Improved Outcomes in Colon and Rectal Surgery part 11 docx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (325.45 KB, 10 trang )
improved outcomes in colon and rectal surgery
to more reliable and reproducible data, these catheters are more
easily and reliably cleaned from patient to patient. Pressure data
is recorded continuously to computer based software that assists
in creating the interpretation and the report. One critical obser-
vation of the water perfused systems is that the patient may react
to the sensation of water dripping from the anus with increased
tone. Scrupulous technique may avoid this.
Because various technologies and methods exist for measur-
ing anal canal pressures, no universally accepted set of normal
values exist. Simpson et al. addressed this issue in a study com-
paring five different catheters and techniques of manometry in
both normal and incontinent patients.(6) Although their sample
size was small, 10 normal and 11 patients with incontinence, the
authors found no significant difference between five commonly
employed devices. They were; a water perfused end-hole catheter,
a catheter water perfused with four radially arranged side holes,
water filled microballoon, microtransducer, and an air-filled port-
able microprocessor controlled device.
sphincter pressure measurement
Although written consent is not required as the patient is fully
awake, at our institution we obtain full informed consent and con-
firmation of patient identity, condition being evaluated, and their
understanding of the tests they are about to undergo. The patients
take one or two small volume cleansing enemas at home before
the exam. Anal canal pressures are measured with the patient lying
comfortably in the left lateral decubitus position with knees as hips
flexed 90°. Some emphasis is placed on comfort and relaxation as
anxiety, talking and anything that increases the intraabdominal
pressure may affect the results. We employ a stationary pull through
technique. The catheter is placed transanally with the measuring
points (balloons, holes or microtransducers) to a distance of 6 cm
above the anal verge. Measurements are taken in the anterior, pos-
terior, left, and right lateral positions. (Figure 10.2c) Pressures are
recorded at relaxation and at maximum “squeeze” for 10 seconds.
The patient must be instructed to try to isolate squeeze of the anus
and not employ the gluteal or any other accessory muscles. The
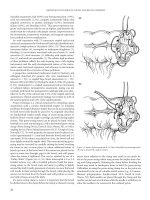
Figure 10.1 Typical Anorectal Physiology Lab with Manometry, Transanal
ultrasound, Pudendal Nerve Terminal Motor Latency testing, Biofeedback, storage
and equipment for sterilization.
(a)
(b)
(C)
Figure 10.2 (A) Manometry Catheter, with balloon. (B) Manometry Catheter, detail microtransducers. (C) Manometry tracing, computer display.
limitations of anorectal physiology testing
catheter is repositioned 1 cm distally and the process is repeated.
The process is repeated in step-wise fashion until the entire canal
had been tested. An alternative to this “station pull out” technique
is recording pressures during a continuous pullout of the catheter
at a controlled steady rate. The following parameters are recorded:
length of the anal high pressure zone, mean resting tone, maximum
squeeze pressure.
In an effort to study the symmetry and detailed overall pressure
profile of the anal sphincter, pressure vectography, a technique
that provides graphical representation of radial pressure profile
of anal canal, was developed.
the recto-anaL inhibitory refLex
The presence or absence of the Recto-Anal inhibitory reflex
(RAIR) is identified by rapid distention of the rectum by insuf-
flation of the balloon at the tip of the catheter with 10 cc of air.
Simultaneous recording taken in the middle of the anal canal high
pressure zone are made for 10 seconds. If the RAIR is present, a
reflex relaxation of internal sphincter and resultant decrease in
anal canal pressure should be observed. Balloon insufflation may
be repeated with more air at 10 cc increments up to 60 cc until a
reflex is observed.
rectaL capacity and sensation
The balloon at the end of the catheter may also be filled with
water in an incremental fashion to assess rectal sensation and
compliance. Measurements are made at the minimum volume of
first rectal sensation, the volume required to produce a sustained
feeling of the need to defecate and a maximum volume that cre-
ates significant discomfort or an irresistible need to defecate.
vaLue and Limitations of
manometry for incontinence
Anal manometry has become a staple in the evaluation of fecal
incontinence. Though routinely performed in many centers, man-
ometry lacks standardization of technique, data collection, and
methods of interpretation. This makes it extremely difficult to
compare data obtained at different centers. The range of accepted
normal values is wide varying for gender, parity, age, and numer-
ous other factors. Despite the fact that the newer catheters are
more comfortable and easier to maintain, the test remains mildly
invasive and uncomfortable for the patient.
There are several technical caveats that may lead to consider-
able alteration in results. Patients with megarectum may require
a higher volume to illicit RAIR and may be falsely labeled as
RAIR negative if the usual volume of 30–40 cc is used to illicit
RAIR.(7) The balloon material can influence the results as latex
balloons tend to deform along their axis, resulting in a falsely
elevated rectal compliance.(8) Rectal compliance testing depends
entirely upon patient’s input, thus patient’s psychological status
plays a very important role in data acquisition during this test.
(7) Furthermore the results of rectal compliance may differ if the
test is performed on “prepared”, i.e., after enema evacuation vs.
unprepared rectum.(7) The rate at which water is injected into
the balloon may also affect the rectal sensitivity testing.(9) Thus,
it is recommended that slow filling should be accomplished at
a rate of 1 ml/second.(7) Whatever the method used, the same
technique should be applied to all patients in order to obtain
reproducible and comparable results.
Caution should be exercised while making treatment decisions
based on manometric findings as normal or abnormal values in
incontinent patients do not necessarily correlate with severity of
symptoms. In a large prospective study Lieberman et al. evaluated
90 incontinent patients, including 6 males with a specific goal at
determining what impact physiology testing including manom-
etry had on treatment and outcome. After appropriate history
and physical exam patients were selected for medical or surgical
management. Following this determination they underwent anal
physiology testing including manometry, pudendal nerve termi-
nal motor latency (PNTML), and anal ultrasound (AUS). Overall
only 9 (10%) had a change in their management plan. Based on the
results of these tests, 5 of 45 patients initially assigned to medical
management were offered surgery instead. On the other hand, 3 of
45 patients assigned to undergo surgical treatment were switched
to the medical group. Almost all of these alterations in manage-
ment were based on AUS. Manometry was found to be abnormal
in one-third of both management groups and there was no correla-
tion between manometric results and change in management plan.
There did not appear to be an association between manometry,
AUS and PNTML results.(10) In an elaborate study of 350 patients
including 80 controls, Felt-Bersma et al. found that the most sig-
nificant difference between continent and incontinent patients was
maximum squeeze pressure.(11) However, the authors surmised
that continent function could not be predicted based on anal man-
ometry alone and suggested that these results should only be inter-
preted in conjunction with other tests.
Although it creates a rather striking and impressive graphical
representation of anal canal pressure profile, pressure vector dia-
grams have been shown to be of questionable clinical value for
sphincter evaluation.(12) A study by Yang et al. could not demon-
strate any correlation when vectoral analysis was compared to nee-
dle electromyography (EMG) and ultrasonography.(12) With the
increased use of AUS the utility of vectogrpahy has been negated.
Anorectal manometry remains of value for objective preopera-
tive documentation of anal tone function or muscle weakness. It
is also helpful in excluding patients from surgery.(2) Perhaps the
biggest merit of manometry is its role as the initial diagnostic
test for short segment Hirschsprung’s disease where the presence
of an RAIR effectively rules out the disease.
eLectromyography
EMG is the measurement of the electrical activity generated by mus-
cle fibers during contraction or at rest. In 1930 Beck first described
anal sphincter EMG.(13) Specifically, the EMG measures activity
in a motor group or those muscle cells innervated by a single axon.
Muscles whose nerves have been damaged will demonstrate altered
activity. Myography has been used to map the perianal area for
muscular activity and thereby detect sphincter defects. EMG is also
used to demonstrate nerve conduction and appropriate activation
and relaxation used in biofeedback therapy.
Concentric Needle EMG
A concentric needle electrode is two insulated electrodes, one
within the other. With the needle inserted into the muscle to
0
improved outcomes in colon and rectal surgery
be observed, in this case the external sphincter or the pelvic
floor, the electrical potential from one electrode to the other
is recorded. Information collected includes amplitude, duration
and frequency, as well as the number of phases. Amplitude is
proportional to the number of muscle fibers activated. Normal
values are an amplitude of <600 μV and duration <6 μs.(14,
15) Longer duration or spreading of the signal can indicate dis-
persion of the motor unit potential (MUP). This may repre-
sent denervation or demyelination, or simply aging. The sum
of the activity of many muscle cells creates a shape to the MUP.
Normal MUPs are bi- or triphasic. In general, more phases
within the action potential indicated denervation and rein-
nervation. However four or more phases have been reported in
normal muscle in up to ¼ of the time.
Single Fiber EMG
Individual muscle fiber action potentials can be recorded with
a single fiber EMG. The recording area of the needle is much
smaller, 25 μm. In normally innervated external anal sphinc-
ter muscle only a few fibers will be activated by a single motor
group axon. However, when damage occurs denervated muscle
fibers are recruited by surviving axons. The number of muscle
fibers and thereby signal density within the recording area of the
needle increases, resulting in a more polyphasic signal. The test
is performed by taking multiple readings requiring multiple skin
punctures around the anus.
Surface EMG/biofeedback
Measurement of muscular activity through the insulation of the
skin is far more imprecise but less painful than needle EMG.
Surface EMG is valuable for documentation of overall activity,
especially during attempted voluntary rest, inhibition, or contrac-
tion of a muscle. Surface EMG is helpful to document paradoxical
sphincter activity as part of the diagnosis of disordered defecation.
Two self-adhering surface electrodes can be applied on opposite
sides of the anus over the subcutaneous portion of the external
sphincter, with a grounding electrode placed at a distance on the
patient. Alternatively, a plug electrode is employed within the anal
canal (Figure 10.3). Surface measurement of muscle activity is
more valuable if the muscle is being artificially activated by stimu-
lating the nerve. When the time of nerve stimulation is known and
time of muscle activity measured, nerve conduction velocity can
be assessed. A specific application of nerve stimulation and surface
EMG is measurement of the PNTML.
pudendaL nerve terminaL motor Latency
The pudendal nerve arises from the second, third and fourth sac-
ral nerve roots bilaterally and passes along the inferior pubic rami
through Alcock’s canal. Prolonged labor or the use of forceps for
delivery may injure the pudendal nerve as it exits from the canal.
The conduction time of the nerve can be measured by stimu-
lating the nerve transrectally and observing the time to electri-
cal activity of the external anal sphincter. A St. Marks electrode
attached to a gloved finger provides both stimulation and meas-
urement (Figure 10.4a,b). An absent trace may indicate injury to
the nerve whereas a prolonged PNTML is interpreted to indicate
nerve injury and repair.
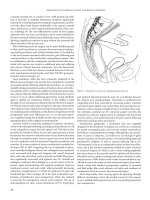
Figure 10.3 Surface EMG electrode.
(a)
(b)
Figure 10.4 St. Marks electrode.
1
limitations of anorectal physiology testing
Limitations of eLectomyography in
incontinence
The EMG delineation of anatomic sphincter defect has been largely
supplanted by imaging studies such as ultrasound and pelvic mag-
netic resonance imaging (MRI). Concentric needle EMG and single
fiber EMG testing is uncomfortable, or in some cases, frankly pain-
ful for the patient. The equipment is expensive and difficult to mas-
ter. Results are variable based on the cooperation of the patient, the
experience of the examiner, and the patience of both.(16) Surface
EMG is mildly uncomfortable to the patient and technically chal-
lenging to perform. Identification of the nerve tracing can be sub-
jective. Pudendal nerve latency testing is operator dependent. Since
PNTML measures the fastest remaining fibers, a normal latency
time does not exclude injury. The latency values obtained are also
affected by the distance between the electrode and the pudendal
nerve; shortest latencies being obtained by placing the electrode as
close to the nerve as possible.(9) This is usually accomplished with
subtle movements of the electrode bearing finger inside the anal
canal while observing waveforms for the shortest latency thus gen-
erated in response to repeated electrical stimuli. This method may
result in significant patient discomfort in some cases. There is some
bilateral crossover innervation of the sphincter therefore a unilat-
erally abnormal test does not preclude normal function. Earlier
studies indicated that significantly abnormal bilateral results were
predictive of poor outcome with sphincter repair.(17–19) However,
other authors have not found PNTML to be helpful in this regard.
(10, 20) Increased pudendal nerve terminal velocities have been
previously associated with patients with idiopathic incontinence.
(21) Newer literature, however, suggests that this association might
not be entirely true. Ricciardi et al. showed that only a small per-
centage of patients with idiopathic fecal incontinence had associ-
ated pudendal neuropathy.(22)
Anal Ultrasonography
High quality circumferential images of the anal sphincter complex
can be obtained using anal ultrasonography (AUS). Although a
number of probes are available, the most commonly used for evalu-
ation of the anal sphincter is a rotating probe that creates a 360°,
two-dimensional transverse image. The transducer generally used is
a combined 7 or 10 MHz transducer, rotating within a water-filled
rigid cap covered with a balloon or condom. Newer probes are fully
self-contained. They still require protection with a condom and
some type of interface media such as gel or water.(Figure 10.5)
In many outpatient anorectal physiology labs the procedure
is performed in the left lateral decubitus position in conjunc-
tion with anal manometry. For a patient scheduled to undergo
multiple anorectal physiology studies the same day, we follow
a policy of performing manometry initially followed by other
investigations as the sphincter stretch induced by 12 mm son-
ogram probe may produce erroneous manometric findings.
As such, the patient may have had limited preparation with a
small volume enema. This is not required for AUS alone. Some
authors prefer the prone or lithotomy positions feeling that the
lateral position deforms the anatomy.(23) The clinical signifi-
cance of this is unclear. AUS can distinguish the internal and
external sphincters individually, with an intact internal sphinc-
ter representing a continuous hypoechoic band. The external
sphincter is more heterogenous but distinctly more hyperechoic.
Although images can be taken throughout the anal canal, images
are traditionally documented and preserved at proximal, mid
and, distal anal canal. Defects in either the internal or external
sphincters are identified as a disruption in the continuous ring.
The external sphincter naturally splits proximally as it extends
to the levator sling and the pelvic floor musculature. Disrupted
tissue heals with a scar which appears amorphous, more echo-
genic than internal sphincter, but less so than external. It is seen
bridging the gap in the defect between the disrupted ends of
the sphincters. The presence of a sphincter defect on AUS cor-
relates well with a history of obstetrical trauma, as well as with
physical exam findings and manometric findings.(10, 20, 24)
Interobserver agreement is excellent and when an anatomic
defect is present AUS sensitivity approaches 100%, specifically
for internal anal sphincter defects in the mid anal canal.(25)
Different techniques have been employed to either improve or
make easier definition of the anal anatomy. Some authors claim
anal squeeze, and relaxation improves the yield of sonographic
exam while others have no benefit.(23) A finger placed in the
posterior wall of the vagina used to measure the thickness of
the perineal body has been shown to aid in the evaluation of
anterior sphincter defects.(25)
Figure 10.5 St. Marks electrode attached to glove.
improved outcomes in colon and rectal surgery
Global deficiencies or thinning of the sphincters rather than
defects are more difficult to define with AUS. The internal
sphincter is normally between 2 to 4 mm. Since it is more distinct
on AUS, excessive thickness or thinness can be identified. One
elusive objective is to identify atrophy of the external sphincter as
this correlates with poor outcome from sphincter repair.(26)
Three dimensional axial endosonography is now available.
The probe spirals and moves through the sphincter at a fixed
rate collecting a three dimensional block of echo-data that can
be represented on a computer screen and evaluated through any
plane through the block. In a study involving 33 women with sus-
pected sphincter injury, two different observers compared 2-D
AUS with 3-D evaluation. There was an identifiable improvement
in the confidence of the examiner in detecting sphincter defects
with 3-D evaluation over 2-D images. Interobserver correlation
was also improved by 3-D evaluation but not to a significant
degree.(27) Nevertheless, 3-D AUS has not been indisputably
demonstrated to be more sensitive or specific, than transverse
planar AUS.
Limitations of uLtrasound in incontinence
Ultrasound is the most important test in the evaluation of fecal
incontinence with few limitations. Anorectal ultrasound entails a
significant learning curve (28) and results are operator and experi-
ence dependent. The external sphincter is less distinct than internal
sphincter and smaller, <90°, defects are harder to demonstrate.
(29) Patients with minimal symptoms and limited defects may
not require surgery; therefore the clinical significance of a defect
is determined by the combination of physical exam, anorectal
physiology (ARP) testing, and AUS (Figure 10.4). The presence of
atrophy of the external sphincter is similarly hard to prove. This is
due to the fact that atrophic external sphincter becomes replaced
with fat making sonographic delineation of the sphincter from
the surrounding fat tissue more difficult.(30) Many investigators
believed that 3-D ultrasound, by virtue of its superior resolu-
tion, may result in improved identification of external sphincter
atrophy. However a comparative study showed no correlation
between 3-d AUS and MRI in 18 incontinent women with MRI
evidence of sphincter atrophy.(31)
Biofeedback for fecal incontinence
Biofeedback is a process by which the patient is given an audi-
tory or visual representation of anorectal information, pres-
sure or muscle activity, which they cannot otherwise perceive or
correctly interpret. Techniques of biofeedback have successfully
used in the treatment of fecal incontinence for over 25 years. The
practice parameters of the American Society of Colon and Rectal
Surgeons (ASCRS) give a grade “B” recommendation for its use
as a first line therapy and in patients that have incomplete success
after sphincter repair.(2)
Limitations of biofeedback for incontinence
Despite some encouraging earlier reports describing success of
biofeedback in the management of incontinence, the Cochrane
system review of treatments for incontinence in 2006 did not
support its use.(32) The authors reported, “The 11 trials reviewed
were of very limited value because they were generally small, of
poor or uncertain quality, and compare different combinations
of treatments”. Overall success with biofeedback varies from 65%
to 89%.(2, 33, 34) Two large randomised controlled trials include
more than 100 subjects. Both concluded that biofeedback pro-
vided no additional benefit over office counseling therapy such
as advice, education, dietary modification, digital guidance, and
medication. Despite the lack of demonstrated benefit, both trials
showed improvement in severity of symptoms, fecal incontinence
scores, and quality of life.(35–37) These benefits were seen in both
the treatment and control groups indicating the role of patient
motivation and ongoing medical involvement in the treatment
of fecal incontinence. The most important predictors of success
were completion of the program, and age over 60 years. Higher
body mass index was associated with a worse outcome.
Summary: Value and limitations of ARP
testing for evaluation of fecal incontinence
The perceived value of ARP testing in the evaluation and man-
agement of fecal incontinence varies greatly on the perspective of
the examiner and the expectations of the patient. The most fre-
quently employed test include anal manometry, transanal ultra-
sound, and pudendal nerve terminal motor latency. Techniques
and normal values are not universally accepted. Abnormal results
do not equate with specific disease, injury, or symptomatology.
Transanal ultrasonography and MRI provide excellent anatomic
definition to aid in the planning of surgical intervention. At best
manometry serves for documentation of preoperative function
and may assist in patient selection for surgery. PNTML is still
controversial as to its role in the treatment of fecal incontinence.
investigations for constipation and
disordered defecation
Constipation is one of the common ailments presented to the color-
ectal surgeon. It usually entails unsatisfactory defecation resulting
from decreased frequency of defecation of difficulty in passing
stools or both. Prevalence in the general population in United
States has been reported to be as high as 2–15%.(38, 39) Women
are affected 2–3 times more commonly with incidence increasing
with age. As with incontinence, the etiology is multifactorial and
complex. Etiological factors associated with constipation include
lifestyle issues and medications; especially narcotics, antidepres-
sants, and calcium channel blockers. Pelvic outlet obstruction
(puborectalis dysfunction, rectocele) is also a common underlying
abnormality. Other causes include neurological or endocrine dys-
function for example, Parkinson’s disease, diabetes mellitus, and
hypothyroidism. Finally, dysfunction of enteric nervous system
seen in Hirschsprung’s and Chagas disease and psychological
factors may also play an important role in the pathogenesis.
Refractory constipation that fails to respond to dietary modifi-
cation and conservative management warrants a formal work-up.
From management perspective, constipation is usually referred
to as either slow transit constipation or obstructed defecation.
The initial history and physical examination, in most cases, is
able to indicate if the patient is experiencing slow transit con-
stipation vs. obstructed defecation. Colonic transit studies are
usually the first tests to be ordered in cases where slow transit
is suspected to be the underlying etiology whereas in patients
limitations of anorectal physiology testing
with obstructed defecation, a defecogram should be offered as
the initial diagnostic study. However, studies have shown that
there is little, if any, correlation between these two diagnostic
modalities and clinical picture does not necessarily reciprocate
the radiological findings.(40)
defecography
Since the 1960s, continuously recorded fluoroscopy has been used
to evaluate the dynamic function of the pelvic floor. Defecating
proctography or ciné defecography is a method whereby semi-solid
radiopaque contrast material is placed retrograde into the rectum
and lateral images are obtained in real time. Creating a realistic
“pseudo stool” has been a challenge. A commercially available prod-
uct was available but was transiently taken off the market. Many
institutions create there own contrast as needed using a combina-
tion of barium and potato starch. At the authors institution we use
a unique recipe based on breadcrumbs. The material must be thick
enough to simulate stool but able to be passed transanally.
Once the enema is administered the subject is seated in a lat-
eral orientation on a radiolucent commode. Before defecation
measurements are made of the angles of the proximal and distal
rectum. In women the vagina may be delineated with a tampon
soaked with water soluble contrast, and in certain circumstances
the small bowel is opacified with oral contrast. If further deline-
ation is required sterile water soluble contrast can be placed
intraperitoneally to define the lower peritoneal reflection, fur-
thermore, in patients with suspected cystocele, instillation of dye
into the bladder may increase the diagnostic yield of the study.
The anorectal angle is created in part by the tone and function of
the puborectalis muscle. Measurements are taken as the patient is sit-
ting at rest, during forced contraction, straining without defecation
(Valsalva maneuver), and during defecation. Perineal decent is
defined as the change in distance of the line drawn perpendicularly
from the anorectal junction to the pubococcygeal line. This line is
drawn from the tip of the coccyx to the posterior-inferior margin
of the pubic ramus. In addition perianal skin can be marked with a
metal marker and the motion or decent of the perineum measured.
Normal reported values vary widely. One author offers a broad
range, 70°–140° at rest, 100° to 180° defecating, and 75° to 90°
squeezing (41, 42), where another is more specific 92° +/- 1.5°
resting and 137° +/- 1.5° straining (5). The change in the angle
may be more important than the absolute numbers. In our prac-
tice the test is of most value if the surgeon reviews the study with
the radiologist while the test is being performed.
Abnormal findings include perineal descent of more than 3 cm
while resting or more than 3 cm while straining. Paradoxical con-
traction of the puborectalis and disordered defecation is indicated
by an observed ascent of the perineum or a static or more acute
anorectal angle during attempted defecation. Additional findings
may include internal intussusception to frank prolapse, rectocele,
or enterocele. Small, <2 cm rectoceles, are commonly seen in
asymptomatic patients and are regarded as a normal finding.
Limitations of defecography in constipation
It must be remembered that defecography is not a “physiological”
study as the study is not performed in response to a natural desire
to defecate, instead patients are asked to evacuate in a rather alien,
uncomfortable environment. Amongst other criticisms regarding
defecography are poor interobserver agreement.(43, 44) To com-
plicate issues further, abnormal defecographic findings are com-
mon in asymptomatic patients.(45, 46) The significant degree
of overlap between defecographic findings in patients with con-
stipation and asymptomatic controls raises questions regarding
the cause and effect relationship between clinical symptoms and
defecographic findings. One of the radiological signs frequently
documented during these studies is contrast retention within the
rectoceles. The clinical significance of this “Barium trapping”
seen has also been questioned.(47)
In a study by Shorvon et al. one half of asymptomatic subjects
had some aspect of mucosal prolapse and intussusception, 17 of 21
women demonstrated some degree of rectocele.(48) In addition,
before that work, no work had employed normal, healthy volun-
teers as controls and “normal” was determined retrospectively by
lack of anatomic abnormality. Other studies were performed in
patients undergoing barium enemas for other, nonanorectal condi-
tions.(48, 45, 49) Anorectal angle assessment and its interpretation
should be performed with utmost caution. As alluded to earlier,
there is a wide variation in normal values for anorectal angle and
many investigators believe that it is the change in angle rather than
the absolute values that serves as a useful guide to therapy.(7)
Patients with urge incontinence may frequently show increased
threshold for urge to defecate. It is unclear if this finding is the
result rather than the cause of constipation (9) and the clinical
implication of this finding remains uncertain. Abnormal pub-
orectalis function noted at defecography has also been a topic of
considerable debate. Many normal individuals have been shown
to have puborectalis abnormalities on defecograms (50), thus the
clinical relevance of these findings are questionable and thera-
peutic decisions should be based on clinical rather than mere
abnormal findings on radiological studies.
coLonic transit studies (sitz marker®)
Colonic transit studies play a pivotal role in the assessment of con-
stipation. Majority of colorectal surgeons agree that transit studies
supply the most pertinent information out of all the physiology
testing modalities available for constipation.(51) The most widely
accepted technique involves ingestion of 24 radio-opaque rings
followed by X-ray at days 1,3 and 5. A normal study entails passage
of more than 80% of the rings. A quick way to help evaluate the
patient for the presence of gastroparisis as well as small dysmotily
is to make sure that they take the sitzmaker pill right before bed
and have the day one x-ray as early in the morning as possible.
While not useful in evaluating colonic transit, all of the makers
should be out of the upper GI tract. The mean colonic time has
been shown to 31 hours in males and 39 hours in females.(15)
Based on the location of retained rings, abnormal studies may be
labeled as “outlet obstruction” if 20% or more rings are retained at
day 5 in the rectosigmoid region or “colonic inertia” if more than
20% rings are dispersed throughout entire colon. Clinical effi-
cacy of colonic transit studies to detect segmental bowel motility
remains controversial.(9) No bowel prep is administered before
the study and patients are directed to avoid using laxatives and
promotility agents including dietary fiber for at least a week before
the study and during the duration of the study.
improved outcomes in colon and rectal surgery
smaLL boweL transit studies
Since it is generally accepted that slow transit constipation is over-
whelmingly attributed to colonic dysfunction, small bowel transit
studies are infrequently requested. However when clinical suspicion
exists, such as patients with gastroparesis and dilated small bowel
on plain x-rays, small bowel motility studies should be undertaken
before undertaking a surgical intervention. Several techniques are
available to assess small bowel transit. Nondigestible carbohy-
drates are broken down into hydrogen and fatty acids upon reach-
ing the colon. Hydrogen and fatty acids are then absorbed into the
blood stream. Therefore interval between ingestion of substrate
and increments in exhaled hydrogen levels estimate small bowel
transit. Similarly, orally administered sulfasalazine is broken down
by colonic bacteria into mesalazine and sulfapyridine and then
absorbed. Colonic transit can be measured by serum detection of
sulfapyridine. Radio nucleotide scintigraphy has also been used to
assess small bowel transit function. However, the clinical applica-
tion of these tests is limited by their complexity and variation in
bacterial flora in different subjects.
mri
Magnetic Resonance Imaging (MRI) of the pelvic floor is the
newest addition to the diagnostic armamentarium available for
pelvic floor evaluation. MRI obviates the exposure to radiation.
Technique involves filling rectum with ultrasound gel. Images can
be obtained in “static” manner or in the form of dynamic pelvic
MRI which involves patient to perform maneuvers that are simi-
lar to those performed during conventional defecography. During
these maneuvers, multiple images are obtained which are then
viewed as a cine loop. MRI provides excellent spatial orientation
of the sphincter complex and provides superior delineation of
the surrounding structures. MRI appears to be superior to ultra-
sonography in discerning external sphincter abnormalities.(30)
Additionally, dynamic MRI defecography appears to be supe-
rior to conventional defecography in the evaluation descending
perineum syndrome as it provides excellent spatial assessment of
pelvic floor musculature.(52)
Limitations of MRI in constipation
Dynamic pelvic floor MRI shares similar limitations as conven-
tional MRI: cost, claustrophobia, and availability. There are how-
ever, some specific limitations related to the diagnostic modality.
Studies comparing dynamic MRI with conventional defecography
have yielded conflicting results. Healy et al. (53) found significant
correlation between dynamic MRI findings and defecography in
ten patients examined employing both techniques. On the con-
trary, Matsouka et al. (54), in their study of 22 patients, reported
defecography to be more sensitive than dynamic MRI and recom-
mended against the routine use of this expensive modality. Most
centers perform pelvic floor imaging with patient in supine posi-
tion. Patients are asked to strain in a position which is far from
physiologic and raises concerns regarding the reliability of the
test. The influence of patient positioning has been investigated.
Bertschinger et al. (55) performed a prospective comparison of
38 patients who underwent closed MRI in supine position fol-
lowed by open MRI in a sitting position. Four rectal descents,
two enteroceles, four small cystoceles, and four small anterior
rectoceles were missed at supine MRI. The clinical significance
of these findings, however, remains questionable. As mentioned
earlier, the lack of “normal controls” makes it difficult to assess
the efficacy of this test.
baLLoon expuLsion test
Balloon expulsion test is an infrequently used method to test
motor defecatory function of the rectum. There is complete lack
of standardization of methods used in various anorectal manom-
etry laboratories.(55A) Various size balloons have been used for
this purpose. Commonly 50–100 cc deformable balloons are used.
Alternatively, smaller, more rigid balloons may also be employed.
The impact of size and compliance of balloon on the final inter-
pretation of test is unclear. In general, it is easier to evacuate larger
balloons.(56) Many investigators believe that volume of balloon
should be individualized to induce a constant desire to defecate.
Consequently, use of lower volumes may result in false positive
results.(57) There is a wide variation in what is considered to be a
normal test. Inability to expel balloon in a sitting position within
30–60 seconds is considered abnormal in most centers. Balloon
expulsion has been shown to be of importance in differentiating
between constipation caused by slow transit from that caused by
pelvic floor dyssynergia.(57)
biofeedback for constipation
Biofeedback training is widely utilized to teach relaxation of the
pelvic floor in patients with pelvic floor dyssynergia. A critical
review of the available literature by Heymen et al. (58) includ-
ing thirty eight studies showed that mean success rate with pres-
sure biofeedback was 78% compared to mean success rate of 70%
seen with electromyography feedback. The authors surmised that
despite the reported success rates, quality research is lacking.
The most controversial area involving biofeedback training
for constipation is questionable longevity/sustainability of the
results. Ferrara et al. (59) reported a clear loss of benefits over
time despite initial success.
patients perspective
Inherent to the evaluation of fecal incontinence is patients’ feel-
ings of shame, embarrassment and discomfort. These sensations
are felt by the incontinent patient resulting in depression and
social isolation. A number of quality of life tools have been devel-
oped to quantify the results of evaluation and treatment of fecal
incontinence. No one tool is universally accepted and these tools
have been difficult to validate.(60, 61)
In addition the testing incontinent patients are subjected to
may be embarrassing and uncomfortable. Deutekom et al. con-
ducted a cohort study of 240 consecutive patients undergoing
evaluation of fecal incontinence in 16 Dutch centers. Each patient
underwent manometry, defecography, AUS, PNTML, and MRI.
Two hundred forty of the 270 self-administered questionnaires
were returned. Patients were asked to evaluate anxiety, discom-
fort, embarrassment, and pain. Answers were scaled from 1(not
0), none to 5, severe. Results were also summarized as total test
burden. Overall test results were surprisingly low, with aver-
age scores in each category not exceeding 2. Overall MRI was
the most preferred and least uncomfortable test. Defecography
limitations of anorectal physiology testing
was the most inconvenient and uncomfortable. Anorectal com-
bined testing; manometry, PNTML, and AUS, also scored low for
discomfort and overall test burden but more so than MRI.(62)
concLusion
Physiological studies of anorectal function can provide valuable
information in carefully selected cases. While performing these
studies, one should be cognizant of the fact that these procedures
can be embarrassing, and at best are far from patient’s usual habits.
It is unnerving for many patients to perform the act of defecation
in the presence of an audience and it is conceivable that “perform-
ance anxiety” may lead to results which may not be truly repre-
sentative of actual patient status. Thus, these studies should be
interpreted with a grain of salt. Despite the plethora of literature
available, the clinical usefulness of these tests remains vague and
there is limited evidence that anorectal imagining guides man-
agement in pelvic floor disorders.(63) There are multiple well-
designed studies, which unfortunately report conflicting results.
We therefore recommend that the most decisive factor govern-
ing treatment decisions is history and physical exam. Physiology
testing should always be used as an adjunct rather than a primary
determinant.
references
1. Whitehead WE, Wald A, Norton NJ. Treatment options for
fecal incontinence. Dis Colon Rectum 2001; 44: 131–42.
2. Tjandra JJ, Dykes SL, Kumar RR et al. Practice parameters for
the treatment of fecal incontinence. Dis Colon Rectum 2007;
50: 1497–507.
3. Person B, Kaidar-Person O, Wexner SD. Novel approaches in
the treatment of fecal incontinence. Surg Clin N Am 2006;
86(4): 969–86.
4. Thompson WG, Longstreth GF, Drossman A et al. Functional
bowel disorders and functional abdominal pain. Gut 1999;
45(Suppl 2): 43–7.
5. Gordon PG. Anatomy and physiology of the anorectum. In
Fazio VW, Church JM Delaney CP, eds. Current Therapy in
Colon and Rectal Surgery. 2nd edn Philadelphia Elsevier-
Mosby, 2005: 1–9.
6. Simpson RR, Kennedy ML, Nguyen MH, Dinning PG,
Lubowski DA. Anal manometry: a comparison of techniques.
Dis Colon Rectum 2006; 49: 1033–8.
7. Wexner SD, Sardinha TC, Gilliand R. Setting up a colorectal
physiology laboratory. In Corman ML, ed. Colon and Rectal
Surgery 5th edn. Philadelphia Lipincot, Williams & Wilkins,
2005: 129–67.
8. Madoff RD, Orrom WJ, Rothenberger DA, Goldberg SM.
Rectal compliance: a critical reappraisal. Int J Colorectal Dis
1990; 5(1): 37–40.
9. Barnett JL, Hasler WL, Camilleri M. American Gastro-
enterological Association medical position statement on
anorectal testing techniques. American Gastroenterological
Association. Gastroenterology 1999; 116(3): 732–60.
10. Liberman H, Faria J, Ternent CA et al. A prospective
Evaluation of the value of anorectal physiology in the man-
agement of fecal incontinence. Dis Colon Rectum 2001; 44:
1567–74.
11. Felt-Bersma RJ, Klinkenberg-Knol EC, Meuwissen SG.
Anorectal function investigations in incontinent and con-
tinent patients. Differences and discriminatory value. Dis
Colon Rectum 1990; 33(6): 479–85.
12. Yang YK, Wexner SD. Anal pressure vectography is of no
apparent benefit for sphincter evaluation. Int J Colorectal
Dis 1994; 9(2): 92–5.
13. Beck A. Electromyographische untersuchungen am sphincter
ani. Phlugers Arch 1930; 224: 278–92.
14. Ferrara A, Lujan JH, Cebrian J et al. Clinical Manometric and
EMG characteristics of patients with fecal incontinence. Tech
Coloproctol 2001; 5: 13–8.
15. Smith LE, Blatchford GJ. Physiologic Testing. In: Wolff BG,
Fleshman JW, Beck DE et al, eds. The ASCRS Textbook of
Colon and Rectal Surgery New York, Springer, 2006: 40–56.
16. Timmcke AE. Limitations of anal physiologic testing. In: Hicks
TC, Beck DE, Opelka FG, Timmcke AE, eds. Complications
of Colon and Rectal Surgery. Baltimore Williams & Wilkins,
1996: 419–30.
17. Jacobs PPM, Scheuer M, Juijpers JHC Vingerhoets MH.
Obstetric fecal incontinence: role of pelvic floor denerva-
tion and results of sphincter repair. Dis Colon Rectum 1990;
33(6): 494–7.
18. Laurberg S, Swash M, Henry MM. Delayed external sphincter
repair for obstetric tear. Br J Surg 1998; 75: 786–8.
19. Wexner SD, Marchetti F, Jagelman DG. The role of sphinc-
teroplasty for fecal incontinence reevaluated: a prospective
physiologic and functional review. Dis Colon Rectum 1991;
34: 22–30.
20. Buie WD, Lowry AC, Rothenberger DA, Madoff RD. Clinical
rather than laboratory assessment predicts continence after
anterior sphincteroplasty. Dis Colon Rectum 2001; 44:
1255–60.
21. Kiff ES, Swash M. Slowed conduction in the pudendal nerves
in idiopathic (neurogenic) faecal incontinence. Br J Surg
1984; 71(8): 614–6.
22. Ricciardi R, Mellgren AF, Madoff RD et al. The utility of
pudendal nerve terminal motor latencies in idiopathic
incontinence. Dis Colon Rectum 2006; 49(6): 852–7.
23. Frudinger A, Bartram CI, Halligan, Kamm M. Examination
techniques for endosonography of the anal canal. Abdom
Imaging 1998; 23: 301–3.
24. Nazir M, Carlsen E, Jacobsen AF, Nesheim BI. Is there any
correlation between objective anal testing, rupture grade,
and bowel symptoms after primary repair of obstetric anal
sphincter rupture: an observational cohort study. Dis Colon
Rectum 2002; 45: 1325–31.
25. Zetterstrom JP, Mellgren A, Madoff RD, Kim DG, Wong WD.
Perineal body measurement improves evaluation of ante-
rior sphincter lesions during endoanal ultrasonography. Dis
Colon Rectum 1998; 41: 705–13.
26. Briel JW, Stoker J, Rociu E et al. External anal sphincter
atrophy on endoanal magnetic resonance imaging adversely
affects continence after sphincteroplasty. Br J Surgery 1999;
86: 1322–7.
27. Christensen AF, Nyhuus B, Nielson MB, Christensen H. Three-
dimensional anal endosonography may improve diagnostic
improved outcomes in colon and rectal surgery
confidence of detecting damage to the anal sphincter complex.
Brit J Radiol 2005; 78: 308–11.
28. Badger SA, Devlin PB, Neilly PJ, Gilliland R. Preoperative
staging of rectal carcinoma by endorectal ultrasound: is there
a learning curve? Int J Colorectal Dis 2007; 22(10): 1261–8.
29. Dobben AC, Terra MP, Duetekom M et al. Anal inspection
and digital examination compared to anorectal physiol-
ogy tests and endoanal ultrasonography in evaluating fecal
incontinence. Int J Colorectal Dis 2007; 22: 783–90.
30. Terra MP, Stoker J. The current role of imaging techniques in
faecal incontinence. Eur Radiol 2006; 16: 1727–36.
31. West RL, Dwarkasing S, Briel JW et al. Can three-dimensional
endoanal ultrasonography detect external anal sphincter
atrophy? A comparison with endoanal magnetic resonance
imaging. Int J Colorect Dis 2005; 20: 328–33.
32. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter
exercises for the treatment of faecal incontinence in adults.
Cochrane Database Syst Rev 2006, 3: CD002111.
33. Jensen L, Lowry A. Biofeedback improves functional outcome
after sphincteroplasty. Dis Colon Rectum 1997; 40: 197–200.
34. Heyman S, Jones KR, Ringel Y, Scarlett Y, Whitehead WE.
Biofeedback treatment of fecal incontinence: a critical review.
Dis Colon Rectum 2001; 44: 728–36.
35. Solomon MJ, Pager CK, Rex J, Roberts R, Manning
J. Randomized, controlled trial of biofeedback with anal
manometry, transanal ultrasound, or pelvic floor retrain-
ing with digital guidance alone in the treatment of mild to
moderate fecal incontinence. Dis Colon Rectum 2003; 46:
703–10.
36. Pager CK, Solomon MJ, Rex J, Roberts RA. Long-term outcomes
of pelvic floor exercise and biofeedback treatment for patients
with fecal incontinence. Dis Colon Rectum 2002; 45: 997–1003.
37. Norton C, Chelvanayagam S, Wilson-Barnett J, Redfern
S, Kamm MA. Randomized controlled trial of biofeedback
for fecal incontinence. Gastroenterology 2003; 125: 1320–9.
38. Stewart WF, Liberman JN, Sandler RS et al. Epidemiology of
constipation (EPOC) study in the United States: relation of clin-
ical subtypes to sociodemographic features. Am J Gastroenterol
1999; 94(12): 3530–40.
39. Sonnenberg A, Koch TR. Epidemiology of constipation in
the United States. Dis Colon Rectum 1989; 32(1): 1–8.
40. Infantino A, Masin A, Pianon P et al. Role of proctography in
severe constipation. Dis Colon Rectum 1990; 33(8): 707–12.
41. Moieira H, Wexner SD. Anorectal Physiologic testing. In:
Beck DE and Wexner SD, eds. Fundamentals of anorectal
surgery. 2nd ed Philadephia WB Saunders, 1998: 37–53.
42. Finlay IG, Bartolo DCC, Bartram CI et al. Proctography
(symposium). Int J Colorectal Dis 1998; 3: 67–98.
43. Penninckx F, Debruyne C, Lestar B, Kerremans R. Observer
variation in the radiological measurement of the anorectal
angle. Int J Colorectal Dis 1990; 5(2): 94–7.
44. Ferrante SL, Perry RE, Schreiman JS, Cheng SC, Frick MP.
The reproducibility of measuring the anorectal angle in
defecography. Dis Colon Rectum 1991; 34(1): 51–5.
45. Bartram CI, Turnbull GK, Lennard-Jones JE. Evacuation proc-
tography: an investigation of rectal expulsion in 20 subjects
without defecatory disturbance. Gastrointest Radiol 1988;
13(1): 72–80.
46. Turnbull GK, Bartram CI, Lennard-Jones JE. Radiologic
studies of rectal evacuation in adults with idiopathic consti-idiopathic consti-
pation. Dis Colon Rectum 1988; 31(3): 190–7.
47. Halligan S, Bartram CI. Is barium trapping in rectoceles sig-
nificant? Dis Colon Rectum 1995; 38(7): 764–8.
48. Shorvon PJ, McHugh S, Diamant NE, Somers S, Stevenson
GW. Defecography in normal volunteers: results and impli-
cations. Gut 1989; 30: 1737–49.
49. Roe AM, Bartolo DCC, Mortensen NJ. Techniques in evacu-
ation proctography in the diagnosis of intractable constipa-
tion and related disorders. J Roy Soc Med 1986; 79: 331–3.
50. Jones PN, Lubowski DZ, Swash M, Henry MM. Is paradoxi-
cal contraction of puborectalis muscle of functional impor-
tance? Dis Colon Rectum 1987; 30(9): 667–70.
51. Karulf RE, Coller JA, Bartolo DC et al. Anorectal physiology
testing. A survey of availability and use. Dis Colon Rectum
1991; 34(6): 464–8.
52. Healy JC, Halligan S, Reznek RH et al. Magnetic resonance
imaging of the pelvic floor in patients with obstructed defae-
cation. Br J Surg 1997; 84(11): 1555–8.
53. Healy JC, Halligan S, Reznek RH et al. Dynamic MR imaging
compared with evacuation proctography when evaluating
anorectal configuration and pelvic floor movement. AJR Am
J Roentgenol 1997; 169(3): 775–9.
54. Matsuoka H, Wexner SD, Desai MB et al. A comparison
between dynamic pelvic magnetic resonance imaging and
videoproctography in patients with constipation. Dis Colon
Rectum 2001; 44(4): 571–6.
55. Bertschinger KM, Hetzer FH, Roos JE et al. Dynamic MR
imaging of the pelvic floor performed with patient sitting in
an open-magnet unit versus with patient supine in a closed-
magnet unit. Radiology 2002; 223(2): 501–8.
55A. Beck DE. A simplified balloon expulsion test. Diseases Colon
Rectum 1992; 35: 597–8.
56. Azpiroz F, Enck P, Whitehead WE. Anorectal functional test-
ing: review of collective experience. Am J Gastroenterol 2002;
97(2): 232–40.
57. Minguez M, Herreros B, Sanchiz V et al. Predictive value of
the balloon expulsion test for excluding the diagnosis of pel-
vic floor dyssynergia in constipation. Gastroenterology 2004;
126(1): 57–62.
58. Heymen S, Jones KR, Scarlett Y, Whitehead WE. Biofeedback
treatment of constipation: a critical review. Dis Colon
Rectum 2003; 46(9): 1208–17.
59. Ferrara A, De Jesus S, Gallagher JT et al. Time-related decay
of the benefits of biofeedback therapy. Tech Coloproctol
2001; 5(3): 131–5.
60. Wexner SD, Jorge JM, Lee E et al. Etiology and management
of fecal incontinence. Dis Colon Rectum 1993; 36: 139–45.
61. Rockwood TH, Church JM, Fleshman JW et al. Fecal inconti-
nence quality of life scale: quality of life instrument for patients
with fecal incontinence. Dis Colon Rectum 2000; 43: 9–17.
62. Deutekom M, Terra MP, Dukgraff MGW et al. Patients per-
ception of tests in the assessment of faecal incontinence. Brit
J Radiology 2006; 79: 94–100.
63. Bharucha AE, Fletcher JG. Recent advances in assessing
anorectal structure and functions. Gastroenterology 2007;
133(4): 1069–74.
11
Limitations of colorectal imaging studies
Travis J Blanchard, Wilson B Altmeyer, and Charles C Matthews
CHALLENGING CASE
A 53 year-old woman presents to the emergency room with fever,
left lower quadrant abdominal pain, and tenderness. Her tem-
perature is 39 degrees Centigrad and her white blood cell count is
17,000 cells per cubic milliliter. What is the best radiologic test to
confirm her diagnosis?
CASE MANAGEMENT
A CT scan of the abdomen and pelvis will evaluate her to con-
firm the diagnosis of acute diverticulitis. In the absence of acute
diverticulitis it may very well provide anothe explanation for her
symptoms.
INTRODUCTION
Years of technical developments, organizational
changes, and
educational advances have inexorably altered the
nature and com-
position of colorectal imaging. Conventional radiology or “plain
films” and barium fluoroscopy studies are still important, but the
developments of CT, US, MRI, nuclear medicine, and interven-
tional radiology have greatly expanded the scope of radiology.
This chapter will discuss the various imaging modalities, focusing
on the capabilities and limitations of each modality to diagnose
various disease processes important to the colorectal surgeon.
ABDOMINAL RADIOGRAPHY (PLAIN FILMS)
Abdominal radiography typically consists of a single-view abdom-
inal x-ray of the kidneys, ureters, and bladder (KUB) or an
acute abdominal series (AAS). An AAS includes an upright chest
radiograph, as well as upright and supine radiographs of the
abdomen. An AAS can identify large masses, radiopaque for-
eign bodies, and radiopaque densities (including gallstones and
kidney stones).
Pneumoperitoneum
As little as 1–2 cc of pneumoperitoneum (free intraperitoneal air)
can be seen on an upright chest (Figure 11.1) or lateral decubitus
film.(1) Postoperative pneumoperitoneum usually resolves in 3 to 7
days. Failure of progressive resolution or an increase in the amount
of air present suggests a bowel anastomosis leak or abscess/sepsis.
Signs of pneumoperitoneum (Figure 11.2) on supine radiographs
include the “Rigler” sign or “double lumen” sign (gas on both sides
of the bowel wall), and gas outlining the falciform ligament.(2)
Bowel Obstruction and Dilatation
The 3, 6, 9 rule can be used to identify bowel dilatation. The small
bowel is dilated when its diameter is 3 cm; the colon when it is
6 cm, and the cecum when it is 9 cm. Abdominal plain films can
diagnose small bowel obstruction (SBO), in 50–60% of cases
with approximately 20% false-negative rate.(3) SBO can be com-
plete/high grade or partial. Dilated loops of small bowel, air-fluid
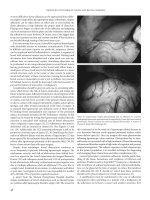
Figure 11.1 Pneumoperitoneum. Upright radiograph of the abdomen demonstrates a
collection of air within the peritoneal space between the liver and the diaphragm.
Figure 11.2 Pneumoperitoneum. Plain radiograph demonstrates the “Rigler”
sign or “double lumen” sign (gas on both sides of the bowel wall).