient with a retained small needle. The morbidity associ-
ated with localizing and removing a small needle must be weighed
against the potential risk of leaving it alone, and the patient can
assist in this decision. Review of biplanar radiographs may assist
in localizing a retained item, and early identification will minimize
the morbidity associated with the object’s removal. Retention of
operative foreign bodies is uncommon, and a surgical team that
adheres to careful practices should avert its occurrence.
“time out” and sided surGery concerns
Wrong-side/wrong site, wrong-procedure, wrong-patient adverse
events (WSPEs) constitute some of the worst medical errors that cli-
nicians and patients experience. These events often result in patient
harm and litigation, but literature on frequency and root causes
is limited.(99) The Institute of Medicine report To Err Is Human
painted a broad picture of the magnitude of medical errors in the
United States and gave directions for safety improvements.(100)
While, sided surgery is less of a concern in colorectal patients, incor-
rect surgery due to inadequate lesion location remains a challenge. In
July 2004, the Joint Commission on the Accreditation of Healthcare
Organizations (JCAHO) implemented the universal protocol for the
prevention of WSPEs.(101) The protocol uses preoperative verifica-
tion of patient, site, and procedure, marking the operative site, and
a time-out immediately before starting the procedure. Prevention of
WSPEs requires new and innovative technologies, reporting of case
occurrence, and learning from successful safety initiatives (such as
in transfusion medicine and other high-risk nonmedical industries),
while reducing the shame associated with these events.
future directions
As the push to not only prevent complications but detect them
earlier continues to expand, molecular markers may become an
increasingly utilized component. Welsch and associates evaluated
the use of C-reactive protein (CRP) in the postoperative course
of 383 rectal resections with primary anastomosis in patients
with rectal cancer and found that using a cutoff of levels above
140 mg/dl on postoperative days 3 and 4 was associated with a
sensitivity of 80 and 54% respectively and specificities of 81 and
92%, respectively, for the presence of all infectious complications.
(102) Although this is just one example of a vast field, it highlights
a growing trend toward bridging the gap between molecular and
bench research with clinical application in attempt to change the
way surgeons approach patients and improve outcomes.
conclusion
As the field of colon and rectal surgery continues to evolve as a
specialty, emphasis on optimization of outcomes through pre-
vention and early identification, and treatment of complications
is imperative. As many complications are a result of multiple dif-
ferent components, sometimes all working in concert to lead to
untoward results, surgeons must also use a multifaceted approach
to ensure a successful perioperative course.
references
1. Sparling KW, Ryckman FC, Schoettker PJ et al. Financial
impact of failing to prevent surgical site infections. Qual
Manag Health Care 2007; 16(3): 219–25.
2. Jensen MP, Chen C, Brugger AM. Postsurgical pain outcome
assessment. Pain 2002; 99(1–2): 101–9.
3. Sim R, Cheong DM, Wong KS, Lee BM, Liew OY. Prospective
randomized, double-blind, placebo-controlled study of
pre- and postoperative administration of a COX-2 specific
inhibitor as opioid-sparing analgesia in major colorectal
surgery. Colorectal Dis 2007; 9(1): 52–60.
4. Cepeda MS, Carr DB, Miranda N et al. Comparison of mor-
phine, ketorolac, and their combination for postoperative
pain: results from a large, randomized, double-blind trial.
Anesthesiology 2005; 103(6): 1225–32.
5. Armstrong DN, Ambroze WL, Schertzer ME, Orangio GR.
Harmonic Scalpel
®
vs. electrocautery hemorrhoidectomy: a pro-
spective evaluation. Dis Colon Rectum 2001; 44(4): 558–64.
6. Place RJ, Coloma M, White PF et al. Ketorolac improves
recovery after outpatient anorectal surgery. Dis Colon
Rectum 2000; 43(6): 804–8.
7. Sun MY, Canete JJ, Friel JC et al. Combination propofol/
ketamine is a safe and efficient anesthetic approach to ano-
rectal surgery. Dis Colon Rectum 2006; 49(7): 1059–65.
8. Read TE, Henry SE, Hovis RM et al. Prospective evaluation
of anesthetic technique for anorectal surgery. Dis Colon
Rectum 2002; 45(11): 1553–8.
9. Polglase AL, McMurrick PJ, Simpson PJ et al. Continuous
wound infusion of local anesthetic for the control of pain
after elective abdominal colorectal surgery. Dis Colon
Rectum 2007; [Epub ahead of print].
10. Beaussier M, El’Ayoubi H, Schiffer E et al. Continuous
preperitoneal infusion of ropivacaine provides effective
analgesia and accelerates recovery after colorectal surgery:
a randomized, double-blind, placebo-controlled study.
Anesthesiology 2007; 107(3): 461–8.
11. Baig MK, Zmora O, Derdemezi J et al. Use of the ON-Q
pain management system is associated with decreased post-
operative analgesic requirement: double blind randomized
placebo pilot study. J Am Coll Surg 2006; 202(2): 297–305.
12. Marret E, Remy C, Bonnet F. Postoperative Pain Forum
Group. Meta-analysis of epidural analgesia versus parenteral
opioid analgesia after colorectal surgery. Br J Surg 2007; 94(6):
665–73.
improved outcomes in colon and rectal surgery
13. Gendall KA, Kennedy RR, Watson AJ, Frizelle FA. The
effect of epidural analgesia on postoperative outcome after
colorectal surgery. Colorectal Dis 2007; 9(7): 584–98.
14. Carli F, Trudel JL, Belliveau P. The effect of intraoperative
thoracic epidural anesthesia and postoperative analgesia
on bowel function after colorectal surgery: a prospective,
randomized trial. Dis Colon Rectum 2001; 44(8): 1083–9.
15. Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative
pain–a systematic review of randomized controlled trials.
Pain 2006; 126(1–3): 91–101.
16. Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical
patients benefit from perioperative gabapentin/pregabalin? A
systematic review of efficacy and safety. Analg 2007; 104(6):
1545–56.
17. Hayssen T, Luchtefeld M, Senagore A. Limited hemorrhoid-
ectomy: results and long-term follow-up. Dis Colon Rectum
1999; 42(7): 909–15.
18. Khan S, Pawlak SE, Eggenberger JC et al. Surgical treatment
of hemorrhoids: prospective, randomized trial comparing
closed excisional hemorrhoidectomy and the Harmonic
Scalpel technique of excisional hemorrhoidectomy. Dis
Colon Rectum 2001; 44(6): 845–9.
19. Chung CC, Ha JP, Tai JP, Tsang WW, Li MK. Double-blind,
randomized trial comparing Harmonic Scalpel
TM
hem-
orrhoidectomy, bipolar scissors hemorrhoidectomy, and
scissors excision. Dis Colon Rectum 2002; 45: 789–94.
20. Mynster T, Nielsen HJ, Harling H et al. Blood loss and trans-
fusion after total mesorectal excision and conventional rectal
cancer surgery. Colorectal Dis 2004; 6(6): 452–7.
21. Kiran RP, Delaney CP, Senagore AJ, Millward BL, Fazio
VW. Operative blood loss and use of blood products after
laparoscopic and conventional open colorectal operations.
Arch Surg 2004; 139(1): 39–42.
22. Gencosmanoglu R, Sad O, Koc D, Inceoglu R. Hemorrhoidectomy:
Open or closed technique? A prospective, randomized clinical
trial. Dis Colon Rectum 2002; 45: 70–5.
23. Harrison JL, Hooks VH, Pearl RK et al. Muscle fragment
welding for control of massive presacral bleeding dur-
ing rectal mobilization: a review of eight cases. Dis Colon
Rectum 2003; 46: 1115–7.
24. Nivatvongs S, Fang DT. The use of thumbtacks to stop massive
presacral hemorrhage. Dis Colon Rectum 1986; 29: 589–90.
25. Khan FA, Fang DT, Nivatvongs S. Management of presacral
bleeding during rectal resection. Surg Gynecol Obstet 1987;
165: 274–6.
26. Hill AD, Menzies-Gow N, Darzi A. Methods of controlling
presacral bleeding. J Am Coll Surg 1994; 178: 183–4.
27. Losanoff JE, Richman BW, Jones JW. Cyanoacrylate adhe-
sive in management of severe presacral bleeding. Dis Colon
Rectum 2002; 45: 1118–9.
28. Cosman BC, Lackides GA, Fisher DP, Eskenazi LB. Use of
tissue expander for tamponade of presacral hemorrhage.
Report of a case. Dis Colon Rectum 1994; 37: 723–6.
29. Qinyao W, Weijin S, Youren Z et al. New concepts in severe
presacral hemorrhage during proctectomy. Arch Surg 1985;
120: 1013.
30. Corman ML. Colon & Rectal Surgery. 5th ed. Philadelphia.
Lippincott Williams & Wilkins 2004.
31. Blumetti J, Luu M, Sarosi G et al. Surgical site infections
after colorectal surgery: do risk factors varying depending
on the type of infection considered? Surgery 2007; 142(5):
704–11.
32. Gendall KA, Raniga S, Kennedy R, Frizelle FA. The impact
of obesity on outcome after major colorectal surgery. Dis
Colon Rectum 2007; [Epub ahead of print].
33. Fujita S, Saito N, Yamada T et al. Randomized, multicenter
trial of antibiotic prophylaxis in elective colorectal surgery:
single dose vs. 3 dose of a second-generation cephalosporin
without metronidazole and oral antibiotics. Arch Surg 2007;
142(7): 657–61.
34. Espin-Basany E, Sanchez-Garcia JL, Lopez-Cano M et al.
Prospective, randomised study on antibiotic prophylaxis in
colorectal surgery. Is it really necessary to use oral antibiot-
ics? Int J Colorectal Dis 2005; 20(6): 542–6.
35. Kobayashi M, Mohri Y, Tonouchi H et al. Mie Surgical
Infection Research Group. Randomized clinical trial com-
paring intravenous antimicrobial prophylaxis alone with oral
and intravenous antimicrobial prophylaxis for the prevention
of a surgical site infection in colorectal cancer surgery. Surg
Today 2007; 37(5): 383–8.
36. Lewis RT. Oral versus systemic antibiotic prophylaxis in
elective colon surgery: a randomized study and meta-
analysis send a message from the 1990s. Can J Surg 2002;
45(3): 173–80.
37. Ishida H, Yokoyama M, Nakada H, Inokuma S, Hashimoto
D. Impact of oral antimicrobial prophylaxis on surgi-
cal site infection and methicillin-resistant Staphylococcus
aureus infection after elective colorectal surgery. Results of
a prospective randomized trial. Surg Today 2001; 31(11):
979–83.
38. Belda FJ, Aguilera L, Garcia de la Asuncion J et al. Spanish
Reduccion de la Tasa de Infeccion Quirurgica Group.
Supplemental perioperative oxygen and risk of surgical
wound infection: a randomized controlled trial. JAMA
2005; 294(16): 2035–42.
39. Greif R, Akca O, Horn EP, Kurz A, Sessler DI. Supplemental
perioperative oxygen to reduce the incidence of surgical-
wound infection. Outcomes Research Group. N Engl J Med
2000; 342(3): 161–7.
40. Niël-Weise BS, van den Broek PJ. Antibiotic policies for
short-term catheter bladder drainage in adults. Cochrane
Database Syst Rev 2005; (3): CD005428.
41. Benoist S, Panis Y, Denet C et al. Optimal duration of urinary
drainage after rectal resection: a randomized controlled trial.
Surgery 1999; 125(2): 135–41.
42. Zaheer S, Reilly WT, Pemberton JH, Ilstrup D. Urinary
retention after operations for benign anorectal diseases. Dis
Colon Rectum 1998; 41(6): 696–704.
43. Changchien CR, Yeh CY, Huang ST et al. Postoperative uri-
nary retention after primary colorectal cancer resection
via laparotomy: a prospective study of 2,355 consecutive
patients. Dis Colon Rectum 2007; 40: 1688–96.
44. Ratnaval CD, Renwick P, Farouk R, Monson JR, Lee PW.
Suprapubic versus transurethral catheterisation of males
undergoing pelvic colorectal surgery. Int J Colorectal Dis
1996; 11(4): 177–9.
general postoperative complications
45. Branagan GW, Moran BJ. Published evidence favors the
use of suprapubic catheters in pelvic colorectal surgery. Dis
Colon Rectum 2002; 45(8): 1104–8.
46. Cataldo PA, Senagore AJ. Does alpha sympathetic blockade
prevent urinary retention following anorectal surgery? Dis
Colon Rectum 1991; 34(12): 1113–6.
47. Petros JG, Bradley TM. Factors influencing postoperative
urinary retention in patients undergoing surgery for benign
anorectal disease. Am J Surg 1990; 159(4): 374–6.
48. Toyonaga T, Matsushima M, Sogawa N et al. Postoperative
urinary retention after surgery for benign anorectal dis-
ease: potential risk factors and strategy for prevention. Int
J Colorectal Dis 2006; 21(7): 676–82.
49. Hoff SD, Bailey HR, Butts DR et al. Ambulatory surgical
hemorrhoidectomy—a solution to postoperative urinary
retention? Dis Colon Rectum 1994; 37(12): 1242–4.
50. Main E, Prasad A, Schans C. Conventional chest physio-
therapy compared to other airway clearance techniques
for cystic fibrosis. Cochrane Database Syst Rev 2005; (1):
CD002011.
51. Berlly M, Shem K. Respiratory management during the first
five days after spinal cord injury. J Spinal Cord Med 2007;
30(4): 309–18.
52. Orringer MB, Marshall B, Chang AC et al. Two thousand
transhiatal esophagectomies: changing trends, lessons
learned. Ann Surg 2007; 246(3): 363–72.
53. Freitas ER, Soares BG, Cardoso JR, Atallah AN. Incentive
spirometer for preventing pulmonary complications after
coronary artery bypass grafting. Cochrane Database Syst
Rev 2007; (3): CD004466.
54. Thomas JA, McIntosh JM. Are incentive spirometry, inter-
mittent positive pressure breathing, and deep breathing
exercises effective in the prevention of postoperative pul-
monary complications after upper abdominal surgery? A
systematic overview and meta-analysis. Phys Ther 1994;
74(1): 3–10.
55. Pasquina P, Tramer MR, Granier JM, Walder B. Respiratory
physiotherapy to prevent pulmonary complications after
abdominal surgery: a systematic review. Chest 2006; 130(6):
1887–99.
56. Duggan M, Kavanagh BP. Atelectasis in the perioperative
patient. Curr Opin Anaesthesiol 2007; 20(1): 37–42.
57. Johnson RG, Arozullah AM, Neumayer L et al. Multivariable
predictors of postoperative respiratory failure after gen-
eral and vascular surgery: results from the patient safety in
surgery study. J Am Coll Surg 2007; 204(6): 1188–98.
58. Nutescu EA. Assessing, preventing, and treating venous
thromboembolism: evidence-based approaches. Am J Health
Syst Pharm 2007; 64(11 Suppl 7): S5–13.
59. Anaya DA, Nathens AB. Thrombosis and coagulation: deep
vein thrombosis and pulmonary embolism prophylaxis.
Surg Clin North Am 2005; 85(6): 1163–77.
60. Gangireddy C, Rectenwald JR, Upchurch GR, Wakefield
TW, Khuri S, Henderson WG, Henke PK. Risk factors and
the clinical impact of postoperative symptomatic venous
thromboembolism. J Vas Surg 2007; 45(2): 335–41.
61. Blann AD, Lip GYH. Venous thromboembolism. BMJ 2006;
332(7535): 215–9.
62. Davidson HC, Mazzu D, Gage BF, Jeffrey RB. Screening for
deep venous thrombosis in asymptomatic postoperative
orthopedic patients using color Doppler sonography: anal-
ysis of prevalence and risk factors. AJR Am J Roentgenol
1996; 166(3): 659–62.
63. Gaitini D. Current approaches and controversial issues in
the diagnosis of deep vein thrombosis via duplex Doppler
ultrasound. J Clin Ultrasound 2006; 34(6): 289–97.
64. Wells PS. Integrated strategies for the diagnosis of venous
thromboembolism. J Thromb Haemost 2007; 5(Suppl 1):
41–50.
65. Schoepf UJ, Schneider AC, Das M et al. Pulmonary embolism:
computer-aided detection at multidetector row spiral com-
puted tomography. J Thorac Imaging 2007; 22(4): 319–23.
66. Büller HR, Agnelli G, Hull RD et al. Antithrombotic ther-
apy for venous thromboembolic disease: the seventh ACCP
conference on antithrombotic and thrombolytic therapy.
Chest 2004; 126(3 Suppl): 401S–428S.
67. Wille-Jorgensen P, Rasmussen MS, Andersen BR, Borly L.
Heparins and mechanical methods for thromboprophylaxis
in colorectal surgery. Cochrane Database Syst Rev 2003; (4):
CD001217.
68. Borly L, Wille-Jorgensen P, Rasmussen MS. Systematic
review of thromboprophylaxis in colorectal surgery—an
update. Colorectal Dis 2005; 7(2): 122–7.
69. Leonardi MJ, McGory ML, Ko CY. The rate of bleeding com-
plications after pharmacologic deep venous thrombosis pro-
phylaxis: a systematic review of 33 randomized controlled
trials. Arch Surg 2006; 141(8): 790–7.
70. Hui AJ, Wong RM, Ching JY et al. Risk of colonoscopic
polypectomy bleeding with anticoagulants and antiplatelet
agents: analysis of 1657 cases. Gastrointest Endosc 2004;
59(1): 44–8.
71. Friedland S, Soetikno R. Colonoscopy with polypectomy in
anticoagulated patients. Gastrointest Endosc 2006; 64(1):
98–100.
72. Dunn AS, Turpie AG. Perioperative management of patients
receiving oral anticoagulants: a systematic review. Arch Int
Med 2003; 163(8): 901–8.
73. Kearon C, Hirsh J. Management of anticoagulation before
and after elective surgery. New Engl J Med 1997; 336(21):
1506–11.
74. Habib AS, Gan TJ. Evidence-based management of postop-
erative nausea and vomiting: a review. Can J Anaesth 2004;
51(4): 326–41.
75. McKenzie R, Sharifi-Azad S, Dershwitz M et al. A random-
ized, double-blind pilot study examing the use of intrave-
nous ondansetron in the prevention of postoperative nausea
and vomiting in female inpatients. J Clin Anesth 1993; 5(1):
30–6.
76. Kovac AL, O’Connor TA, Pearman MH et al. Efficacy of
repeat intravenous dosing of ondansetron in controlling
postoperative nausea and vomiting: a randomized, double-
blind, placebo-controlled multicenter trial. J Clin Anesth
1999; 11(6): 453–9.
77. Kovac AL, Pearman MH, Khalil SN et al. Ondansetron
prevents postoperative emesis in male outpatients. S3A-379
Study Group. J Clin Anesth 1996; 8(8): 644–51.