Improved Outcomes in Colon and Rectal Surgery part 15 pps
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (204.51 KB, 10 trang )
1
improved outcomes in colon and rectal surgery
154. Maglinte DD, Balthazar EJ, Kelvin FM, Megibow AJ. The
role of radiology in the diagnosis of small-bowel obstruc-
tion. AJR Am J Roentgenol 1997; 168: 1171–80.
155. Maglinte DD, Kelvin KM, Rowe MG, Bender GN, Rouch
DM. Small-bowel obstruction: optimizing radiologic inves-
tigation and nonsurgical management. Radiology 2001;
218: 39–46.
156. Maglinte DD, Kelvin FM, Sandrasegaran K et al. Radiology
of small bowel obstruction: contemporary approach and
controversies. Abdom Imaging 2005; 30: 160–78.
157. Maglinte DD, Heitkamp DE, Howard TJ, Kelvin FM, Lappas
JC. Current concepts in imaging of small bowel obstruc-
tion. Radiol Clin North Am 2003; 41: 263–83.
158. Frager D, Medwid SW, Baer JW, Mollinelli B, Friedman M.
CT of small-bowel obstruction: value in establishing the
diagnosis and determining the degree and cause. AJR Am
J Roentgenol 1994; 162: 37–41.
159. Furukawa A, Yamasaki M, Takahashi M et al. CT diagnosis
of small bowel obstruction: scanning technique, interpreta-
tion and role in the diagnosis. Semin Ultrasound CT MR
2003; 24: 336–52.
160. Suri S, Gupta S, Sudhakar PJ et al. Comparative evaluation
of plain films, ultrasound and CT in the diagnosis of intes-
tinal obstruction. Acta Radiol 1999; 40: 422–8.
161. Boudiaf M, Soyer P, Terem C et al. CT evaluation of small
bowel obstruction. RadioGraphics 2001; 21: 613–24.
162. Maglinte DD, Gage SN, Harmon BH et al. Obstruction of
the small intestine: accuracy and role of CT in diagnosis.
Radiology 1993; 188: 61–4.
163. Maglinte DD, Reyes BL, Harmon BH et al. Reliability and role
of plain film radiography and CT in the diagnosis of small
bowel obstruction. AJR Am J Roentgenol 1996; 167: 1451–5.
164. Gazelle GS, Goldberg MA, Wittenberg J et al. Efficacy of CT in
distinguishing small-bowel obstruction from other causes of
small-bowel dilatation. AJR Am J Roentgenol 1994; 162: 43–7.
165. Balthazar EJ. CT of small-bowel obstruction. AJR Am
J Roentgenol 1994; 162: 255–61.
166. Furukawa A, Yamasaki M, Furuichi K et al. Helical CT in the
diagnosis of small bowel obstruction. Radiographics 2001;
21: 341–55.
167. Jaffe TA, Martin LC, Thomas J, Adamson AR, DeLong
DM, Paulson EK. Small-Bowel Obstruction: Coronal
Reformations from Isotropic Voxels at 16-Section Multi–
Detector Row CT. Radiology 2005; 238: 135–42.
168. Sandhu PS, Bonnie JN, Coakley FV et al. Bowel Transition
Points: Multiplicity and Posterior Location at CT are Associated
with Small-Bowel Volvulus. Radiology 2007; 245: 160–7.
169. Sheedy SP, Earnest IV F, Fletcher JG, Fidler JL, Hoskin TL.
CT of Small-Bowel Ischemia Associated with Obstruction in
Emergency Department Patients: Diagnostic Performance
Evaluation. Radiology 2006; 241: 729–36.
170. Balthazar EJ, Liebeskind ME, Macari M. Intestinal ischemia
in patients in whom small bowel obstruction is suspected:
evaluation of accuracy, limitations, and clinical implications
of CT in diagnosis. Radiology 1997; 205: 519–22.
171. Frager D, Baer JW, Medwid SW, Rothpearl A, Bossart P.
Detection of intestinal ischemia in patients with acute
small-bowel obstruction due to adhesions or hernia: effi-
cacy of CT. AJR Am J Roentgenol 1996; 166: 67–71.
172. Zalcman M, Sy M, Donckier V, Closset J, Gansbeke DV.
Helical CT signs in the diagnosis of intestinal ischemia in
small-bowel obstruction. AJR Am J Roentgenol 2000; 175:
1601–7.
173. Ha HK, Kim JS, Lee MS et al. Differentiation of simple and
strangulated small-bowel obstructions: usefulness of known
CT criteria. Radiology 1997; 204: 507–12.
174. Balthazar EJ. George W. Holmes Lecture: CT of small-bowel
obstruction. AJR Am J Roentgenol 1994; 162: 255–61.
175. Rockey DC, Paulson E, Niedzwiecki D et al. Analysis of air
contrast barium enema, computed tomographic colonog-
raphy, and colonoscopy: prospective comparison. Lancet
2005; 365: 305–11.
176. Johnson CD, MacCarty RL, Welch TJ et al. Comparison
of the relative sensitivity of CT colonography and double-
contrast barium enema for screen detection of colorectal
polyps. Clin Gastroenterol Hepatol 2004; 2: 314–21.
177. Yee J, Akerkar GA, Hung RK et al. Colorectal neoplasia: per-
formance characteristics of CT colonography for detection
in 300 patients. Radiology 2001; 219: 685–92.
178. Fenlon HM, Nunes DP, Schroy PC 3rd et al. A comparison
of virtual and conventional colonoscopy for the detection
of colorectal polyps. N Engl J Med 1999; 341: 1496–503.
179. Macari M, Bini EJ, Xue X et al. Colorectal neoplasms: pro-
spective comparison of thin-section low-dose multi-detec-
tor row CT colonography and conventional colonoscopy
for detection. Radiology 2002; 224: 383–92.
180. Sosna J, Morrin MM, Kruskal JB et al. CT colonography
of colorectal polyps: a metaanalysis. AJR Am J Roentgenol
2003; 181: 1593–8.
181. Pickhardt PJ, Choi JR, Hwang I et al. Computed tomographic
virtual colonoscopy to screen for colorectal neoplasia in
asymptomatic adults. N Engl J Med 2003; 349: 2191–200.
182. Copel L, Sosna J, Kruskal JB et al. CT Colonography in 546
patients with incomplete colonoscopy. Radiology 2007; 244:
471–8.
183. Kim DH, Pickhardt PJ, Hinshaw JL et al. Prospective blinded
trial comparing 45-ml and 90-ml doses of oral sodium
phosphate for bowel preparation prior to CT colonography.
J Comput Assist Tomogr 2007; 31: 53–8.
184. Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD.
Acute phosphate nephropathy following oral sodium phos-
phate bowel purgative: an underrecognized cause of chronic
renal failure. J Am Soc Nephrol 2005; 16: 3389–96.
185. Pickhardt PJ, Choi JR. Electronic cleansing and stool tagging
in CT colonography: advantages and pitfalls encountered
with primary three-dimensional evaluation. AJR 2003; 181:
799–805.
186. Gluecker TM, Johnson DC, Harmsen WS et al. Colorectal
cancer screening with CT colonography, colonoscopy, and
double-contrast barium enema examination: prospec-
tive assessment of patient perceptions and preferences.
Radiology 2003; 227: 378.
187. Pickhardt PJ, Barish MA, Barlow DS et al. Significant compli-
cations at CT colonography: survey results from the Working
1
limitations of colorectal imaging studies
Group on virtual colonoscopy. Sixth international symposium
on virtual colonoscopy. Boston, Mass, October 17–18, 2005.
188. Burling D, Halligan S, Slater A, Noakes MJ, Taylor SA.
Potentially serious adverse events at CT colonography
in symptomatic patients: national survey of the United
Kingdom. Radiology 2006; 239(2): 464–71.
189. Sosna J, Blachar A, Amitai M et al. Colonic perforation at
CT colonography: assessment of risk in a multicenter large
cohort. Radiology 2006; 239(2): 457–63.
190. Kozarek RA, Sanowski RA. Use of pressure release valve to
prevent colonic injury during colonoscopy. Gastrointest
Endosc 1980; 26: 139–42.
191. Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH.
Patient-controlled room air insufflation versus automated
carbon dioxide delivery for CT colonography. AJR 2006;
186: 1491–6.
192. Johnson CD, Harmsen WS, Wilson LA et al. Prospective
blinded evaluation of computed tomographic colonography
for screen detection of colorectal polyps. Gastroenterology
2003; 125: 311–9.
193. Cotton PB, Durkalski VL, Pineau BC et al. Computed tomo-
graphic colonography (virtual colonoscopy): a multicenter
comparison with standard colonoscopy for detection of
colorectal neoplasia. JAMA 2004; 291: 1713–9.
194. Rockey DC, Paulsen EK, Niedzwiecki D et al. Analysis of air
contrast barium enema, computed tomographic colonog-
raphy, and colonoscopy: prospective comparison. Lancet
2005; 365: 305–11.
195. Kim DH, Pickhardt PJ, Taylor AJ et al. CT Colonography ver-
sus Colonoscopy for the Detection of Advanced Neoplasia.
N Engl J Med 2007; 357: 1403–12.
196. Fruhmorgen P, Demling L. Complications of diagnostic and
therapeutic colonoscopy in the Federal Republic of Germany:
results of an inquiry. Endoscopy 1979; 11: 146–50.
197. Farley DR, Bannon MP, Zietlow SP et al. Management of
colonoscopic perforations. Mayo Clin Proc 1997; 72: 729–33.
198. Anderson ML, Pasha TM, Leighton JA. Endoscopic per-
foration of the colon: lessons from a 10-year study. Am
J Gastroenterol 2000; 95: 3418–22.
199. Gatto NM, Frucht H, Sundarajan V et al. Risk of perforation
after colonoscopy and sigmoidoscopy: a population-based
study. J Natl Cancer Inst 2003; 95: 230–6.
200. Bowles CJ, Leicester R, Romaya C et al. A prospective study
of colonoscopy practice in the UK today: are we adequately
prepared for national colorectal cancer screening tomorrow?
Gut 2004; 53: 277–83.
201. Bond JH. Clinical evidence for the adenoma-carcinoma
sequence, and the management of patients with colorectal
adenomas. Semin Gastrointest Dis 2000; 11: 176–84.
202. Odom SR, Duffy SD, Barone JE, Ghevariya V, McClane SJ.
The rate of adenocarcinoma in endoscopically removed
colorectal polyps. Am Surg 2005; 71: 1024–6.
203. Schoenfeld P, Cash B, Flood A et al. Colonoscopic screen-
ing of average-risk women for colorectal neoplasia. N Engl
J Med 2005; 352: 2061–8.
204. Pickhardt PJ, Hassan C, Laghi A et al. Cost-effectiveness of
colorectal cancer screening with computed tomographic
colonography: the impact of not reporting diminutive
lesions. Cancer 2007; 109: 2213–21.
205. Imperiale TF, Wagner DR, Lin CY et al. Risk of advanced
proximal neoplasms in asymptomatic adults according to the
distal colorectal findings. N Engl J Med 2000; 343: 169–74.
206. Regula J, Rupinski M, Kraszewska E et al. Colonoscopy in
colorectal-cancer screening for the detection of advanced
neoplasia. N Engl J Med 2006; 355: 1863–72.
207. Rex DK, Lieberman D. ACG colorectal cancer prevention
action plan: update on CT-colonography. Am J Gastroenterol
2006; 101: 1410–3.
208. Zalis ME, Barish MA, Choi JR et al. CT colonography
reporting and data system: a consensus proposal. Radiology
2005; 236: 3–9.
209. Manfredi S, Bouvier AM, Lepage C et al. Incidence and pat-
terns of recurrence after resection for cure of colonic cancer
in a well defined population. Br J Surg 2006; 93: 1115–22.
210. Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Impact
on survival of intensive follow-up after curative resection
for colorectal cancer: systematic review and meta-analysis
of randomised trials. BMJ 2002; 324: 813.
211. Choi YJ, Park SH, Lee SS et al. CT colonography for fol-
low-up after surgery for colorectal cancer. AJR 2007; 189:
283–9.
212. Fletcher JG, Johnson CD, Krueger WR et al. Contrast-
enhanced CT colonography in recurrent colorectal carci-
noma: feasibility of simultaneous evaluation for metastatic
disease, local recurrence, and metachronous neoplasia in
colorectal carcinoma. AJR 2002; 178: 283–90.
213. Filippone A, Ambrosini R, Fuschi M et al. Preoperative T
and N staging of colorectal cancer: accuracy of contrast-
enhanced multi–detector row CT colonography—initial
experience. Radiology 2004; 231: 83–90.
214. You YT, Chang Chien CR, Wang JY et al. Evaluation of
contrast-enhanced computed tomographic colonography
in detection of local recurrent colorectal cancer. World
J Gastroenterol 2006; 12: 123–6.
215. Rubesin ES, Levine MS, Laufer I, Herlinger H. Double-
contrast barium enema examination technique. Radiology
2000; 215: 642.
216. Mutch MG, Birnbaum EH, Menias CO. Diagnostic
Evaluations-Radiology. In ASCRS Textbook of Colon and
Rectal Surgery, Chapter 6. New York: Springer-Verlag,
2006: 77.
217. Blakeborough A, Anthony H. Chapman AH, Swift S et al.
Strictures of the sigmoid colon: barium enema evaluation.
Radiology 2001; 220: 343.
218. Kelvin FM, Gardiner R, Vas W, Stevenson GW. Colorectal carci-
noma missed on double contrast barium enema study: a prob-
lem in perception. AJR Am J Roentgenol 1981; 137: 307–13.
219. Brady AP, Stevenson GW, Stevenson I. Colorectal cancer
overlooked at barium enema examination and colonos-
copy: a continuing perceptual problem. Radiology 1994;
192: 373–8.
220. Levine MS, Rubesin SE, Igor Laufer IL, Herlinger H.
Diagnosis of colorectal neoplasms at double-contrast bar-
ium enema examination. Radiology 2000; 216: 11.
1
improved outcomes in colon and rectal surgery
221. Levine MS, Kam LW, Rubesin SE, Ekberg O. Internal hem-
orrhoids: diagnosis with double-contrast barium enema
examinations. Radiology 1990; 177: 141–4.
222. MacCarty RL. Colorectal cancer: the case for barium enema.
Mayo Clin Proc 1992; 67: 253–7.
223. Kahn S, Rubesin SE, Levine MS, Laufer I, Herlinger H.
Polypoid lesions at the anorectal junction: barium enema
findings. AJR Am J Roentgenol 1993; 161: 339–42.
224. Harned RK, Consigny PM, Cooper NB. Barium enema
examination following biopsy of the rectum or colon.
Radiology 1982; 145: 11–6.
225. Maglinte DDT, Strong RC, Strate RW et al. Barium enema
after colorectal biopsies: experimental data. AJR Am
J Roentgenol 1982; 139: 693–7.
226. Thoeni RF, Menuck L. Comparison of barium enema
and colonoscopy in the detection of small colonic polyps.
Radiology 1977; 124: 631–5.
227. Ott DJ, Gelfand DW, Wu WC, Kerr RM. Sensitivity of dou-
ble-contrast barium enema: emphasis on polyp detection.
AJR Am J Roentgenol 1980; 135: 327–30.
228. Ott DJ, Chen YM, Gelfand DW, Wu WC, Munitz HA. Single-
contrast vs double-contrast barium enema in the detection
of colonic polyps. AJR Am J Roentgenol 1986; 146: 993–6.
229. Glick S, Wagner JL, Johnson CD. Cost-effectiveness of
double-contrast bariumenema in screening for colorectal
cancer. AJR Am J Roentgenol 1998; 170: 629–36.
230. Beggs I, Thomas BM. Diagnosis of carcinoma of the colon
by barium enema. Clin Radiol 1983; 34: 423–5.
231. Fork FT. Reliability of routine double contrast examination
of the large bowel: a prospective study of 2590 patients. Gut
1983; 24: 672–7.
232. Rex DK, Rahmani EV, Haeman JH. Relative sensitivity of
colonoscopy and barium enema for detection of colorec-
tal cancer in clinical practice. Gastroenterology 1997; 112:
17–23.
233. Byers T, Levin B, Rothenberger D, Dodd GD, Smith RA.
American Cancer Society guidelines for screening and sur-
veillance for early detection of colorectal polyps and cancer:
update 1997. Ca Cancer J Clin 1997; 47: 154–60.
234. Rice RP. Lowering death rates from colorectal cancer: chal-
lenge for the 1990s. Radiology 1990; 176: 297–301.
235. Hizawa K, Iida M, Kohrogi N et al. Crohn’s disease: early
recognition and progress of aphthous lesions. Radiology
1994; 190: 451–4.
236. Nolan DJ, Traill ZC. The current role of barium examina-
tions of the small intestine. Clin Radiol 1997; 52: 809–20.
237. Najjar SF, Jamal MK, Savas JF et al. The spectrum of colove-
sical fistula and diagnostic paradigm. Am J surg 2004; 188:
617–21.
238. Szucs RA, Turner MA. Gastrointestinal tract involvement by
gynecologic disease. Radiographics 1996; 16: 1251–70.
239. Thompson, WM. Imaging and findings of lipomas of the gas-
trointestinal tract. AJr Am J Roentgenol 2005; 184: 1163–71.
240. Mutch MG, Birnbaum EH, Menias CO. Diagnostic
Evaluations-Radiology. In ASCRS Textbook of Colon and
Rectal Surgery, Chapter 6. New York: Springer-Verlag, 2006:
82–3.
241. Marcello PW, Roberts PL, Schoetz DJ et al. Long-term
results of the ileoanal pouch procedure. Arch Surg 1993;
128: 500–3.
242. Fazio VW, Ziv Y, Church JM et al. Ileal pouch–anal anas-
tomoses complications and function in 1005 patients. Ann
Surg 1995; 222: 120–7.
243. Alfisher MM, Scholz FJ, Roberts PL, Counihan T. Radiology of
ileal pouch–anal anastomosis: normal findings, examination
pitfalls, and complications. RadioGraphics 1997; 17: 81–98.
244. Prudhomme M, Dozois RR, Godlewski G, Mathison S,
Fabbro-Peray P. Anal canal strictures after ileal pouch–anal
anastomosis. Dis Colon Rectum 2003; 46: 20–3.
245. Dolinsky D, Levine MS, Stephen E. Utility of contrast enema
for detecting anastomotic strictures after total proctocolec-
tomy and ileal pouch–anal anastomosis. AJR 2007; 189: 25–9.
246. Houston JD, Davis M. Fundamentals of Fluoroscopy.
Philadelphia: W.B. Saunders Company, 2001: 88–90.
247. Halper RD. Gastrointestinal Imaging: The Requisites.
Philadelphia: Mosby, 2006: 322–4.
248. Hicks HC et al. Complication of Colon & Rectal Surgery.
Lippincott Williams & Wilkins, 1996: 71–3.
249. Gore RM, Levine MS. Textbook of Gastrointestinal
Radiology, 2nd edition. Philadelphia: W.B. Saunders
Company, 2000: 905–13, 112.
250. Middleton WD, Kurtz AB, Hertzberg BS. Ultrasound: The
Requisites, 2nd edition. St. Louis: Mosby, 2004: 220–4.
251. Boutkan H, Luth W, Meyer S et al. The impact of intraop-
erative ultrasonography of the liver on the surgical strategy
of patients with gastrointestinal malignancies and hepatic
metastases. Eur J Surg Oncol 1992; 18: 342–6.
252. Kruskal JB, Kane RA. Intraperative US of the Liver:
Techniques and Clincal Applications. RadioGraphics, 2006:
1067–84.
253. Bipat S, Glas AS, Slors FJ et al. Rectal cancer: local staging
and assessment of lymph node involvement with endolumi-
nal US, CT, and MR Imaging – A Meta-Analysis. Radiology
2004; 232(3): 773–83.
254. Yamada T, Alpers DH, Laine L, et al. Textbook of Gastro-
enterology 4th Edition. Philadelphia: Lippincott Williams &
Wilkins, 2003: 3139–55, 3184–98.
255. Gore RM, Levine MS. Textbook of Gastrointestinal Radiology,
2nd edition. Philadelphia: W.B. Saunders Company, 2000:
1031–3.
256. MacCarthy EP et al.
2007/gadolinium_DHCP.pdf. www.fda.gov. 9/12/2007.
257. Haaga JR, Lanzieri CF, Gilkeson RC. CT and MR Imaging
of the Whole Body 4th Edition. St. Louis: Mosby, 2003:
1238–58.
258. Gore RM, Levine MS. Textbook of Gastrointestinal Radiology,
2nd edition. Philadelphia: W.B. Saunders Company, 2000:
86–97.
259. Beets-Tan RG, Beets GL. Rectal Cancer: Review with
Emphasis on MR Imaging. Radiology 2004; 232(2):335–46.
260. Bipat S, Glas AS, Slors FJ et al. Rectal Cancer: Local
Staging and Assessment of Lymph Node Involvement with
Endoluminal US, CT, and MR Imaging – A Meta-Analysis.
Radiology 2004; 232(3): 773–83.
11
limitations of colorectal imaging studies
261. Ziessman HA et al. Nuclear Medicine: The Requisites.
Philadelphia: Mosby, 2006: 302–45.
262. Mettler FA, Guiberteau MJ. Essentials of Nuclear Medicine
Imaging. Philadelphia: Suanders Elsevier, 2006: 359–422.
263. Gore RM, Levine MS. Textbook of Gastrointestinal
Radiology, 2nd edition. Philadelphia. W.B. Saunders
Company, 2000: 1043.
264. Ziessman HA et al. Nuclear Medicine: The Requisites.
Philadelphia: Mosby, 2006: 346–83.
265. Gore RM, Levine MS. Textbook of Gastrointestinal Radiology,
2nd edition. Philadelphia. W.B. Saunders Company, 2000:
1033–34.
266. Mettler FA, Guiberteau MJ. Essentials of Nuclear Medicine
Imaging. Philadelphia: Saunders Elsevier, 2006: 215–9,
322.
267. Hicks HC et al. Complication of Colon & Rectal Surgery.
Lippincott Williams & Wilkins, 1996: 69–70.
268. Darcy M. Clinical management of gastrointestinal bleeding.
In: Murphy TP, Benenati JF, Kaufman JA, eds. Patient Care
in Interventional Radiology, SCVIR, Fairfax, VA, 1999.
269. Hastings GS. Angiographic localization and transcatheter
treatment of gastrointestinal bleeding. Radiographics 2000;
20: 1160–8.
270. Gomes AS, Lois JF, McCoy RD. Angiographic treatment of
gastrointestinal hemorrhage: comparison of vasopressin
infusion and embolization. AM J Roentgenol 1986; 146:
1031–7.
271. Lambiase RE. Percutaneous abscss and fluid drainage: a
critical review. Cardiovasc Interv Radiol 1991; 14: 143–57.
272. Cinat ME, Wilson SE, Din AM. Determinants for successful
percutaneous image-guided drainage of intra-abdominal
abscess. Arch Surg 2002; 137: 845–9.
273. Catalano OA, Hahn PF, Hooper DC, Mueller PR. Efficacy of
percutaneous abscess drainage in patients with vancomy-
cin-resistant enterococci. AJR 2000; 175: 533–6.
274. Boland GW, Lee MJ, Dawson SI et al. Percutaneous abscess
drainage complications. Semin Interv Radiol 1994; 11: 267–75.
275. Lambiase RE, Cronan JJ, Dorfman GS et al. Postoperative
abscesses with enteric communication percutaneous treat-
ment. Radiology 1989; 171: 497–500.
276. Bernardino ME. Percutaneous biopsy. Am J Roentgenol
1984; 142: 41–5.
277. Charboneau JW, Reading CC, Welch TJ. CT and sonograph-
ically guided needle biopsy: current techniques and new
innovations. Am J Roentgenol 1990; 154: 1–10.
278. McGahan JP, Ddd GD. Radiofrequency ablation of the liver
current status. Am J Roentgenol 2001; 176: 3–16.
279. Wood BJ, Ramkaransingh JR, Fojo T, Walther MM, Libutti
SK. Percutaneous tumor ablation with radiofrequency.
Cancer 2002; 94: 443–51.
280. Sullivan KI. Hepatic artery chemoembolization. Semin
Oncol 2002; 29: 145–51.
281. Perler BA, Becker GJ eds. Vascular Intervention: a Clinical
Approach. Thieme medical Publisher, New York, 1998.
282. Kerr DJ, McArdle CS, Ledermann J et al. Intrahepatic arterial
versus intravenous fluorouracil and folinic acid for colorec-
tal cancer liver metastases: a multicenter randomized trial.
Lancet 2003; 361: 368–73.
Transanal endoscopy
Terry C Hicks
CHALLENGING CASE
A 60-year-old woman with a strongly positive family history
of colorectal cancer undergoes a colonoscopy. She has a 1.5 cm
pedunculated polyp snared from the transverse colon. Five days
after the colonoscopy the patient experienced two bloody bowel
movements. She presents to the emergency room with a heart
rate of 120 and a blood pressure of 100/70.
CASE MANAGEMENT
You have two large bore intravenous lines started and begin rapid
infusion of 2 L of lactated Ringers. Blood is drawn for a CBC,
basic metabolic profile, coagulation studies, and type and cross
for 4 units of packed RBC. A nasogastric tube is placed and billous
heme negative fluid is aspirated. A proctoscopy reveals blood and
clots in rectum, but no source of bleeding.
A tagged RBC scan is obtained which is immediately positive
in the cecum. An angiogram of the ileocolic artery reveals active
bleeding in the cecum. Using a micro catheter, the interventional
radiologist is able to embolize a segmental vessel and the bleeding
ceases. The patient is transferred to the ICU for observation.
INTRODUCTION
Endoscopy is commonly used to evaluate the gastrointestinal (GI)
tract. Endoscopy of the lower GI tract may include colonoscopy, flex-
ible sigmoidoscopy, rigid proctoscopy, and anoscopy. While each of
these procedures may have associated risks, discussion of colonos-
copy will address the other procedures. This chapter will address the
technical and nontechnical issues associated with this procedure.
Colonoscopy is a procedure commonly used to diagnose and treat
colonic conditions. It is a natural extension of the colon and rectal
physical exam, and it has significant advantages over other examina-
tions of the lower GI tract. This procedure allows direct inspection of
the mucosal surface with the potential to identify and/or treat polyps,
neoplasia, vascular lesions, and inflammatory bowel disease. The suc-
cess of a colonoscopy is dependent on operator experience, patient
selection, as well as the timing and extent of the bowel preparation.
Although colonoscopy is an invasive procedure, complications
are fortunately infrequent. However, major complications associ-
ated with colonoscopy can result in significant morbidity or even
death. Serious complications can arise with either a diagnostic or
therapeutic colonoscopy and include: perforation, hemorrhage,
postpolypectomy coagulation syndrome, problems related to
mechanical bowel preparation, infections, and anesthetic mala-
dies (intravenous medications).(1)
NONTECHNICAL COMPLICATIONS
Colonic complications may occur that are essentially unrelated to
the actual technical performance of the procedure. These com-
plications include patient selection, method of preparation of
the colon, and administration of medication for sedation before
and during the procedure, as well as the clinical decisions that are
made concerning those patients who are on anticoagulation.
Minimizing preventable colonoscopic complications begins
with the patient selection process. The endoscopist needs to
identify those patients who have enhanced risks for endoscopist
injury. This can usually be accomplished by obtaining an appro-
priate medical history and physical examination, along with any
necessary laboratory studies.
Before performing an endoscopic examination of the colon,
the physician must consider the patient’s general condition. The
risk of potential injury must be balanced against the anticipated
therapeutic gain, and this includes being cognizant of the patient’s
ability to tolerate injury. For example, a patient with signs or
symptoms suggestive of a colorectal carcinoma who requires a
colon evaluation, but who has recently had a myocardial infarc-
tion, may be better evaluated with CT colography or barium
enema. Consultation with a patient’s obstetrician is appropriate
to insure that the test is merited and falls within acceptable safety
guidelines for patients in their last two trimesters of pregnancy.
Other relative contraindications to a colonoscopy include large
abdominal aortic aneurysms or substantial splenomegaly.
COLORECTAL PREPARATION
Every effort should be utilized to cleanse the colon of feces and
particulate matter before the examination as an adequate bowel
preparation is one of the most important factors in avoiding injury
and maximizing the quality of the exam. The ultimate success of the
colonoscopy depends on operator experience as well as the timing
and extent of bowel preparation. Inadequate colon preparation is
reported in approx. 25% of cases and leads to lower cecal intubation
rates and decreased polyp detection.(2–4) Other potential problems
associated with a suboptimal bowel preparation include: increased
rate of complications, longer procedural times, or the need to for
repeat examination.(4, 5)
Among the many factors that lead to inadequate bowel prepa-
rations, poor compliance due to incomplete consumption of the
preparation is the most frequent etiology.(6) Reports of poor com-
pliance are usually attributed to the patient’s intolerance of the
high volume of ingested cleansing solutions.(7) Some preparations
are associated with specific adverse effects in some subpopulations,
including those who have renal insufficiency, preexisting electrolyte
abnormalities, or congestive heart failure. The clinician must be
cognizant of the advantages and disadvantages for different bowel
preparations in order to obtain not only the best clinical exam but
also to protect the patient from complications.
The variety of colonoscopy preparations in clinical use involve
combinations of diet restriction and laxatives. As an adjunct, most
bowel preparations include a period of time during which the
patient is restricted to a clear liquid or low residue diet, to reduce the
amount of stool in the colon. However, dietary restriction by itself
transanal endoscopy
is insufficient to adequately cleanse the colon. Current cleansing
preparations include lavage solutions (usually polyethylene glycol—
electrolyte lavage solution) and hypertonic electrolyte solutions.
Oral Lavage
Oral lavage methods have been developed to reduce the time required
for mechanical cleansing (usually only 2–4 hours are required). Oral
lavage consists of the ingestion of a large volume of osmotically bal-
anced, nonabsorbable solutions that act as a purgative, clearing the
colon of stool through mechanical forces. Polyethylene glycol (PEG)
containing preparations have become the most preferred method of
colonic preparation.(8) A meta-analysis of eight trials reported that
PEG preparations were associated with either an adequate or excel-
lent preparation in 70% of patients.(9) Unfortunately, up to 20%
of patients are unable to complete the PEG preparation because of
the poor palatability of the solution or its required large volumes.
Recently, there have been new strategies to increase the efficacy in
patient tolerability of PEG containing solutions. Recent studies
have evaluated whether or not there is increased tolerability when
using flavor versus nonflavor preparations.(10) There are also recent
reports of investigations into the use of lower volume PEG solutions
(i.e., 2 L rather than the standard 4 L), with or without the utiliza-
tion of adjunct purgatives.(11) Other variations include the addition
of adjunct purgatives (senna, magnesium citrate, or bisacodyl) in an
effort to increase the efficacy of PEG solutions.(12, 13)
To assist patients with the volume of fluid that must be ingested
some endoscopists have tried adding metoclopramide, in the hopes
that it would reduce nausea and increasing bowel motility. A study
by Brady et al. (14) (a small placebo trial) utilizing metclopramide
as an adjunct reported no significant benefits in the terms of bowel
preparation or decrease in abdominal discomfort.
Contraindication to oral lavage solutions include significant gas-
tric retention, suspected or established mechanical bowel obstru-
ction, severe colitis, or the presence of ileus.
Hypertonic Electrolyte Solutions
Salt-based agents for bowel preparation are known as saline laxa-
tives and include those containing magnesium cations or phosphate
anions. Salt-based agents work by exerting a hyperosmotic effect in
the intestines. The poorly absorbed magnesium or phosphate ions
within the small intestine result in retention of water that directly
stimulates stretched receptors and increases peristalsis.
Sodium phosphate (NaP) is one of the more commonly used
saline laxatives and is available in liquid or tablet form. This
hyperosmotic product draws fluid into the intestinal tract result-
ing in a purgative action. Proponents of the utilization of NaP
refer to studies that show that in healthy individuals the prep is
safe, better tolerated, and equally or more effective than PEG.
(15, 16) It is imperative to note that the downside of using oral
sodium phosphate solutions is the potential for significant fluid
shifts which can precipitate intravascular volume depletion.
A few cases of nephrocalcinosis with subsequent renal insuffi-
ciency have also been reported.(17) The effect seems to be age and
dose related. Additional risk factors for this unusual occurrence
include underlying renal disease, dehydration, hypercalcemia,
or hypertension with the use of angiotensin-converting enzyme
(ACE) inhibitors or angiotensin receptor blockers (ARBs).
These issues makes it imperative that (NaP) not be used in
patients with congestive heart failure, decompensated cirrho-
sis, renal failure, or those presenting with baseline electrolyte
abnormalities.(18) There is also the current concern for the
development of hyperphosphatemia. Patients who have renal
insufficiencies (familial filtration rate of <50% of normal) can
develop life threatening hyperphosphatemia. Most patients with
normal renal function find the sodium phosphate NaP prepara-
tion safe. Rejchrt and his colleagues reported on the utilization
of NaP preparations and its effects on the colonic lining. They
found the preparation induced mucosal lesions, erosions, and
aphthous lesions in up to 3% of patients.(19) They concluded
that this preparation should not be utilized for patients under-
going diagnostic evaluation for potential inflammatory bowel
disease because it may lead to misinterpretation secondary to the
mucosa lesions induced by the preparation.(20)
As discussed previously, multiple studies have indicated the
necessity for good bowel cleansing before endoscopic evaluation, as
it adds not only to the quality of the examination, but also reduces
potential complications. At present, the choice of bowel preparation
is dependent on the clinical context, and the presence or absence
of associated risk factors. The endoscopist should be cognitive of
these issues before prescribing a colonoscopy preparation.
Intravenous Sedation
Conscious sedation reduces patient symptoms during endos-
copy, but accounts for significant risks including vasovagal reac-
tions and cardiopulmonary events. Conscious sedation can in
fact cause respiratory depression, hypotension, tachycardia, or
brachycardia.(33, 34) Patients who develop severe hypotension
or hypoxia associated with sedation are also at risk for myocardial
infarction. To reduce these risks and prevent excessive sedation, it
is important that the physician and/or nurse providing the medi-
cation carefully titrate it throughout the procedure.(35) In the
United States, it is standard to monitor blood pressure, pulse, and
oxygen saturation on a timely basis throughout the procedure. It
is also common to administer supplemental oxygen via nasal can-
nula. It is interesting to note that a prospective study of private
patients (men and women), less than one-third were willing to
undergo colonoscopy without sedation.(36)
At present, the most commonly utilized agents for colonoscopic
sedation are meperidine, fentanyl, and midazolam. Meperidine
and fentanyl are used for analgesia. Fentanyl has a shorter time
of onset and recovery, while meperdine appears to potentiate the
sedative effect of benzodiazepines. Midazolam provides an anter-
ograde amnesia and possesses a short half life, which is a distinct
advantage for the safety of the patient. Midazolam also poten-
tiates the narcotic effect and permits the reduction of narcotic
doses and their associated complications. The utilization of these
agents affects the psycho motor function of the patient for hours,
and thus postprocedure monitoring is necessary before their dis-
charge from endoscopy unit. Some centers are now evaluating the
use of propofol (Diprivan). Propofol lacks analgesic effect but is
a rapid onset and effective sedative. It also has a shorter recovery
time. Propofol’s most serious potential side effect is sudden respi-
ratory depression, which may require intubation in order to con-
trol the airway. Therefore, utilization of this drug usually requires
improved outcomes in colon and rectal surgery
administration by an anesthesiologist, a nurse anesthetist, or a
dedicated physician. Most endoscopy centers, with an adequate
number of procedure rooms and recovery space have not found
this drug cost-effective.
Hemodynamic depression is managed with increased intrave-
nous fluids, while respiratory depression is treated with supple-
mental oxygen and sedation reversal. Naloxone (Narcan); 0.4 mg
intravenously (or 0.2 mg intravenously and 0.2 mg intramuscu-
larily) will reverse the narcotic effect. Flumazenil (Romazicon);
0.2 to 1.0 milligrams intravenously, will reverse sedation from
Midazolam. Excessive colonic distention may produce a vasova-
gal reaction which responds to increased intravenous fluids and
decompression of colon gas. Significant bradycardia, secondary
to the sedation may require administration of atropine (0.5 mg
IV every 3–5 minutes to a dose of 3 mg).
Infectious Disease Complications
Colonoscopy can produce infectious complication by transmis-
sion of disease from patient to patient, from patient to examiner, or
from bacteremia related to the procedure. Current national recom-
mendations for mechanical cleansing of endoscopic equipment,
if followed properly, should prevent the transmission of such dis-
eases as HIV and hepatitis. Transmission of disease from patient to
examiner can also be prevented by appropriate eye, facial, and hand
protection and endoscopic suites should be equipped with goggles,
disposable aprons and gloves, or facial splash guards.
The incidence of bacteremia associated with colonoscopy has
been reported from 1 to 20%. Despite the potential risks of bacter-
emia, there are presently only five reported cases of endocarditis
associated with colonoscopy, despite the millions of colonoscop-
ies performed annually.(37, 38) The American Heart Association
(AHA) had previously recommended antibiotic treatment for
patients that were described as high-risk (patients with pros-
thetic heart valves, congenital cardiac malformations, surgically
constructed systemic pulmonary shunts, and previous history
of endocarditis). More recently, the AHA SBE prophylaxis panel
after extensive study, now recommends that the risk of antibiotic
complications greatly outweighs the potential for bacteremia
leading to endocarditis. They conclude that no antibiotics should
be administered for SBE prophylaxis during colonoscopy.(39)
TECHNICAL COMPLICATIONS
Hemorrhage
Hemorrhage after colonoscopy is most commonly associated
with polypectomy, but can occur with diagnostic procedures.
Hemorrhage is most frequently associated with intraluminal bleed-
ing, but can also arise from extraluminal sources, such as a mesen-
tery laceration, secondary to mechanical forces produced during
instrumentation. Splenic injury or rupture results in intraperi-
toneal hemorrhage (see miscellaneous complications section for
more details). Hemorrhage following colonoscopic polypectomy
has a prevalence that ranges between 1–2.5%, and is the most
common complication of polypectomy.(21) The hemorrhage
may be an immediate or delayed event and has been reported up
to 14 days postpolypectomy.(22) Those hemorrhages occurring
during the endoscopic procedure represent 1.5% of polypecto-
mies, and those in the delayed setting, after the completion of the
procedure represent 2% of polypectomies.(23)
Immediate hemorrhage upon transection of the pedicle of a
pedunculated polyp occurs because of inadequate coagulation
of the feeding vessels. Pedunculated polyps, >1 cm in diameter
with fixed stalks have the highest risk for immediate hemorrhage,
as they have substantial vessels.(24) The utilization of the cold
biopsy technique can result in capillary bleeding, which is usually
of no clinical significance. The corollary to this observation is that
significant bleeding can occur with cold or hot biopsy techniques
if the patient is being treated with anticoagulants or antiplatelet
medications. Though most postpolypectomy hemorrhage is self-
contained, the endoscopist must respect the clinical potential of
the bleeding to produce enough hypotension as to cause stroke,
myocardial infarction, or frank shock.
With persistent and significant ongoing bleeding, the endo-
scopist my have difficulty in locating the residual stalk. This
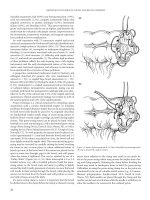
Figure 12.1 Management of postpolypectomy hemorrhage.
transanal endoscopy
makes it imperative that the source be quickly controlled at the
onset of bleeding before the field being obscured by blood and
clot. Initially, the endoscopist can utilize the polypectomy snare
to regrasp the stalk and hold it taut for approximately 15 minutes
without the utilization of any electrocautery. This maneuver if
unsuccessful can be performed again and this usually suffices to
control the bleeding. Other options are: the placement of clips or
detachable snares. If these are not available, the residual stalk can
be injected with 1–10,000 solution of epinephrine plus saline, or
the base of the residual stalk can be recoagulated without enough
energy to transect the stalk. Many endoscopists now suggest that
if one identifies a potentially significant polyp that might produce
postpolypectomy bleeding (i.e., large in size or patient’s condition
mandates they continue anticoagulation), that the utilization of
clips or a detachable snare in advance of the resection may be
beneficial. It should be noted that if one elects to use electrocau-
tery after initial snaring, that they should be careful to prevent full
thickness injury at the site.
Delayed hemorrhage occurs when the retained scar from a
polyp site separates prematurely from the coagulation bed, lead-
ing to hematochezia. This type of bleeding usually occurs within
2–15 days of the after the procedure, typically within the first
7–10 days. Postpolypectomy bleeding can be significant enough
to require in-hospital fluid resuscitation and potential therapeu-
tic intervention. These patients usually have arterial bleeding, and
pass bright red bloody bowel movements spaced at close intervals
(i.e., 30–60 minutes).(25) The active bleeder (after appropriate
resuscitation) may benefit from a prompt colonoscopy without
bowel prep to identify the site of bleeding. If the bleeding site is
located it can be treated with judicious multipolar cautery, injec-
tion of epinephrine solution, detachable snare, or placement of
hemoclips. Often times, it is clinically difficult to determine if the
hemorrhage has ceased because the hematochezia may continue
for several hours afterwards. If the hemorrhage appears persistent,
despite local efforts or if the patient needs urgent intervention,
the location of the active bleeding may be identified with a tagged
RBC scan.(26) If the scan is positive, arteriography can confirm
the bleeding location and offers potential treatment modalities
for either selective arterial vasopressin infusion, or emboliza-
tion. The choice depends upon the patient’s clinical history and
presentation as well as the skill and experience of the radiogra-
pher. Figure 12.1 describes the authors’ and editors’ management
algorithm for postpolypectomy bleeding.
Prophylactic techniques during polypectomy may decrease the
incidence of postpolypectomy bleeding.(27) During the tech-
nique of taking a large polyp, some endoscopists use a saline inter-
mucosal lift or an epinephrine solution injection into the stalk of
the polyp in efforts to control hemostasis. Detachable snares and
clips have also been used with a similar goal in mind. Although
successful, even in skilled hands, clips may slip or transmit cur-
rent if cautery makes contact with the metal. Detachable snares
may slip from their initial position, or if pulled too tight can cut
through the base of the polyp, leading to the problem that one is
trying to prevent. The absence of national guidelines concerning
the prophylactic approaches to postpolypectomy bleeding, makes
each endoscopist responsible for evaluating the clinical situation
and being cognitive of his level of expertise.
Another factor in postpolypectomy hemorrhage relates to the
management of patients on antiplatelet agents or anticoagulants.(28,
29) The American Society for Gastrointestinal Endoscopy has rec-
ommended that aspirin need not to be stopped before polypectomy
as there is insufficient evidence supporting an increased risk with
its utilization.(30) However, many endoscopists will hold a patient’s
aspirin for 7 days if their indication for taking aspirin is weak. If the
patient can tolerate it, the author and editors withhold clopidogrel
(Plavi
®
, Sanofi-Aventic, Bridgewater, NJ) for a minimum of 7 days
before the colonoscopy and hold warfarin for a minimum of 3 days
before the procedure and check a prothrombin time (PT) before the
colonoscopy.(31) Therapeutic procedures are usually safe with an
INR of <2.(32) If the patient’s INR is above this level, the procedure
may be delayed until the INR is lower or if the anticipated need
for a therapeutic procedure is low (e.g., a screening indication) a
diagnostic procedure may be performed with the understanding
that if significant lesions are identified, therapeutic maneuvers (i.e.,
biopsy or polypectomy) will be deferred.
Patients who cannot tolerate reversal of anticoagulation (a
determination usually made by the patient’s cardiologist or neu-
rologist) can often be managed with a bridging with enoxaparin
sodium (Lovenox
®
, Sanofi-Aventic, Bridgewater, NJ) or consid-
ered for alternate procedures such as CT colography.
Recommendations for restarting anticoagulation or antiplatelet
agents postpolypectomy are difficult clinical decisions that must
be based on the patient’s risk-benefit ratio, (i.e., the risk of stroke,
or coagulation of cardiac stents versus the risk of postpolypectomy
hemorrhage). Unfortunately, there is little prospective data to sup-
port recommendations. The author and editors restart these medi-
cations at their normal daily dose the day following a diagnostic
procedure. Recommendations after a polypectomy depend on the
size and number of polyps removed and the level of anticoagula-
tion at the start of the procedure. Warfarin is usually started at the
normal daily dose, 1–5 days after the procedure, while clopidogrel
is restarted 1 week after the procedure.(32) Anticoagulant recom-
mendations are summarized in Table 12.1.
Perforation
The most serious complication of colonoscopy is overt perfo-
ration.(40) Perforation can result from mechanical forces dur-
ing colonoscopic insertion or from barotrauma during colonic
insufflation, or during the process of polyp removal. Perforation
Table 12.1 Anticoagulant recommendations.
Drug
Before Procedure
After Diagnostic
Procedure
After Therapeutic
Procedure
Aspirin
Continued or
stopped 7 days
prior
Continued or
started day of
procedure
Restarted 5–7 days
after
Clopidogel
Held for > 7 days
prior
Restarted
day after
procedure
Restarted 7 days
after
Warfarin
Held for > 3 days
prior and check
PT
Restarted
day after
procedure
Restarted 1–5 days
after
Note: PT Prothrombin time.
improved outcomes in colon and rectal surgery
occurs in 0.6% to 0.8% of diagnostic procedures and 0.5% to
3% (See Table 12.2).(41, 42) Perforation is diagnosed during the
procedure by observation of extraluminal fat or other intraab-
dominal contents (e.g., small bowel, liver) via the colonoscope.
These patients usually report immediate pain and demonstrate
signs of peritoneal irritation. Patients that develop symptomatol-
ogy postprocedure vary from, asymptomatic free intraabdomi-
nal air, a tense abdomen, or florid peritonitis and sepsis. Patients
presenting with localized symptoms can be observed and treated
with intravenous fluids, antibiotics, and bowel rest.(43) Patients
who present with or develop signs of generalized peritoneal irri-
tation during observation, should receive a laparotomy. Repair
or resection of the perforation is performed with or without a
diverting ostomy. An algorithm for the management of colono-
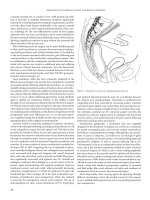
scopic perforations is presented in Figure 12.2.
It is important to remember that a perforation can come from
an unsuccessful encounter with the colon wall with the tip,
the deflection bend, and/or the shaft of the colonoscope. The
inexperienced examiner can drive the tip of the scope through
a large diverticulum; or may, while advancing the scope form a
significant loop, allowing the shaft of the scope to make a lacera-
tion in the bowel wall, away from the tip of the scope, which may
go on unrecognized. Significant clinical experience, along with
judgment and good technique, serve as the best preventative tools
against a perforation.(50, 51)
MISCELLANEOUS COMPLICATIONS
Though the major complications associated with colonoscopy
are hemorrhage and perforation, there exists a large body litera-
ture that has reported rarely encountered complications. These
include incarceration of the colonoscope within an inguinal
hernia, (52) cecal volvulus with subsequent perforation, (53)
ischemic colitis, (54) aortic thrombosis in a patient with Bechcet’s
syndrome, (55) and splenic injury (56).
There have been approximately 59 clinical reports which
detail 67 cases of splenic injury, following diagnostic or thera-
peutic colonoscopy.(56, 57) The authors note the most likely
etiology of splenic injury is related to the performance of the
procedure rather than any therapeutic maneuver. It is theorized
that the mechanism of injury is thought to be excessive traction
on the splenocolic ligament or adhesions. This theory has been
confirmed by laparotomy in several of the reported cases. It is
interesting to note that in most of the injuries, the endoscopists
felt the procedure had been performed without difficulty. The
presentation for these injuries span between 6–24 hours, and
vary from vague abdominal pain of the left upper quadrant,
with mild orthostatic hypotension, and a decreased hematocrit
to severe hypotension and shock. It is suggested that an abdomi-
nal CT scan is the most helpful diagnostic test as it may show a
splenic laceration, with free intraperitoneal blood or a splenic
hematoma. Most of the cases, required surgery, and the patient’s
overall condition dictated whether an emergency intervention
was necessary. Observation and conservative management was
rarely successful. Awareness of the potential for splenic injury
during colonoscopy is important as it may help avoid any delay
in diagnosis. Unfortunately, these cases are so rare that no iden-
tifiable risk factors have been documented that potentially could
help prevent this complication.
Patients with reducible hernias, merit additional attention by
the endoscopist who must consider the benefits of a diagnostic or
therapeutic procedure versus the potential for injury or the delay
Table 12.2 Colonoscopy perforation rates.
Author, Year
(Reference) Colonoscopies, n Perforations, n (%) Setting
Lo and Beaton,
1994 (44)
26,708 12 (0.045) University,
teaching
Farley et al.,
1997 (43)
57,028 43 (0.075) Mayo clinic,
teaching
Anderson et al.,
2000 (44)
10,486 10 (0.19) Mayo clinic,
teaching
Araghizadeh
et al., 2001 (45)
34,620 31 (0.09) Ochsner
Clinic,
teaching
Korman, et al.,
2003 (46)
116,000 37 (0.03) ASC, private
practice
Cobb et al.,
2004 (47)
43,609 14 (0.032) Teaching
Lqbal et al.,
2005 (48)
78,702 66 (0.084) Mayo clinic,
teaching
Levin et al.,
2006 (49)
16,318 15 (0.09) Kaiser
Permanente
Figure 12.2 Management of colonoscopic perforation.
transanal endoscopy
until the hernia is repaired. For patients with reducible inguinal
hernias, the procedure can be performed with general pressure
on the hernia sac during the colonoscopy or with the utilization
of a truss. Should the colonoscope become incarcerated in an
inguinal hernia, a “pulley” technique has been described where a
relatively large easily graspable colonoscope loop is created within
the hernia sac and then is withdrawn over the pulley “hand” one
limb at a time.(52) Development of a cecal volvulus, may occur
with a hypermobile cecum.(53)
Postpolypectomy Syndrome
The postpolypectomy syndrome is believed to result from a
transmural burn to the colonic wall and usually presents with
a syndrome similar to that of diverticulitis.(58) This represents
the second most common polypectomy complication with rates
ranging from 0.5% to 1%.(59) Usually this complication occurs
in the thin right-sided bowel after removal of a sessile polyp or
performance of a hot biopsy. It is felt that the factors leading to
this syndrome include the utilization of high cumulative quality
and longer duration of electrocautery. It is also possible for the
electric current to enter the mucosa opposite the polyp if the tip
of the polyp is allowed to touch the opposing wall during the
application of the cautery. Moving the polyp back-and-forth dur-
ing cautery may dissipate the current. This syndrome is usually
seen in sessile polyps that are >2 cm in diameter.(60)
The patients may present within hours of the procedure but
some may not show some significant symptomology until 5–6
days postprocedure. Patients usually present with pain which
may or may not include fever, an elevated white count, and local-
ized tenderness. Initial studies include plain x-rays or a CT scan,
which confirm by definition an absence of free air. Patients are
started on broad spectrum antibiotics and bowel rest, then most
importantly patients are examined over appropriate intervals to
make sure there is no progression from the localized tenderness
to frank peritonitis.(61) Fortunately, most of the patients that are
properly managed progress to recovery without surgical inter-
vention, and they can be discharged when their pain has resolved,
and their white blood cell count has normalized. Prevention of
postpolypectomy syndrome aided by adherence to the previously
described guidelines for a safe polypectomy.
PROCTOSCOPIC PERFORATIONS
Proctoscopic perforations are rare, serious complications of
intestinal endoscopy.(62) Nelson et al. reported only three per-
forations in 16,325 proscopic exams.(63) The injury most com-
monly occurs when the bowel wall has been weakened by disease
such as colitis, a rectal tumor, or constricting lesion. The line of
force during insertion guides the scope to the anterior rectal wall
where the usual site of injury is located between the peritoneal
reflection and the rectosigmoid junction.(64) The perforation
is usually recognized as the scope enters the peritoneal cavity or
the endoscopist encounters the perirectal fat or local bleeding.
(52) Significant rectal trauma with a rectal perforation requires
operative treatment. Most authors believe that delayed treatment
increases the mortality from 8% to 20%.(65) The major mor-
bidity and mortality are associated with the fact that the proxi-
mal colon typically is not mechanically cleansed. The treatment
involves resection of associated pathology, repair of the perfora-
tion site, rectal washout, pelvic drainage, and a diverting colos-
tomy (see chapter 35).(66)
SUMMARY
Training, experience, and conservative technique help to mini-
mize complications associated with endoscopic procedures of the
colorectum. Prompt recognition and appropriate management of
complications help to minimize the patient’s morbidity.
REFERENCES
1. Rogers BHG Silvis SE, any Bel OT et al. Complications of
Flexible Fibroptic Colonoscopy and Polypectomy. Gastropint
Endosc 1975; 22: 73–7.
2. Harewood GC, Sharma VK, de Garmop. Impact of
Colonoscopy Preparation Quality on Detection of Suspected
Colonic Neoplasia. Gastroinest Endosc 2003; 58: 76–9.
3. Aslinia F, Uradomo L, Steele A et al. Quality Assessment of
Colonoscopic Cecal Intubation: Analysis of six years of con-
tinuous practice at University Hospital. An J Gastrointestal
2006; 101: 721–31.
4. Froehich F, Wietilsbach V, Gonvers JJ et al. Impact of Colon
and Colonic Cleansing on Quality and Diagnostic yield of
Colonoscopy; The European panel of appropriateness of
gastrointestinal endoscopy European Multi-Center Study,
Gastroenterol Endosc 2005; 61; 378–84.
5. Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact
of bowel preparation on the efficacy and cost of colonoscopy.
ANJ Gastroenterol 2002; 97: 1696–700.
6. Hsu CW, Imperiale TF. Meta-analysis and cost comparison of
polyethylene glycol lavage vs. sodium phosphate for colonos-
copy preparation. Gastrointest Endosc 1998; 48: 276–82.
7. Harewood GC, Wiersema MJ, Melton LJ III. A prospective con-
trol assessment of factors influencing the acceptance of screen-
ing colonoscopies. An J Gastroenterol 2002; 97: 3186–94.
8. Lieberman DA, Holb J, Eise G et al. Utilization of colonoscopy
in the U.S.: Results from a national consortium. Gastroinest
Endosc 2005; 62: 875–83.
9. HSU CW, Imperiale TF. Met-analysis and cost comparisons
of polyethylene glycol lavage vs. sodium phosphate for colon
preparation. Gastro Endosc 1998; 48: 276–82.
10. Diab FH, Marshal JB. The palatability of five colonic lavage
solutions that’s in Aliment Pharmacol Ther 1996; 10: 815–19.
11. Hookey LC, Depew WT, Vonner SJ. Combined low volume
polyethylene glycol solution plus stimulant laxative vs. stan-
dard volume polyethylene glycol solution: A perspective,
randomized study of colon cleansing before colonoscopy.
Can J Gastroenterol 2006; 20: 101–5.
12. Clarkston WK, Smith RJ. The use of golytely and docolax in
combination in outpatient colonoscopy. J Slin Gastroenterol
1993; 17: 146–8.
13. Sharma VK, Steinberg EN et al. Randomized control study
of pretreatment with magnesium citrate and the quality
of colonic preparation with polyethylene glycol electrolyte
lavage solution. Gastrointest Endosc 1997; 46: 541–3.
14. Brady CE, DiPalma JA, Pierson WP. Golytely lavage – Is meto-
clopramide necessary? AM J Gastroenterol 1985; 80: 180–4.