Improved Outcomes in Colon and Rectal Surgery part 10 ppt
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (136.72 KB, 10 trang )
8
improved outcomes in colon and rectal surgery
78. Petrelli NJ, Stulc JP, Rodriguez-Bigas M, Blumenson L.
Nasogastric decompression following elective colorectal surgery:
a prospective randomized study. Am Surg 1993; 59(10): 632–5.
79. Pickleman J, Lee RM. The management of patients with
suspected early postoperative small bowel obstruction. Ann
Surg 1989; 210(2): 216–9.
80. Miller G, Boman J, Shrier I, Gordon PH. Readmission for
small-bowel obstruction in the early postoperative period:
etiology and outcome. Can J Surg 2002; 45(4): 255–8.
81. Beck DE, Opelka FG, Bailey HR, Rauh SM, Pashos CL. Incidence
of small-bowel obstruction and adhesiolysis after open colorec-
tal and general surgery. Dis Colon Rectum 1999; 42(2): 241–8.
82. Senagore AJ. Pathogenesis and clinical and economic con-
sequences of postoperative ileus. Am J Health Syst Pharm
2007; 64(20 Suppl 13): S3–7.
83. Lobo DN, Bostock KA, Neal KR et al. Effect of salt and water
balance on recovery of gastrointestinal function after elective
colonic resection: a randomized controlled trial. Lancet 2002;
359(9320): 1812–8.
84. Khoo CK, Vickery CJ, Forsyth N, Vinall NS, Eyre-Brook IA. A
prospective randomized controlled trial of multimodal periop-
erative management protocol in patients undergoing elective
colorectal resection for cancer. Ann Surg 2007; 245(6): 867–72.
85. Bradshaw BG, Liu SS, Thirlby RC. Standardized periopera-
tive care protocols and reduced length of stay after colon
surgery. J Am Coll Surg 1998; 186(5): 501–6.
86. Fukuya T, Hawes DR, Lu CC, Chang PJ, Barloon TJ. CT
diagnosis of small-bowel obstruction: efficacy in 60 patients.
Am J Roentgenol 1992; 158(4): 765–9.
87. Wolff BG, Weese JL, Ludwig KA et al. Postoperative ileus-
related morbidity profile in patients treated with alvimopan
after bowel resection. J Am Coll Surg 2007; 204(4): 609–16.
88. Viscusi ER, Goldstein S, Witkowski T et al. Alvimopan, a
peripherally acting mu-opioid receptor antagonist, compared
with placebo in postoperative ileus after major abdominal
surgery: results of a randomized, double-blind, controlled
study. Surg Endosc 2006; 20(1): 64–70.
89. Senagore AJ, Bauer JJ, Du W, Techner L. Alvimopan acceler-
ates gastrointestinal recovery after bowel resection regardless
of age, gender, race, or concomitant medication use. Surgery
2007; 142(4): 478–86.
90. Smith AJ, Nissan A, Lanouette NM et al. Prokinetic effect of eryth-
romycin after colorectal surgery: randomized, placebo-controlled,
double-blind study. Dis Colon Rectum 2000; 43(3): 333–7.
91. Bonacini M, Quiason S, Reynolds M et al. Effect of intrave-
nous erythromycin on postoperative ileus. Am J Gastroenterol
1993; 88(2): 208–11.
92. Roberts JP, Benson MJ, Rogers J et al. Effect of cisapride
on distal colonic motility in the early postoperative period
following left colonic anastomosis. Dis Colon Rectum 1995;
38(2): 139–45.
93. Tollesson PO, Cassuto J, Rimbäck G et al. Treatment
of postoperative paralytic ileus with cisapride. Scand
J Gastroenterol 1991; 26(5): 477–82.
94. Brown TA, McDonald J, Williard W. A prospective, ran-
domized, double-blinded, placebo-controlled trial of
cisapride after colorectal surgery. Am J Surg 1999; 177(5):
399–401.
95. Reissman P, Teoh TA, Cohen SM et al. Is early oral feeding
safe after elective colorectal surgery? A prospective random-
ized trial. Ann Surg 1995; 222(1): 73–7.
96. Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition
within 24 hours of colorectal surgery versus later commence-
ment of feeding for postoperative complications. Cochrane
Database Syst Rev 2006; (4): CD004080.
97. Han-Geurts IJ, Hop WC, Kok NF et al. Randomized clinical
trial of the impact of early enteral feeding on postoperative
ileus and recovery. Br J Surg 2007; 94(5): 555–61.
98. Stamos MJ, Theuer CP, Headrick CN. General postop-
erative complications. In Hicks TC, Beck DE, Opelka FG,
Timmcke AE. eds, Complications of Colon & Rectal Surgery.
Baltimore: Williams & Wilkins, 1996: 118–39.
99. Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human:
Building a Safer Health System. Washington, DC: Institute
of Medicine, National Academy Press; 2000.
100. Seiden SC, Barach P. Wrong-side/wrong site, wrong-
procedure, wrong-patient adverse events. Are they pre-
ventable? Arch Surg 2006; 141: 931–9.
101. Joint Commission on Accreditation of Healthcare
Organizations. Guidelines for implementing the universal
protocol for preventing wrong site, wrong procedure, wrong
person surgery. JCAHO Web site. ntcommis-
sion.org/PatientSafety/UniversalProtocol/. Accessed June
15, 2006.
102. Welsch T, Muller SA, Ulrich A et al. C-reactive protein
as early predictor for infectious postoperative complica-
tions in rectal surgery. Int J Colorectal Dis 2007; 22(12):
1499–507.
Care paths and optimal postop management
Surya P M Nalamati and Eric J Szilagy
CHALLENGING CASE
A 45-year-old man undergoes an open sigmoid colectomy for
diverticular disease and is placed on a postoperative care path.
On postoperative day three, the patient is ambulating, has mild
nausea, and has not passed flatus.
CASE MANAGEMENT
The care path is modified to continue intravenous fluids. The
patient-controlled analgesia is stopped and the patient is started on
small doses of intravenous narcotics. Ambulation is continued.
INTRODUCTION
The constant endeavor of modern medicine has been to improve
the quality of patient care. Over the past century there have
been tremendous technological advances in medicine and sur-
gery. The pattern of care has evolved from being a single physi-
cian managing one patient’s care to team care involving one or
more physicians from same or different fields of specialization,
residents, nurses, physician assistants, social workers, and case
managers. However, these advances have increased health care
delivery costs, which in turn demanded a system of care to be
developed that is more efficient without compromising patient
safety and the quality of health care. Care paths are one of the
most wide spread tools used to enhance outcomes and contain
costs.(1) Introduced in early1990s in the United Kingdom and
United States, they have rapidly gained acceptance and are now
being used all over the world.
Care paths are structured, multidisciplinary plans of anticipated
care, set in an appropriate time frame, to help patients with spe-
cific conditions or sets of symptoms, move progressively through
a clinical experience to a positive outcome. Numerous synonyms
exist for care paths including clinical pathways, critical care path-
ways, integrated care pathways, critical paths, multidisciplinary
pathways of care, and care maps.(2, 3) Fast-track surgery and ERAS
(enhanced recovery after surgery) programs are recent evolutions of
the care paths concept.(4–6) Critical pathways, successfully utilized
in several different business sectors, including construction and
automotive industries, have been adapted and applied to medical
field.(7–11) They are designed to support the implementation and
translation of national guidelines, or an evidence-based standard of
care, into local protocols. The anticipated result is the subsequent
application of pathways to clinical practice, clinical and nonclinical
resource management, clinical audit, and financial management.(2)
They provide detailed guidance for each stage in the management
of a patient (diagnosis, treatment, interventions etc.,) for a specific,
given condition over a period of time. They encompass the progress
of patient care and document details of outcome.(12)
Clinical pathways have four main components: a time line, the
categories of care or activities and their interventions, interme-
diate and long-term outcome criteria, and the variance records,
which allows deviations to be documented and analyzed.(2)
Care paths differ substantially from clinical guidelines, proto-
cols, and algorithms. Clinical guidelines are consensus state-
ments that are systemically developed to assist practitioners
in making patient management decisions related to particular
clinical circumstance.(13) Protocols are treatment guidelines
that are developed based on clinical guidelines.(14) Although
anchored in clinical guidelines, care paths are designed to be
used by multidisciplinary teams and focus on details of the
process of care and highlight inefficiencies. Care paths in con-
trast to guidelines contain a continuous monitoring and data
evaluation component. This helps in identifying the rate limit-
ing steps and to make any evidence-based changes in the care
path to improve the overall process of care.
CARE PATHS IN COLON AND RECTAL SURGERY
Care paths in surgery are used in the management of patients under-
going commonly performed surgical procedures.(15) In colon and
rectal surgery, care paths have been successfully initiated since the
early1990s. Care paths have been used in the management of peri-
operative care where they were more procedure specific. They have
been successfully used in management of standard colon resection,
laparoscopic colon resections, complex cases such as restorative
proctocolectomy, and complex anorectal reconstructions.(16–20)
The objectives of care paths, as reviewed by Pearson et al., were to
select the best demonstrated practice when practice varied unneces-
sarily.(21) He outlined the process guidelines as follows:
Define the standards for the expected duration of hospital
stay and for the use of tests and treatments. These standards are
evidence-based or based on guidelines for the specific clinical cir-
cumstance. For example, Stephen et al. noted in their article, that
before implementation of pathways patients had their nasogastric
tubes removed after they passed flatus.(22) During development
of their care path for colonic resection, evidence for the routine
use of nasogastric tube which was a rate-limiting step for early
recovery was reviewed. Evidence showed that routine prophylac-
tic decompression with nasogastric tube decompression is of no
use and should be abandoned.(23) This led to incorporating the
step to remove the nasogastric tube on the day of the surgery,
which has become now widely accepted clinical practice.
To examine the interrelations among the different steps in the
•
care process and find ways to coordinate or decrease the time
in the rate-limiting step.
To give all the care providers a common plan from which to view •
and understand their various roles in overall care process.
To provide a framework for collecting data on the care pro- •
cess so that providers can learn and analyze how often and why
patients do not follow an expected course.
To decrease documentation burdens and improve patient satis- •
faction with care by educating patients and their families about
the plan of care.
improved outcomes in colon and rectal surgery
DEFINING AND IMPROVING OUTCOME MEASURES
Surgical care outcomes have been defined by a variety of measures.
Complication rates relating to abnormal outcomes, such as infection,
hemorrhage, organ system dysfunction, and reconstruction failure,
are common benchmarks for surgical performance. These rates have
been the targets of quality improvement because they have impact
not only on morbidity but also mortality. However, these outcome
measures, although significant, do not necessarily reflect the effort of
the entire surgical team or the efficiency of the care process.
Length of stay, rate of return of physiologic function, and quality
of life measures are cumulative standards that also take into account
the impact of multiple caregivers. They may not only correlate with
lower morbidity and mortality, but provide additional metrics for
the result of a multidisciplinary team approach.(24) The cumula-
tive effect of introducing efficiency in the multidisciplinary effort is
improved utilization of resources, with higher quality, using fewer
resources at a lower cost.
Traditionally, the hospital stay after colonic resection varied be -
tween 5 and 10 days with a median of 7 days and a complication
rate of 10–20%.(25, 26) Implementation of care paths has signifi-
cantly reduced this number. Archer et al. reported a mean length of
stay decrease from 10.3 to 7.5 days, and average hospital charges from
$21,650 to $17,958 (20) whereas Billingham et al., in their series of 263
patients, reported stay of 5.5 vs. 8.2 days and hospital charges $12,672
vs. $16,665 (19). Even though the decreased length of stay and cost
benefits appear to be obvious, it is not clear in these studies if there is
significant shifting of costs from hospital care to home care.
BENEFITS OF CARE PATHS
Care paths provide explicit and well-defined standards for care. They
support introduction of evidence-based medicine and use of clini-
cal guidelines thus reducing variations in patient care and improving
clinical outcomes.(27) They improve multidisciplinary communica-
tion, interprofessional collaboration, teamwork, and care planning.
(28) Care paths implement continuous clinical audit, providing a
means of continuous quality improvement, thus providing a base-
line for future initiatives to modify the pathway. Care paths reduce
risk, support training, optimize resources, and reduce costs, which
contribute to shortening the hospital stay. Care paths are not pre-
scriptive; they do not override clinical judgment. On the contrary, the
surgeon can at any time elect not to follow the pathway based on his
clinical judgment. One of the most useful characteristic of care paths
is that they provide a visual overview of each patient’s care with spe-
cific outcomes stated. This can be reviewed and acted on by every one
caring for the patients, as well as by patients themselves.(20) Using
care paths designed around evidence-based data and standards of
practice, one could expect a decrease in overall malpractice risk.(29)
DEVELOPMENT OF CARE PATH
The development and implementation of care paths consist of
the following steps as reviewed in numerous publications.(1, 14,
19, 21, 22, 30, 31)
Select a Topic
Topic selection for formulating a care path could be either disease
or procedure specific. High volume, high-cost diagnoses or proce-
dures are ideal. Critical pathways development in colorectal surgery
concentrated on high volume and high cost procedures like colec-
tomies, restorative total proctocolectomies, and complex anorectal
reconstructions.(5, 16, 19, 20, 22) Most of the care paths developed
in colon and rectal surgery are procedure specific and aimed at
perioperative management. These procedures are more suitable for
pathway development because of the predictable course of events
before and after hospitalization, and variations in care associated
with them. Development of care paths makes the goal of decreased
variation and improved resource utilization possible.
Select a Team
A multidisciplinary team is the most critical element of any care
path. Historically, care paths were developed by and for nurses
and other nonphysician hospital-based workers. However, the
lack of physician participation led to failure of that model of care
pathway.(32, 33) The active role of surgeons in a leadership role
is crucial to development and implementation of pathways. In
addition, it is vital to involve representatives from all groups that
will play a role in implementation of pathway. The team should
discuss all the elements of the pathways. The team should meet
regularly to develop the pathway and after implementation to dis-
cuss variances and make appropriate revisions to it.
Colon and rectal care path teams consist of surgeons, house
staff, physician assistants, stoma nurses, office nurses for preop-
erative care planning, ward nurses, physical therapists, and social
workers to plan the home care needs and arrange them in a
timely fashion. Timely intervention by each of the team members
is essential for success of the care path.
Evaluate the Current Process of Care
A careful review of the medical records should be performed to
identify the critical intermediate outcome, rate limiting steps, and
high cost areas on which to focus. Evaluation can be automated
where electronic medical systems are available. This should
include review of the preop process (preanesthesia work up,
obtaining necessary consults, stoma training if needed, antibiot-
ics, anti coagulation) and the postop process. In our initial review
process, during the development of care pathways, we found that
nasogastric intubation, postoperative feeding, GI function recov-
ery time, and mobilization to be the important rate limiting steps.
Further development will be based on intraoperative anesthesia
care, and postoperative pain management.
Evaluate Medical Evidence and External Practices
After defining the rate limiting steps, the team should evaluate lit-
erature for evidence of the best-demonstrated practice. For most
of the rate limiting steps in colon rectal surgery there is data avail-
able. In the absence of evidence, comparison with other institutes
to set up a benchmark is the most reliable method. These steps
should be then incorporated into the care path.
Establish Goals and Endpoints
A reasonable objective should be established as a goal, which
serves as an endpoint for the care path. Multiple goals can be
established involving each aspect of care in the pathway, and the
successful achievement of each of these landmarks would define
the success of care path. For example, in our care path for colon
surgery (see Figures 9.1 and 9.2), the goal of the pathway is to
care paths and optimal postop management
Aspect of Care PRE-HOSPITAL PRE-OP/DAY OF POST_OP/DAY OF POST_OP/DAY 1 POST_OP/DAY 2
SURGERY Date_____ SURGERY Date_____ Date________ Date________
CONSULTS Surgery/PCP
Anesthesia class3 Anesthesia ostomy/ET consult
ET for elective stoma
TESTS CBS,chem 7, Platelets CBS,chem 7 (only if blood
EKG & CXR, as Type and Screen loss or metabolic issue)
needed only
Bed rest, Reposition q 2-4 hr
Ambuiate X 4 Ambuiate X 4
Increase
Dangle Post-op eve______
1. 2. 3. 4 1. 2. 3. 4
ACTIVITY
Ankle Pumps, C&DB
Increase freq & distance Increase freq &
50ft to 100ft distance 50ft to 100ft
Spirometry No NG unless obstructed.
NG_____ IV, Dressing SED
NG_______
NG_______
Incentive spirometry
IV, de dressing, SED
TREATMENTS
IV, Dressing,
Pulse Ok Wean O2
Spirometry
Incentive spirometry, Pulse Ok Incentive
Bowel prep day Analgesia: PCA____
Analgesia: PCA____
Analgesia: PCA____
before surgery Epidural_____
Epidural_____
Epidural_____ dc___
Routine meds as DVT prophylaxis Other_____
Other_____
Other_____ All routine meds
indicated by MD Resume pre-hospitalization
Resume meds
PO if tolerated
meds
Bowel prep day
I&O dc Foley______ (leave
before surgery
Confirm bowel prep Monitor I & O Foley
in if fluid status problem
ELIMINATION
Monitor I & O Foley
or Epidural Bladder scan
post vaid straight cath
Clear Liquids when
DIET Clear fluids per MD NPO after midnight NPO/Ice chips Sips of clear liquids tolerating 800cc clear liquid
advance to PO#1 diet_____
Consult CRM if Consult CRM: estabilish Consult CRM: confirm
DISCHARGE PLAN needs identified Consult CRM discharge dispositional discharge plan
by nurse screen or MD needs
Pre-op education Post-op education, C&DB, SED,
TEACHING re:bowel prep, post op pre-op teaching Incentive spirometry, early Introduce ostomy Ostomy education
activities, pain ambulation education
management_____
REVIEW Days Initials_______ Initials:___Sign/title:____ Initials:___Sign/title:____ Initials:___Sign/title:____
PATH
Eves Sign/title_______ Initials:___Sign/title:____ Initials:___Sign/title:____ Initials:___Sign/title:____
WAY Nights Initials:___Sign/title:____ Initials:___Sign/title:____ Initials:___Sign/title:____
Figure 9.1 Care Path for Colon Surgery.
improved outcomes in colon and rectal surgery
Aspect of Care POST-OP/DAY 3 POSTE-OP/DAY 4 COMMENTS OUTCOMES
Date____________ Date____________
CONSULTS Clear discharge with consulting MD Appropriate referrais pre-op
TESTS
Ambulate in atleast
ACTIVITY
4 times(50 ft —100 ft) Ambulate independently, Elimination of post-op complications
Progressively increase or with aids as required related to immobility
distance/frequency
TREATMENTS Transfer IV to saline lock Monitor No post-op infections
Analgesia: change to PO pain
PO analgesia
Effective pain management
medication if tolerating fluids
Monitor I&O Monitor I&O
ELIMINATION
Monitor bowel function: Monitor bowel function: Effective bowel and bladder functon
Flatus__________ Flatus__________ re-estabilished
DIET
PO # 1 diet
Diet/Low Residue
Adequate nutritional intake
Dietary consult if needed
Consult CRM: Finalize discharge plan
obtain supplies and equipment.
DISCHARGE PLAN
Complete discharge, W-10. Consult CRM: review
Discharge by post-op day #4
Consider discharge if tolerating PO & finalize Discharge plan
Passing flatus if needs identified by nurse
screen or MD
Reinforce ostomy education. Incerase Patient/Family will
TEACHING patient participation. Review diet,
Review diet, activity
understand and participate
activity and medication.
meicaton and home care
in plan
REVIEW Days Initials:___Sign/title:____ Initials:___Sign/title:____ Initials:___Sign/title:____
PATH Eves Initials:___Sign/title:____ Initials:___Sign/title:____ Initials:___Sign/title:____
WAY Nights Initials:___Sign/title:____ Initials:___Sign/title:____ Initials:___Sign/title:____
Figure 9.2 Care Path for Colon Surgery (Continued).
care paths and optimal postop management
discharge the patient on postoperative day 4, and if possible on
day 3. The subgoals that were established were based on the aspect
of care such as to ambulate on day 1 after surgery, remove the
Foley catheter on day 2, etc. Goals should also be established for
achieving patient satisfaction, as measured by survey tools such as
those used by Press Ganey Associates.(34)
Determine Critical Pathway Format
There are multiple formats, which can be used; most of them
have a task-time matrix in which specific tasks are specified
along a time line. Care paths range from different kinds of man-
ual formats to electronic format, where electronic charting, and
pathway compliance are obtained simultaneously.(31, 35)
Implement the Care Path
Education of all the involved caregivers, patients, and their families
is the key to successful implementation of a care path. In-service
education should be given to all the involved groups, especially
if electronic care paths are used.(36) Different individuals in the
care path should be assigned specific functions such as collection
of data, analyzing variance etc.
Document and Analyze Variance
Implementation of the pathway is only the first action of care
path. This must be followed by data collection analysis and then
process improvement to achieve the set goals. A good tool suited
for this purpose is the analysis of variance grids.(27) By examin-
ing the variance sheets that record variances in implementation of
pathway regularly, it will be easier to identify common reasons for
noncompliance. These issues can be discussed with the team to see
if any changes can be made for full implementation of care path.
OUR INSTITUTIONAL EXPERIENCE
Care pathways for colon surgery were initiated in 1995 at our
institute. This was used for both standard and laparoscopic colon
resections. The care path was designed with an aim of providing
optimal care in a cost effective manner. The goal of the pathway
is to discharge patients by postoperative day 4 and when possible
on day 3. The endpoint of the pathway is to discharge the patient
home when tolerating diet and passing flatus. The documenta-
tion for the pathways was of paper format and had boxes to check
and spaces for writing notes to document the progress.
The pathway starts with preoperative patient teaching during
their office visit. Patients are informed about the procedure, the
length of stay and anticipated days for landmarks of progress, such
as ambulation, advancement of diet and return of bowel func-
tion, and discharge. They are also provided with a printed copy
of the care path guide, which is especially designed for patients,
that details preoperative preparation, what to expect on arrival
to hospital, and postoperative care scenario. Patients are encour-
aged to call if they have any doubts regarding the care path. A
complete blood count, chemistries are ordered for every patient.
Electrocardiogram and chest x-rays are ordered for patients if
needed. Patients undergo bowel preparation at home the day
before surgery, using Golytely.
On the day of surgery the patients receive Heparin 5000 units
subcutaneously, and antibiotics in the preoperative period.
Antibiotics are administered for 24 hours and heparin is given
throughout their hospital stay. Antiembolism devices such as
Venodynes are put on preoperatively. The anesthesiologist places
an epidural catheter if the patient consents to it.
Postoperatively, the patient is admitted to a regular surgical
unit, and vitals monitored every 4 hours during the entire length
of hospitalization. The patient is given an incentive spirometer
and its use demonstrated. On postoperative day 1 they are ambu-
lated. Patients are given sips of clear liquids and if tolerated given
a clear liquid tray. Stoma education is introduced on day 2 if
indicated. Patients on postop day 2 are given unrestricted clear
liquids and advanced to regular diet if tolerating 800 cc of clear
liquids. Foley catheter is also removed. In addition, the epidural
or patient controlled anesthesia (PCA) are discontinued and the
patient is started on oral pain medications. Discharge disposition
needs are established. On postoperative day 3, patients are given
a regular diet; discharge paperwork is completed and kept ready.
If the patient tolerates diet and passes flatus they are discharged;
if not, the patient is discharged on postoperative day 4 after
meeting the discharge criteria. Before discharge, the diet, activ-
ity, medication, and homecare instructions are reviewed with the
patient and follow up appointments are set up. The Care path
was routinely analyzed at interdisciplinary conferences. (Refer to
Figures 9.1 & 9.2).
CHALLENGES AND CONCERNS
Many surgeons believe that their responsibility to practice medi-
cine economically is becoming secondary to their responsibility
of practicing medicine effectively.(37)
Common reluctance to accept care paths arises from a preju-
dice in viewing them as a form of “cookbook” medicine.(38, 39)
Some consider them to be intrusive, decreasing the physician’s
autonomy, and lack the element of individualized care for each
patient. However, although care paths encourage standardiza-
tion as a strategy to improve quality and efficiency, physicians,
by helping define these standards, actually would gain greater
control over the patient care rather than losing their autonomy.
Physicians also need to be free to write orders to change the
pathway or to remove the patient from the pathway if needed.
Documenting the reasons to do so would help in analyzing the
pathway processes and lead to improvement in the pathway. This
would increase the acceptance of care paths and also counter the
criticism of deficiency of individual care.
Although many studies report cost savings in implementa-
tion of care paths, most of these do not address the issue of cost
of development of care paths. Macario et al. estimated the cost
of development of the care path for patients undergoing knee
replacement surgery at $21,000.(40) However these did not take
into account the time staff physicians spent on the project.
There is a concern among the academic faculty that the care
paths when used in resident training environments may discourage
experimentation, independent thinking, and application of appro-
priate clinical judgment to individual cases. Those responsible for
house staff education may feel care paths might stifle the question-
ing through which residents learn. However medical training might
be well served by incorporating methods such as critical pathways
to teach students evidence-based and cost-effective practice.(21)
improved outcomes in colon and rectal surgery
Care pathways may serve to frame the educational process. They
are based on expected physiological outcomes, but are monitored
so that variances (i.e., complications) can be addressed with the use
of clinical judgment. The clinical judgment is, in effect, the imple-
mentation of an expanded path or alternate pathway. The alternate
pathway, for example, may be for postoperative myocardial infarc-
tion management. Pathways are structured but dynamic.
There is also a concern that care paths might create an atmos-
phere in which patients will be steered away from clinical research
studies into treatment according to critical pathways. Including
a step in the care path can offset this. If set criteria are met, the
patient should be considered for the appropriate clinical trial
and the research team would be contacted. This would actually
yield in improved recruitment to clinical studies. Research ques-
tions can themselves be embedded in care paths and answers be
obtained on analysis of the care paths.
Another frequently voiced concern is that physicians may be
more vulnerable to malpractice suits if they do not comply with
a care path and a patient has a complication. In essence, litigation
is more likely to occur when there is a failure to follow a pathway
based on standards of care. Careful documentation as to reason
for deviation from the pathway could potentially decrease the
chance of litigation.
REALISTIC EXPECTATIONS
Despite the successful use of care paths for optimal management
of postoperative patients undergoing colon and rectal surgeries,
it should be noted that the pathways are oriented towards ideal
patients with predictable course of care. Enthusiasm to man-
age every patient with the care path without paying attention
to individual circumstance could be counterproductive. Hence,
care paths should be designed to recognize patients with special
needs or comorbidities. Perioperative pathways that consider the
needs of diabetics, cardiac patients, pulmonary patients, or stroke
patients for example, would be a proactive way to institute risk-
reducing strategies that would have a positive impact in reducing
perioperative morbidity and mortality.
Tremendous scope for improvement still exists in implementa-
tion of evidence-based management. In a recent article, Kehlet
et al. reviewed the care after colonic surgery in Europe and the
United states and analyzed the use of evidence-based care in the
perioperative period.(41) They identified mechanical bowel prep-
aration, operative techniques, nasogastric intubation, time frame
for postoperative food, and fluid intake, time to recovery from
GI function, and mobilization as the important variables, which
have positive evidence-based practices. They noted preoperative
bowel preparation was used in >85% of patients. The nasogastric
tube was left in situ postoperatively in 40% vs. 66% of patients
in the United States and Europe, respectively. It took 3–4 days
for 50% of patients tolerating liquids. This suggests that clinical
practice does not optimally reflect published evidence and indi-
cates a potential for major improvement.
FAST TRACK COLON AND RECTAL SURGERY
Fast track surgery is an evolution in the care path approach that
involves rapid progress from perioperative preparation, through
surgery, and discharge from hospital. Synonyms used for this
include accelerated postoperative recovery programs and enhanced
recovery programs. Critical elements of fast track colon surgery
paths include use of the following: extensive preoperative coun-
seling, no bowel preparation, no premedication, administration
of short acting anesthetic drugs, standardized surgical procedure,
minimal access techniques, restriction of drains, and catheters,
early extubation, rewarming and sustained postoperative normo-
thermia, optimal pain control, avoiding opiates for pain control,
early ambulation and discharge, and follow up after discharge.
(2, 5, 16, 17, 42–44)
Factors that limit early discharge include pain, nausea, vomit-
ing, prolonged ileus, mechanical factors such as drains, indwell-
ing catheters, and stress-induced organ dysfunction.(6, 45) Kehlet
and Mogensen, in their study involving 18 patients who under-
went open sigmoid colectomy, addressed these factors by imple-
menting a multimodal rehabilitation program.(43) It involved a
highly scripted preoperative and postoperative care path regu-
lating the introduction of epidural analgesia, diet, and ambula-
tion. The pathway involved mobilization of patients on the day
of surgery, administering cisapride and magnesium, and allowing
free fluid intake on evening of surgery, among numerous other
inter ventions instituted. They described a median postoperative
stay of 2 days, mobilization of patients for 5 hours on second post-
operative day and 10 hours on third postoperative day. They also
showed decreased pain and fatigue scores.(43) Delaney et al. stud-
ied 60 patients undergoing major abdominal and pelvic surgeries
without administration of preemptive epidurals, oral cathartics,
and prokinetic agents. They have described shorter length of stay
than patients having traditional care.(46)
Recent developments of laparoscopic colon surgery showed
significant improvements in average length of stay after colec-
tomy and also better patient satisfaction in terms of pain control.
This further catalyzed the interest in fast track pathways in colon
and rectal surgery. Several authors described in their studies,
multimodal care plans involving different strategies to optimize
preoperative, intraoperative, and postoperative limiting factors,
to achieve an early discharge with no difference in readmission
rate or mortality.(16, 17, 24, 42, 46–48) Wind et al. published a
review of all randomized controlled and controlled clinical trial
on fast track colon surgery. This meta-analysis showed that the
average hospital stay (2.61 days) and morbidity were signifi-
cantly lower for fast track programs.(44) The strategies adapted
in these studies included the following: extensive preoperative
counseling, no bowel preparation, no premedication, antibiotics
administration before surgery, no preoperative fasting, adminis-
tration of carbohydrate loaded liquids until 2 hrs before surgery,
tailored anesthesia encompassing thoracic epidural anesthesia,
and short-acting anesthetics, perioperative high inspired oxygen
concentrations, avoidance of perioperative fluid load, short inci-
sions, minimally invasive surgery, nonopiod pain management,
no routine use of drains and nasogastric tubes, early removal of
bladder catheters, standard laxatives and prokinetics, and early
and enhanced postoperative feeding and mobilization.
There are no randomized controlled trails comparing laparo-
scopic, fast track, and standard approaches. However, recently
LAFA (laparoscopic and or fast track multimodal management
versus standard care) trial was instituted, which was conceived
care paths and optimal postop management
to determine whether laparoscopic surgery, fast track surgery, or
a combination of both is to be preferred over open surgery with
standard care in patients having segmental colectomy for malignant
disease.(49)
SUMMARY
Care paths are tools which promote evidence-based standard
care, improve efficiency, and reduce hospital stay without com-
promising the quality of final outcome of care.
Care paths can be used as either disease specific or process-
specific tools to manage patients throughout the complete disease
cycle. Care path development requires a dedicated multidisci-
plinary team. With increasing popularity of laparoscopic colon
surgeries and other techniques to decrease perioperative stress
response, care paths in colon and rectal surgery are evolving
continuously, into new programs, such as fast-track surgery.
REFERENCES
1. Campbell H, Hotchkiss R, Bradshaw N, Porteous M.
Integrated care pathways. BMJ 1998; 316: 133–7.
2. Napolitano LM. Standardization of perioperative management:
clinical pathways. Surg Clin North Am 2005; 85: 1321–7, xiii.
3. Renholm M, Leino-Kilpi H, Suominen T. Critical pathways.
A systematic review. J Nurs Adm 2002; 32: 196–202.
4. Delaney CP, Fazio VW, Senagore AJ et al. ‘Fast track’ postop-
erative management protocol for patients with high co-mor-
bidity undergoing complex abdominal and pelvic colorectal
surgery. Br J Surg 2001; 88: 1533–8.
5. Hendry P, Fearon KCH. Intraoperative surgical consider-
ations for enhanced recovery after elective colonic surgery.
Transfus Altern Transfus Med 2007; 9: 61–5.
6. Wilmore DW. From Cuthbertson to fast-track surgery:
70 years of progress in reducing stress in surgical patients.
Ann Surg 2002; 236: 643–8.
7. Wagner HM. Principles of Operations Research. 2nd ed.
Englewood Cliffs, NJ: Prentice-Hall; 1975.
8. Buffa E. Modern Production Management. 3rd ed. New York:
John Wiley & sons; 1969.
9. Critical Path Software Smoothness Road for Automotive
supplier. Industria Engineering 1992: 28–9.
10. Kallo G. The reliability of critical path method (CPM) tech-
niques in the analysis and evaluation of delay claims. Cost
Engineering 1996; 38: 35–7.
11. Hatfield M. The case for critical path. Cost Engineering 1998;
40: 17–8.
12. Clinical Pathways: multidisciplinary plans of best clinical
practice. www.openclinical.org.
13. Field MJ LK. Clinical Practice Guidelines: Directions for a
New Program. Washington, DC: National Academy Press;
1990.
14. Nathan R. Critical pathways: a review. AHA Scientific
Statement; 2000.
15. Gadacz TR, Adkins RB Jr, O’Leary JP. General surgical clini-
cal pathways: an introduction. Am Surg 1997; 63: 107–10.
16. Basse L, Hjort Jakobsen D, Billesbolle P, Werner M, Kehlet H.
A clinical pathway to accelerate recovery after colonic resec-
tion. Ann Surg 2000; 232: 51–7.
17. Basse L, Raskov HH, Hjort Jakobsen D et al. Accelerated
postoperative recovery programme after colonic resection
improves physical performance, pulmonary function and
body composition. Br J Surg 2002; 89: 446–53.
18. Edwards SG, Thompson AJ, Playford ED et al. Integrated
care pathways: disease-specific or process-specific? Clin Med
2004; 4: 132–5.
19. Melbert RB, Kimmins MH, Isler JT et al. Use of a critical
pathway for colon resections. J Gastrointest Surg 2002; 6:
745–52.
20. Archer SB, Burnett RJ, Flesch LV et al. Implementation of a
clinical pathway decreases length of stay and hospital charges
for patients undergoing total colectomy and ileal pouch/anal
anastomosis. Surgery 1997; 122: 699–703.
21. Pearson SD, Goulart-Fisher D, Lee TH. Critical pathways as
a strategy for improving care: problems and potential. Ann
Intern Med 1995; 123: 941–8.
22. Stephen AE, Berger DL. Shortened length of stay and hos-
pital cost reduction with implementation of an accelerated
clinical care pathway after elective colon resection. Surgery
2003; 133: 277–82.
23. Cheatham MLMD, Chapman WCMD, Key SPMDa, Sawyers
JLMD. A meta-analysis of selective versus routine nasogastric
decompression after elective laparotomy. Annals of Surgery
1995; 221: 469–78.
24. Khoo CK, Vickery CJ, Forsyth N, Vinall NS, Eyre-Brook IA.
A prospective randomized controlled trial of multimodal peri-
operative management protocol in patients undergoing elective
colorectal resection for cancer. Ann Surg 2007; 245: 867–72.
25. Bokey EL, Chapuis PH, Fung C et al. Postoperative morbidity
and mortality following resection of the colon and rectum for
cancer. Dis Colon Rectum 1995; 38: 480–6.
26. Schoetz DJ Jr, Bockler M, Rosenblatt MS et al. “Ideal” length
of stay after colectomy: whose ideal? Dis Colon Rectum 1997;
40: 806–10.
27. Panella M, Marchisio S, Di Stanislao F. Reducing clinical
variations with clinical pathways: do pathways work? Int
J Qual Health Care 2003; 15: 509–21.
28. Atwal A, Caldwell K, Atwal A, Caldwell K. Do multidisci-
plinary integrated care pathways improve interprofessional
collaboration? Scand J Caring Sci 2002; 16: 360–7.
29. Garnick DW, Hendricks AM, Brennan TA. Can practice
guidelines reduce the number and costs of malpractice
claims? JAMA 1991; 266: 2856–60.
30. Downey LM, Ireson CL, Slavova S, McKee G. Defining ele-
ments of success: a critical pathway of coalition development.
Health Promot Pract 2008; 9: 130–9.
31. Ramos MC, Ratliff C. The development and implementation
of an integrated multidisciplinary clinical pathway. J Wound
Ostomy Continence Nurs 1997; 24: 66–71.
32. Hampton DC. Implementing a managed care framework
through care maps. J Nurs Adm 1993; 23: 21–7.
33. Yandell B. Critical paths at alliant health system. Qual Manag
Health Care 1995; 3: 55–64.
34. Press Ganey Associates, />35. Kopec D, Shagas G, Reinharth D, Tamang S. Development
of a clinical pathways analysis system with adaptive Bayesian
improved outcomes in colon and rectal surgery
nets and data mining techniques. Stud Health Technol
Inform 2004; 103: 70–80.
36. Clarke A. Implementing electronic integrated care pathways:
learning from experience. Nurs Manag (Harrow) 2005; 12: 28–31.
37. Jones JW, McCullough LB, Richman BW. The ethics of clini-
cal pathways and cost control. J Vasc Surg 2003; 37: 1341–2.
38. Audet AM, Greenfield S, Field M. Medical practice guide-
lines: current activities and future directions. Ann Intern
Med 1990; 113: 709–14.
39. Holoweiko M. What cookbook medicine will mean for you.
Med Econ 1989; 66: 118–20, 25–7, 30–3.
40. Macario A, Horne M, Goodman S et al. The effect of a peri-
operative clinical pathway for knee replacement surgery on
hospital costs. Anesth Analg 1998; 86: 978–84.
41. Kehlet H, Buchler MW, Beart RW Jr, Billingham RP, Williamson
R. Care after colonic operation–is it evidence-based? Results
from a multinational survey in Europe and the United States.
J Am Coll Surg 2006; 202: 45–54.
42. Gatt M, Anderson AD, Reddy BS et al. Randomized clinical trial
of multimodal optimization of surgical care in patients under-
going major colonic resection. Br J Surg 2005; 92: 1354–62.
43. Kehlet H, Mogensen T. Hospital stay of 2 days after open sig-
moidectomy with a multimodal rehabilitation programme.
Br J Surg 1999; 86: 227–30.
44. Wind J, Polle SW, Fung Kon Jin PH et al. Systematic review of
enhanced recovery programmes in colonic surgery. Br J Surg
2006; 93: 800–9.
45. Kehlet. Multimodal approach to control postoperative
pathophysiology and rehabilitation. Br J Anaesth 1997; 78:
606–17.
46. Delaney CP, Zutshi M, Senagore AJ et al. Prospective, ran-
domized, controlled trial between a pathway of controlled
rehabilitation with early ambulation and diet and traditional
postoperative care after laparotomy and intestinal resection.
Dis Colon Rectum 2003; 46: 851–9.
47. Ortiz H, Armendariz P, Yarnoz C. Early postoperative feed-
ing after elective colorectal surgery is not a benefit unique to
laparoscopy-assisted procedures. Int J Colorectal Dis 1996;
11: 246–9.
48. Zutshi M, Delaney CP, Senagore AJ, Fazio VW. Shorter
hospital stay associated with fastrack postoperative care
pathways and laparoscopic intestinal resection are not asso-
ciated with increased physical activity. Colorectal Dis 2004;
6: 477–80.
49. Wind J, Hofland J, Preckel B et al. Perioperative strategy in
colonic surgery; LAparoscopy and/or FAst track multimodal
management versus standard care (LAFA trial). BMC Surg
2006; 6: 16.
10
Limitations of anorectal physiology testing
Thomas E Cataldo and Syed G Husain
CHALLENGING CASE
A 65-year-old woman and her 30-year-old daughter both present to
your office with complaints of fecal incontinence. Both are G3 P3,
all vaginal deliveries, and have had at least one delivery of a child
of 8 pounds or more. Both have required an assistance device for
delivery of one child. Both report fecal soiling for the last year. The
older woman reports progressive uncontrolled passage of flatus and
occasional identification of stool in her undergarments that she
was unaware of having passed. The daughter reports incontinence
to moderate amounts of stool despite attempts to delay defecation.
There is additional incontinence associated with athletic activity.
CASE MANAGEMENT
Normal function of the anus and rectum resulting in comforta-
ble passage of stool under voluntary control is a complex balance
of a number of competing factors and requires intricate correct
performance of enteric and colonic physiology, rectal, anal and
pelvic sensory and motor nerves, as well as anatomically intact
and functioning anal and pelvic musculature. Disruption of any
of these factors may result in fecal incontinence. On the other
end of the spectrum, the patient may suffer difficult, painful, or
incomplete evacuation. Anorectal dysfunction is often devastating
to the patient resulting in emotional distress and social isolation.
Fecal continence is defined as the ability to defer defecation
until a socially appropriate time and place. Incontinence has a
number of definitions from simply involuntary passage of stool
to inability to control passage of solid, liquid, or gas. In a 2001
consensus conference report fecal incontinence is defined as,
“recurrent uncontrolled passage of fecal material for at least one
month in an individual with a developmental age of at least four
years”.(1) Reported prevalence varies from 1.4% to 18%, with rates
as high as 45% in elderly, debilitated, or psychiatrically impaired
institutionalized adults. These numbers are generally accepted as
under reported due to patients’ unwillingness to come forward
due to associated social and cultural stigma.(1–3)
Constipation is as difficult to define. It may be as subjective as
any difficulty or infrequency in passing stool as perceived by the
patient. The Rome II criteria define constipation as two or more
of the following for at least 3 months: straining more than 25%
of the time, hard stools more than 25% of the time, incomplete
evacuation more than 25% of the time, two or fewer bowel move-
ments in a 7 day period.(4)
A problem inherent to all anorectal physiology testing is the
scarcity of “normal” values for comparison. There is relative
paucity of literature describing anorectal physiology testing on
normal population and almost all of the available studies are
comprised of small group of subjects.
PHYSIOLOGY OF FECAL CONTINENCE
Normal fecal continence relies on a number of mechanisms.
The first of which is normal enteral and colonic motility and fluid
transport physiology resulting in manageable stool consistency.
Stool consistency may be the most important characteristic that
influences fecal continence.(5) Some patients may be continent to
solid stool but not to liquid or gas. The rectum needs to maintain
adequate reservoir capacity. In addition the rectum, anus, and pelvic
musculature must have adequate ability to sense and differentiate
the presence, of solid, liquid, and gas. Additionally, it is postulated
that the vascular cushions or hemorrhoids create a controllable seal
as a rectal “corpus cavernosum”. This is supported by the identifi-
cation of leakage in some patients after otherwise uncomplicated
hemorrhoidectomy.(5) On a mechanical level, defecation occurs
when the pressure within the rectum exceeds the pressure or resist-
ance provided by the anus. For normal defecation this relies on the
controlled increase in rectal pressure combined with simultaneous
relaxation of the anus and straightening of the rectum through
relaxation of the pelvic muscles. The rectum must also possess cor-
rect mechanical properties of capacity and distensibility. It must be
able to sense the need to empty and the qualities of the contents
within it. Disease processes or injuries that limit the ability of the
rectum to distend to accept stool and air from the sigmoid colon
will alter the urge to defecate, and the ability to defer defecation.
Conversely, a chronically distended capacious rectum may lose the
ability to sense when it is full and thereby overflow. Both cases might
present as incontinence. Alternatively, if the body cannot differenti-
ate between solid, liquid, or gas or if the mechanism by which this is
sampled is altered, the result is often fecal soiling.
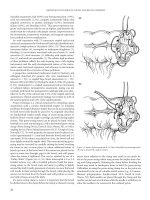
THE ANORECTAL PHYSIOLOGY LAB
A battery of devices and tests has been developed to investigate
many aspects of normal and altered defecation. Many centers have
collected the equipment to accomplish these tests. (Figure 10.1).
Much work remains to fully elucidate the source and thereby the
solutions to disordered defecation.
INVESTIGATIONS FOR INCONTINENCE
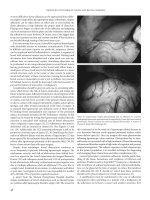
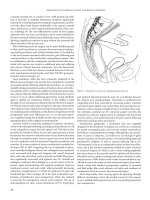
Manometry
Manometry is a technique to measure the pressures which exist
within the anal canal and the pressures that the anus is capable
of achieving voluntarily. Over many years a variety of catheters,
pressure detectors, and recording apparatuses have been devel-
oped. In addition, different operator techniques have been devel-
oped making standardization of results difficult. Throughout the
1960s a variety of catheters were developed with different num-
bers of open tipped channels and microballoons. The number
of channels varied and they were arranged radially or in a spi-
ral orientation. Water within the channels was either static or
continuously perfused. Initial continuous recordings were made
with pen and ink on polygraph devices. Currently, the state of
the art manometers contain solid state micropressure transducers
mounted within the catheter itself (Figure 10.2a,b). In addition