Improved Outcomes in Colon and Rectal Surgery part 19 docx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (544.86 KB, 10 trang )
7
Hemorrhoidal surgery
Dan R Metcalf and Anthony J Senagore
CHALLENGING CASE
A 38-year-old man presents to your office after receiving an urgent
hemorrhoidectomy 1 year previously. He had continued pain and
bleeding with bowel movements. He feels his anus is “too tight”
and continues to be symptomatic despite attempts at dilatation,
daily fiber, and stool softeners. Examination reveals three healed
incisions and anal stenosis. The anus will only admit the tip of
your finger with discomfort.
CASE MANAGEMENT
The patient has anal stenosis due to removal of an excessive
amount of anoderm with his surgery. The management of refrac-
tory posthemorrhoidectomy stenosis usually requires some type
of flap repair. The choice of flap repair selected will depend on the
degree of stenosis and the surgeon’s experience. The editors have
found one or multiple house advancement flaps to be the most
common option chosen in our practice.
INTRODUCTION
Few diseases are more chronicled in human history than sympto-
matic hemorrhoidal disease.(1, 2) Citations of hemorrhoidal dis-
ease have been noted in historic texts dating back to Babylonian,
Egyptian, Greek, and Hebrew cultures.(1, 2) A multitude of treat-
ment regimens have been offered including anal dilation, vari-
ous topical liniments, and the often feared red hot poker.(3, 4)
Although few people have died of hemorrhoidal disease, some
patients wish they had particularly after therapy and this fact led
to the beatification of St. Fiachre, the patron saint of gardeners
and hemorrhoidal sufferers.(5) This chapter will guide the practi-
tioner to a more humane approach to hemorrhoidal disease with
the emphasis on cost-effectiveness and obtaining superior short
and long-term outcomes.
ANATOMY/ETIOLOGY
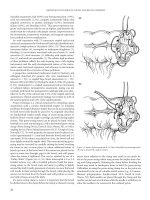
Hemorrhoidal cushions are located within the submucosa of the
upper anal canal and are a normal component of the anorectal
anatomy. These cushions are composed of blood vessels, smooth
muscle (Treitz’s muscle), connective tissue, and elastic tissue.(6)
(Figure 17.1) Anatomically, the hemorrhoidal cushions appear
with marked predictability in the right anterior, right posterior, and
left lateral positions, although there may be intervening secondary
hemorrhoidal complexes which obscure this classic anatomy.(6)
The blood supply to the anal cushions is derived from the superior
rectal artery, a branch of the inferior mesenteric; the middle rectal
arteries arising from the internal iliac arteries; and the inferior rec-
tal arteries arising from the pudendal arteries. The venous drainage
transitions from the portal venous system above the level of the
dentate line to the systemic venous system below this level.(6)
Anal cushions contribute to the maintenance of anal continence
and allow the anal canal to dilate during defecation without tearing.
(6) Consequently, in some patients hemorrhoidectomy may result
in various degrees of incontinence or leakage. Hemorrhoidal disease
occurs as the result of abnormalities within the connective tissue
of these cushions producing bleeding with or without prolapse of
the hemorrhoidal tissue.(7) This can occur as the result of exces-
sive straining, chronic constipation, or low dietary fiber.(8) A clear
understanding of the pathophysiology is important when consider-
ing therapeutic interventions. At the earlier stages of disease, when
the major manifestation is transudation of blood through thin
walled damaged vascular channels, ablation of the vessels should
be adequate. In contrast, when there is significant disruption of the
mucosal suspensory ligament in the late stages of the disease, a tech-
nique resulting in fixation of the mucosa to the underlying muscu-
lar wall is necessary for effective therapy.(9) Internal anal sphincter
dysfunction may play a role, as a number of investigators have dem-
onstrated increased internal anal sphincter tone in patients with
hemorrhoidal disease.(10–12) In reality, a combination of all of the
above factors are important for the ultimate development of large
prolapsing hemorrhoids.
Hemorrhoids are divided into two groups, external and internal.
External hemorrhoids are located distal to the dentate line and are
covered by modified squamous epithelium (anoderm). In contrast,
internal hemorrhoids are covered by columnar or transitional epi-
thelium and are located proximal to the dentate line. Internal hem-
orrhoids are further divided into grades based on size and clinical
Figure 17.1 Sagital section of anal cushion showing internal and external
hemorroids.
hemorrhoidal surgery
symptoms. Grade I internal hemorrhoids bulge into the lumen and
produce bleeding; Grade II internal hemorrhoids protrude with
bowel movements and reduce spontaneously; Grade III internal
hemorrhoids protrude spontaneously or with bowel movements
and require manual reduction: Grade IV internal hemorrhoids are
permanently prolapsed and irreducible.(13) Mixed hemorrhoids are
those with components of both internal and external hemorrhoids.
Although there tends to be a correlation between symptoms and the
grade of hemorrhoidal disease, therapeutic decisions should not be
based solely on these criteria. As will be outlined later, it is impor-
tant to consider the relative role of internal hemorrhoidal tissue in
addition to external hemorrhoidal skin tagging when choosing a
modality for complete resolution of the patient’s symptoms.(7)
CLINICAL EVALUATION
Among the most common symptoms associated with hemor-
rhoidal disease are bleeding, protrusion, and pain. However,
Mazier reported on a series of 500 patients with anorectal com-
plaints they associated with their hemorrhoids and ultimately,
only 35% of patients were found to have any significant hemor-
rhoidal disease at all.(14) Hemorrhoidal bleeding is characteristi-
cally painless and bright red and seen on the toilet paper or in
the commode after a bowel movement. However, more vigorous
bleeding can occur as the hemorrhoids enlarge, particularly in
advanced stages when a portion of the complex is fixed externally,
allowing the blood to drip or spurt into the commode. Generally,
prompt reduction of the protruding mass will alleviate this symp-
tom. Acute thromboses of internal or external hemorrhoids are
usually associated with a palpable mass and severe pain. These
patients typically present with extreme discomfort and on clinical
examination the diagnosis is frequently obvious.
Examination of the patient with hematochezia should be tai-
lored by the age of the patient and include sufficient investigations
to rule out a proximal source of bleeding such as inflammatory
bowel disease or neoplasia. Hemorrhoidal bleeding as a cause of
anemia is an uncommon occurrence with an incidence of 0.5 per
100,000 per year.(15) Consequently, hemorrhoids should not be
dismissed as the cause of iron deficiency anemia.
The authors examine patients in the left lateral position with
the knees drawn up toward the chest as high as possible. This
approach allows relative patient comfort and the ability to clearly
inspect the perianal skin, perform anoscopy, and proctosig-
moidoscopy. A careful digital examination of the anal canal and
distal rectum should be performed with the addition of prostate
examination in male patients. Examination with an anoscope is
essential to adequately inspect the hemorrhoidal tissue and anal
canal. Inspection of the three common locations for hemorrhoids
should be performed with documentation of the size, friability,
and ease of prolapse. Documentation of anal pathology should
be described by anatomic position (anterior, posterior, etc.,) to
avoid confusion regarding the position in which the patient was
examined. Upon completion of this portion of the exam, a deci-
sion should be made regarding the need for more proximal evalu-
ation of the colon and rectum. However, rigid proctoscopy should
be the minimum in all patients. After appropriately grading the
hemorrhoidal disease, discussion can ensue with the patient
regarding the various treatment options.
NONEXCISIONAL OPTIONS
The majority of patients with hematochezia attributable to hem-
orrhoids can be managed conservatively without surgical inter-
vention. Dietary and lifestyle modification, reduction of straining
with defacation, sclerotherapy, infrared coagulation, and rubber
band ligation are described in chapter 18. These options are
considered before considering excisional options.
EXCISIONAL HEMORRHOIDECTOMY
Approximately 5–10% of patients will require surgical manage-
ment of their hemorrhoids.(16) Excisional hemorrhoidectomy
should be considered in those patients with extensive sympto-
matic disease who have failed or are not candidates for medical
and nonexcisional options. In addition to this, the customary
indications for hemorrhoidectomy include frequent or per-
sistent prolapse requiring manual reduction resulting in dis-
comfort and anal seepage, and hemorrhoids associated with
conditions such as fissure, fistula, ulceration, or extensive anal
skin tags. The final indication for excisional hemorrhoidectomy,
although debatable, is the development of acutely thrombosed
and gangrenous internal hemorrhoids. It is apparent however
that similar full excisional hemorrhoidectomy can be per-
formed using standard closed hemorrhoidectomy techniques
without undue complications. Specifically, the risk of steno-
sis appears unwarranted if careful technique is used and the
maximum amount of anoderm is preserved with skin bridges
between excision sites. In the case of limited external hemor-
rhoidal thromboses, surgical excision may also be warranted
for more rapid pain relief and avoidance of a residual skin tag.
(17–20) Limited external thromboses can be easily managed in
the office setting with local anesthesia and complete excision
with or without skin closure.(Figure 17.2)
Options for excisional hemorrhoidectomy include the follow-
ing techniques: Milligan-Morgan hemorrhoidectomy; Ferguson
Closed hemorrhoidectomy; Whitehead hemorrhoidectomy; sta-
pled hemorrhoidectomy; and variations of the Milligan-Morgan
and Ferguson techniques using alternative energy devices. The use
of lasers for excisional hemorrhoidectomy offers no advantage
and in fact causes delayed healing, increased pain, and increased
cost.(21) The procedures are usually performed in the operating
room after minimal preoperative bowel preparation. The choice
of anesthetic is typically left to the anesthesiologist and patient,
however local anesthesia supplemented by the administration of
intravenous narcotics and propofol is very effective and short act-
ing. The use of spinal anesthesia, although effective, may increase
the risk of postoperative urinary retention do to a higher intraop-
erative administration of intravenous fluids.
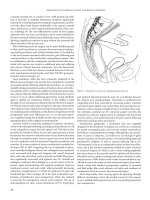
The Milligan-Morgan hemorrhoidectomy (Figure 17.3), which
is widely practiced in Europe, was originally described in 1937
and its efficacy has subsequently been documented in many
series.(22–24) This technique involves resection of the internal
and external hemorrhoid complex, ligation of the arterial pedicle,
and preservation of the intervening anoderm.(22) The distal ano-
derm and external skin are left open to heal by secondary inten-
tion to minimize the risk of infection. This technique has been
proven to be a safe and effective means for managing advanced
hemorrhoidal disease.(22) However, the open wounds typically
7
improved outcomes in colon and rectal surgery
take 4–8 weeks to heal and can be a cause of considerable discom-
fort and prolonged morbidity after this procedure.
The closed Ferguson hemorrhoidectomy (Figure 17.4) was
proposed as an alternative to the Milligan-Morgan technique
and enjoys a similar large body of evidence regarding its safety
and efficacy.(17–20) This technique utilizes an hourglass-shaped
excision of the entire internal and external hemorrhoidal com-
plex (centered at the midportion of the anoderm), preservation
of the internal and external anal sphincters, and primary closure
of the entire wound. Occasionally, it is necessary to undermine
flaps of anoderm and perianal skin to allow excision of inter-
mediate hemorrhoidal tissue, while preserving the bridges of
anoderm between pedicles. This technical adjustment will avoid
postoperative strictures.
The Whitehead hemorrhoidectomy (Figure 17.5), described in
1882, involves a circular incision at the level of the dentate line
with subsequent circumferential excision of the hemorrhoidal
tissue and relocation of the dentate line which is often a com-
ponent of prolapsing hemorrhoids.(25) Although this technique
had a long period of widespread use in the United Kingdom, it
was subsequently largely abandoned because of the high rates of
mucosal ectropion and anal stricture.(26–29) However, using a
modification of the original technique it has enjoyed renewed
support by some surgeons in the United States with minimal
stricture rates and no occurrences of mucosal ectropion.(30–31)
Despite these promising reports, the Whitehead procedure is
technically demanding because of the need to accurately identify
the dentate line and relocate it to its proper location.
Stapled hemorrhoidopexy is a relatively novel technique
with growing acceptance as an alternative to excisional hemor-
rhoidectomy for the treatment of grade III and grade IV hemor-
rhoids. The technique, as described in 1998 by Antonio Longo (32),
involves circumferential excision of the mucosa and submucosa
above the hemorrhoids using a circular stapler resulting in reloca-
tion and fixation of the internal hemorrhoids. Briefly, a circular
anoscope is inserted into the anal canal to reduce the prolapsing
tissue and allow placement of a circumferential purse-string suture
4 cm proximal to the dentate line into the mucosa and submucosa.
A 33 mm hemorrhoidal circular stapler (EthiconEndo-Surgery;
PPH03) with the anvil fully extended is then advanced proximal to
the purse-string which is then gently tightened around the shaft of
the stapler. The free ends of the suture are then threaded through
the lateral channels of the stapler housing to provide traction on
the purse-string as the stapler is closed and advanced into the anal
canal. Once in position the stapler is closed and fired. The staple
line should be inspected for hemostasis and bleeding controlled
with an absorbable suture.(Figure 17.6) Numerous randomized
controlled trials comparing stapled hemorrhoidopexy to conven-
tional hemorrhoidectomy have substantiated the benefits of sta-
pled hemorrhoidectomy, namely reduced operating room time,
less pain and analgesic use, and earlier return to work with similar
symptom control.(33–36) In a prospective, randomized, controlled
multicenter trial comparing stapled hemorrhoidopexy and closed
hemorrhoidectomy Senagore et al. reported less pain, less pain at
first bowel movement, less analgesic use and similar symptom con-
trol using stapled hemorrhoidopexy in which 88% of patients were
treated as outpatients.(36) As demonstrated in a recent systematic
review of 25 randomized, controlled trials comparing stapled hem-
orrhoidopexy to conventional hemorrhoidectomy, stapled hem-
orrhoidopexy is a safe and effective procedure for the treatment
symptomatic hemorrhoids with superior short-term outcomes.
(37) This review indicates that the incidence of recurrent hem-
orrhoids is significantly higher at one or more years after stapled
hemorrhoidopexy (5.7% vs. 1%), however, the overall recurrence
or persistence of hemorrhoidal symptoms was similar between the
groups (SH vs. conventional: 25.3% vs. 18.7%, p = 0.07).(37) In a
retrospective review of 291 patients submitted to stapled hemor-
rhoidopexy with grade III and grade IV hemorrhoids, the overall
recurrence rate after a minimum follow-up of 5 years was 18.2%.
(38) They showed a tendency for higher recurrence in grade IV
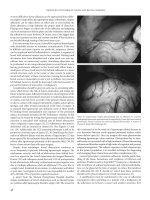
Figure 17.2 Excision of thrombosed external
hemorrhoid.
7
hemorrhoidal surgery
Figure 17.3 Open (Milligan-Morgan) hemorrhoidectomy. (A) External hemorrhoids grasped with forceps and retracted outward. (B) Internal hemorrhoids grasped
with forceps and retracted outward with external hemorrhoids. (C) External skin and hemorrhoid excised with scissors. (D) Suture placed through proximal internal
hemorrhoid and vascular bundle. (E) Ligature tied. (F) Tissue distal to ligature is excised. Insert depicts completed three bundle hemorrhoidectomy.
(A) (B) (C)
(D) (E) (F)
Figure 17.4 Modified Ferguson excisional hemorrhoidectomy. (A) Double ellipitical incision made in mucosa and anoderm around hemorrhoidal bundle with a
scalpel. (B) The hemorrhoid dissection is carefully continued cephalad by dissecting the sphincter away from the hemorrhoid. (C) After dissection of the hemorrhoid
to its pedicle, it is either clamped, secured, or excised. The pedicle is suture ligated. (D) The wound is closed with a running stitch. Excessive traction on the suture is
avoided to prevent forming dog ears or displacing the anoderm caudally.
(A) (B) (C) (D)
7
improved outcomes in colon and rectal surgery
Figure 17.5 Whitehead hemorrhoidectomy. (A) Suture placed through proximal internal hemorrhoid for orientation. Excision started at dentate line and continued to
proximal bundle. (B) Internal hemorrhoidal tissue excised above ligated bundle. (C) Vascular tissue excised from underside of elevated anoderm. (D) End of anoderm
reaproximated with sutures to original location of dentate line. (E) Completed procedure.
(A) (B) (C) (D) (E)
hemorrhoids with a significantly higher reoperation rate.(38) In
some instances this was thought to be related to inappropriate
patient selection. In a large series reported by Jongen et al. stapled
hemorrhoidopexy with good patient selection was associated with
a low rate (3.4%) of reoperation for persistent or recurrent hemor-
rhoidal prolapse.(39)
The data clearly indicate that stapled hemorrhoidopexy is a
safe and effective option to treat symptomatic hemorrhoids with
superior short-term outcomes. Although a higher rate of late
recurrence is reported with this technique in the current literature,
an appropriately designed randomized trial with adequate power
and longer follow-up is needed to ultimately define the durability
of stapled hemorrhoidopexy. Patient selection for stapled hemor-
rhoidopexy may also play an important role in short and long term
outcome analysis.
Improper technique with PPH has led to significant complica-
tions. Placement of the purse-string suture too high (cranial) or
too deep has led to a full thickness excision and occasional anas-
tomotic leaks with subsequent sepsis and some deaths. Placement
of the purse-string suture too low may lead to impaired conti-
nence (inclusion of sphincter muscle in staples) or pain. Chronic
pain following PPH may respond to anti-inflammatory agents or
time. Some success in refractory patients has been obtained with
removal of residual staples (usually done under anesthesia) or
injection of long duration steroids.
In the quest to provide patients with the benefit of less post-
operative pain, alternative devices such as the Harmonic Scalpel®
and LigaSure™ have recently been used to perform excisional
hemorrhoidectomy. There have been four randomized, controlled
trials published in an attempt to assess the efficacy of Harmonic
Scalpel® hemorrhoidectomy.(40–43) Although all studies indicate
that the harmonic scalpel is an effective alternative with a simi-
lar complication profile to more conventional methods, there is
inconsistency regarding the short term benefits such as postop-
erative pain across these studies. Multiple randomized, controlled
trials evaluating LigaSure™ hemorrhoidectomy to conventional
techniques have been performed; (44–50) Most of these stud-
ies demonstrate a reduction in postoperative pain and operating
time when using the LigaSure™. A multicenter, prospective, ran-
domized study by Altomare et al. showed significantly less pain
12 hours after defacation, lower analgesic requirements, and faster
return to work and normal activity with no difference in early or
late complications.(48) Both instruments have been shown to be a
safe and effective alternative to conventional hemorrhoidectomy.
However, the added cost, conflicting short term outcomes, and
lack of long term follow-up prelude recommendations for their
routine use. At the present time, conventional methods of exci-
sional hemorrhoidectomy remain the “gold standard”.
POSTOPERATIVE COMPLICATIONS
Regardless of the excisional technique used for treatment of
advanced hemorrhoidal disease, the key to effective patient
management is avoidance of postoperative complications.
Pain
The anoderm has a rich supply of sensory nerves and pain
arises from involvement of the anoderm below the dentate line.
Posthemorrhoidectomy pain is associated with reflex spasm of the
urethral and anal sphincter muscles. Spasm of these muscles leads
to difficulty voiding and urinary retention and difficulty with evac-
uation and constipation. Both of which are covered later. From the
patient’s perspective, pain is the most feared element of the pro-
cedure. A variety of analgesic regimens have been recommended,
usually consisting of a combination of oral and parenteral nar-
cotics.(51–55) Local anesthetic agents such as 0.5% bupivacaine
solution may provide analgesia for up to 6–8 hours after surgery.
The use of ketorolac has demonstrated considerable efficacy in
managing posthemorrhoidectomy pain.(51) Alternative adminis-
tration routes for narcotics either by patch or subcutaneous pump
have been successful in controlling pain, however due to the risk
of narcotic respiratory depression, administration by these routes
can be risky in the outpatient setting.(53–55) The most appropri-
ate regimen following outpatient hemorrhoidectomy appears to
be intraoperative use of ketorolac, sufficient doses of oral narcotic
analgesics for home administration, and supplementation of the
narcotics by an oral nonsteroidal medication.
Urinary Retention
Urinary retention is a frequent postoperative complication follow-
ing hemorrhoidectomy with an incidence from 1–52%.(16, 56–58)
7
hemorrhoidal surgery
A variety of strategies have been used to treat this problem includ-
ing parasympathomimetics, alpha-adrenergic blocking agents, and
sitz baths.(59, 60) However, prevention seems to be the best strategy
by limiting perioperative fluid administration to 250 ml, avoiding
the use of spinal anesthesia and anal packing, and prescribing an
aggressive oral analgesic regimen.(56) Elderly men with obstructive
uropathy are at increased risk for urinary retention. If catheteriza-
tion becomes necessary, intermittent catherization under sterile
conditions is the option of choice. Urologic consultation may be
sought for patients with persistent symptoms of bladder outflow
obstruction.
Hemorrhage
Early postoperative bleeding (<24 hours) occurs in approxi-
mately 1% of cases and represents a technical error requir-
ing return to the operating room for repair of the offending
wound.(61) Occasionally bleeding may continue undetected,
with blood accumulating in the capacious rectum. The first
Figure 17.6 Stapled anoplasty (procedure for prolapse and hemorrhoids [PPH]). (A) Retracting anoscope and dilator inserted. (B) Monofilament pursestring suture
(eight bites) placed using operating anoscope approximately 3–4 cm above anal verge. (C) Stapler inserted through pursestring. Pursestring suture tied and ends of
suture manipulated through stapler. (D) Retracting on suture pulls anorectal mucosa into stapler. (E) Stapler closed and fired. (F) Completed procedure.
(a)
(d)
(b)
(e)
(c)
(f)
7
improved outcomes in colon and rectal surgery
sign of this complication may be pallor, tachycardia, and hypo-
tension. This patient requires fluid resuscitation and a return
to the operating room for suture ligation or diathermy control
of the bleeding site.
Bleeding from the staple line when using a PPH can be con-
trolled by oversewing the bleeding point of the staple line. This is
less common with the second generation 33 mm hemorrhoidal
circular stapler (Ethicon endosurgery; PPH03) which has a shorter
stapler height.
Delayed hemorrhage occurs in 0.5–4% of cases of excisional
hemorrhoidectomy and often occurs at 5–10 days postoperatively.
(62–64) The etiology has been held to be early separation of the
ligated pedicle before adequate thrombosis in the feeding artery can
occur.(65) Hemorrhage in this situation is frequently significant and
requires some method for control of ongoing hemorrhage. Options
include return to the operating room for suture ligation, or tampon-
ade at the beside by Foley catheter or anal packing.(66–68) The out-
come after control of secondary hemorrhage is generally good with
virtually no risk of recurrent bleeding. It may be helpful to irrigate
out the distal colon and rectum at the time of intraoperative control
of hemorrhage to avoid confusion in the postoperative period.
Constipation and Fecal Impaction
Fecal impaction is a distressing complication of excisional hemo-
rrhoidectomies. Postoperative pain, the patient’s fear of pain
associated with defecation, and the constipating effects of nar-
cotics are contributing factors. Hence, providing adequate anal-
gesia and patient reassurance are important. Patients should be
instructed on the importance of adequate hydration. Many sur-
geons also recommend bulking agents and/or laxatives (e.g., pol-
yethylene glycol solution), and topical anesthetics before a bowel
movement to facilitate evacuation.(69) When fecal impaction
is identified, early, simple irrigating enemas may help clear the
anorectum of impacted feces. If the impactions are soft, an oral
cleansing regime (17 gm of polyethelyene glycol solution in 4 oz
of water every 15–20 minutes until the impaction is cleared) may
be utilized.(70) In more severe cases, manual disimpaction under
conscious sedation or general anesthesia may be necessary.
Infection
Infection of the urinary tract may result from either stasis of urine
or instrumentation of the urinary tract. A 3% incidence has been
reported from one institution following hemorrhoidal surgery.
(16) A urine culture should be obtained before administration of
appropriate antibiotics.
The anoderm harbors an abundance of potentially pathologic
bacterial microorganisms. Despite this, infective complications
after hemorrhoidectomy are infrequent. Bacteremia and sepsis
have been documented after hemorrhoidectomy, but abscess
formation is rare unless a hematoma becomes infected. Isolated
liver abscesses have been reported, and this very rare complica-
tion should be considered in patients with postoperative fever. It
is usually currently identified by abdominal CT scan.
Another potential infectious complication is postoperative pel-
vic sepsis. This can occur after any anorectal procedure includ-
ing rubber band ligation and excisional hemorrhoidectomy.
Classic findings include anorectal pain, fever, and difficulty
with urination. A high index of suspicion is required as delay in
diagnosis can have fatal consequences. As described in chapter
18, patients with suspected pelvic sepsis require resuscitation,
diagnostic evaluations (pelvic CT scans and/or anoscopic evalu-
ation), and treatment (broad spectrum antibiotics and debride-
ment of necrotic tissue).
Anal Stenosis
Anal stenosis results from excessive stripping of anal mucosa,
which may leave inadequate bridges of anoderm for healing to
occur without stenosis.(71) Secondary hemorrhoids should be
managed with either submucosal hemorrhoidectomy or conserv-
ative methods such as sclerotherapy or rubber band ligation at a
subsequent visit. In mild cases, stenosis may simply be a web that
disappears with graduated anal dilatation in the office. In more
severe cases, surgical intervention may be required to relive ste-
nosis. Surgical correction may be accomplished by one of several
reconstructive operations including skin and subcutaneous tissue
flaps. These flap techniques are discussed in chapter 20.
Mucosal Ectropion (Whitehead Deformity)
Mucosal ectropion with the classic “Whitehead deformity”
is commonly seen after an incorrectly performed procedure
described by Whitehead.(22) As described previously, the oper-
ation entails making a circumferential incision at the level of the
dentate line, elevating a flap of anal mucosa, and performing a
submucosal hemorrhoidal excision. Redundant mucosa is then
excised, and the anal canal is reconstructed with sutures. If the
reconstruction does not relocate the dentate line in the correct
location in the anal canal, anal mucosal will be located in the
distal anal canal or perineum. Persistent mucous discharge and
perianal irritation may result. Correction requires resection of
the mislocated anal mucosa and reconstruction, which usually
requires flaps.
Fecal Incontinence
Incontinence of feces results chiefly from damage to the internal
anal sphincter during hemorrhoidectomy.(72) The internal anal
sphincter is a thin, whitish, smooth muscle composed of circular
fibers located just beneath the anal mucosa. It is almost always
absent at the anal verge because its inferior limit is a few milli-
meters proximal to it. During surgery, the hemorrhoidal column
should be lifted off the internal anal sphincter, which must be
identified before excision of hemorrhoidal tissue. It is important
to document the state of continence in patients before surgery.
Soiling and fecal leakage are the chief impairments of continence
resulting from internal anal sphincter damage. Treatment for soil-
ing and leakage includes bulking agents and slowing agents (e.g.,
loperamide) and consideration of biofeedback therapy. Attempts
to surgically repair damaged internal sphincter muscle have been
disappointing.
Anal Fistula
Fistula in ano is an uncommon complication of hemorrhoidec-
tomy and is thought to occur more commonly after a closed pro-
cedure.(73) The fistula is usually a simple submucosal tract that
may be treated by simple unroofing.
7
hemorrhoidal surgery
Anal Tags
Anal skin tags after hemorrhoidal excision are not uncommon.
Usually these tags are edematous areas of anoderm that generally
resolve spontaneously some weeks after surgery. Reassurance will
help allay patient’s concerns. It the tags remain bothersome or are
associated with symptomatic pruritis, they can be excised in the
office using local anesthesia.
SPECIAL SITUATIONS
Postpartum Hemorrhoids
Postpartum hemorrhoids that are refractory to conservative
measures may require surgical management. Hemorrhoidectomy
in this setting is safe, has a low prevalence of complications, and
in many cases will minimize recovery time. Proper patient posi-
tion (usually left lateral Sims) and good anesthetic techniques are
important. As in other urgent hemorrhoidectomies, preservation
of as much anoderm as possible is also critical.
Anorectal Varices
Unlike hemorrhoids, varices result from portal venous hyperten-
sion. Differentiation from hemorrhoids is essential because exci-
sion of varices may result in venous bleeding that may be difficult
to control. A history of anal bleeding in a cirrhotic patient should
arouse suspicion. Varices may be present in the rectum, anal canal,
or anal verge.(74) Duplex Doppler ultrasonography of the anorec-
tum may confirm the diagnosis. Active bleeding from varices will
usually require oversewing with a continuous suture technique.
CONCLUSION
The management of symptomatic hemorrhoidal disease should
be directed at the symptom complex of the patient. The major-
ity of these patients can be effectively treated by reducing strain
at defacation, correcting constipation, the use of any of a variety
of anal ointments. For those patients with persistent symptoms,
either injection or banding of the internal hemorrhoids offers
predictably successful results. Only a minority of patients should
require excisional hemorrhoidectomy by any of the described
techniques. Stapled hemorrhoidopexy, Harmonic Scalpel®, and
LigaSure™ all offer safe and effective alternatives to the tradi-
tional open or closed excisional hemorrhoidectomy, however
more long-term data is needed to provide recommendations for
their routine use.
REFERENCES
1. Holley CJ. History of hemorrhoidal surgery. South Med
J 1946; 39: 536.
2. Madoff RD. Biblical management of anorectal disease.
Presented at the Midwest Society of Colon and Rectal
Surgeons’ meeting. Brechenridge, CO; 1991.
3. Dirckx JH. The Biblical plague of “hemorrhoids”. Am
J Dermatopathol 1985; 7: 341–6.
4. Maimonides M, Rosner F, Munter S. trans. Treatise on
Hemorrhoids. Philadelphia, JB Lippincott; 1969.
5. Rachochot JE, Petourand CH, Riovoire JO. Saint Fiacre: the
healer of hemorrhoids and patron saint of proctology. Am J
Proctol 1971; 22: 175.
6. Thompson WHF. The nature of haemorrhoids. Br J Surg
1975; 62: 542–52.
7. Morgado PJ, Suarez JA, Gomez LG et al. Histoclinical basis
for a new classification of hemorrhoidal disease. Dis Colon
Rectum 1988; 31: 474–80.
8. Burkitt DP, Graham-Stewart CW. Hemorrhoids - postulated
pathogenesis and proposed prevention. Postgrad Med J 1975;
51: 631–6.
9. Haas PA, Fox TA, Haas GP. The pathogenesis of hemorrhoids.
Dis Colon Rectum 1984; 27: 442–50.
10. Hancock BD. Internal sphincter and the nature of haemor-
rhoids. Gut 1977; 18: 651–5.
11. Arabi Y, Alexander-Williams J, Keighley MRB. Anal pres-
sures in hemorrhoids and anal fissure. Am J Surg 1977; 134:
608–10.
12. Arscia SD ed. Morphological and Physiological Aspects of Anal
Continence and Defecation. Bruxelles. Presses Academiques
Europeenes, 1969: 150–1.
13. Banov L Jr, Knoepp LF Jr, Erdman LH, Alia RT.
Management
of hemorrhoidal disease.
J S C Med Assoc 1985; 81: 398–401.
14. Benyon J. Endorectal and Anal Sonography in Surgery of
the Colon, Rectum and Anus. W.B. Saunders, Philadelphia;
1995.
15. Kluiber RM, Wolff BG. Evaluation of anemia caused by hem-
orrhoidal bleeding. Dis Colon Rectum 1994; 37: 1006–7.
16. Bleday R, Pena PJ, Rothenberger DA, Goldberg SM, Buls JG.
Symptomatic hemorrhoids: current incidence and complica-
tions of operative therapy. Dis Colon Rectum 1992; 35: 477–81.
17. Ferguson JA, Heaton JR. Closed hemorrhoidectomy. Dis
Colon Rectum 1959; 2: 176–9.
18. Muldoon JP. The Completely Closed Hemorrhoidectomy: a
reliable and trusted friend for 25 years. Dis Colon Rectum
1981; 24: 211–4.
19. Mc Connell JC, Khubchandani IT. Long-term Follow-Up
of Closed Hemorrhoidectomy. Dis Colon Rectum 1983; 26:
797–9.
20. Ganchrow MJ, Mazier WP, Friend WG, Ferguson JA. Hemo-
rrhoidectomy revisited: a computer analysis of 2,038 cases.
Dis Colon Rectum 1971; 14: 128–33.
21. Senagore A, Mazier WP, Luchtefeld MA et al. The treatment
of advanced hemorrhoidal disease: a prospective random-
ized comparison of cold scalpel vs. contact Nd:YAG laser. Dis
Colon Rectum 1993; 6: 1042–9.
22. Milligan ET, Morgan CN, Lond LE. Surgical anatomy of the
anal canal, and the operative treatment of hemorrhoids.
Lancet 1937; 2: 1119–24.
23. Duhamel J, Romand-Heuer Y. The technique of the Milligan
and Morgan hemorrhoidectomy. Coloproctology 1980; 4:
265–6.
24. Tajana A. Hemorrhoidectomy according to Milligan-
Morgan: ligature and excision technique. Int Surg 1989; 74:
158–61.
25. Whitehead W. The surgical treatment of hemorrhoids. Br
Med J (Clin Res) 1882; 1: 148–50.
26. Andrews E. Disastrous results following Whitehead’s opera-
tion and the so-called American operation. Columbus Med J
1895; 15: 97–106.
7
improved outcomes in colon and rectal surgery
27. Andrews E. Some of the evils caused by Whitehead’s opera-
tion and by its modification, the American operation. Trans
Ill Med Soc 1895: 433–46.
28. Khubchandani M. Results of Whitehead operation. Dis
Colon Rectum 1984; 27: 730–2.
29. Rand AA. The sliding skin-flap graft operation for hemor-
rhoids: a modification of the Whitehead procedure. Dis
Colon Rectum 1969; 12: 265–76.
30. Wolff BG, Culp CE. The whitehead hemorrhoidectomy. Dis
Colon Rectum 1988; 31: 587–90.
31. Bonello JC. Who’s afraid of the dentate line? The whitehead
hemorrhoidectomy. Am J Surg 1988; 156: 182–6.
32. Longo A. Treatment of hemorrhoidal disease by reduction of
mucosa and hemorrhoidal prolapse with a circular suturing
device: a new procedure. Proceeding of the 6th world con-
gress of endoscopic surgery, Rome, 1998: 777–84.
33. Shalaby R, Desoky A. Randomized clinical trial of stapled
versus Milligan-Morgan haemorrhoidectomy.
Br J Surg 2001;
8: 1049–53.
34. Pavlidis T, Papaziogas B, Souparis A et al. Modern stapled
Longo procedure vs. conventional Milligan-Morgan hemor-
rhoidectomy: a randomized controlled trial.
Int J Colorectal
Dis 2002; 17: 50–3.
35. Palimento D, Picchio M, Attanasio U et al. Stapled and open
hemorrhoidectomy: randomized controlled trial of early
results. World J Surg 2003; 27: 203–7.
36. Senagore AJ, Singer M, Abcarian H et al. A prospective, randomized,
controlled multicenter trial comparing stapled hemorrhoidopexy
and Ferguson hemorrhoidectomy: perioperative and one-year
results. Dis Colon Rectum 2004; 47: 1824–36.
37. Tjandra JJ, Chan MK. Systematic review on the procedure
for prolapse and hemorrhoids (stapled hemorrhoidopexy).
Dis Colon Rectum 2007; 50: 878–92.
38. Ceci F, Picchio M, Palimento D et al. Long-term outcome of
stapled hemorrhoidopexy for Grade III and Grade IV hem-
orrhoids. Dis Colon Rectum 2008; 51: 1107–12.
39. Jongen J, Bock JU, Peleikis HG, Eberstein A, Pfister K.
Complications and reoperations in stapled anopexy: learn-
ing by doing. Int J Colorectal Dis 2006; 21: 166–71.
40. Chung CC, Ha JP, Tai YP, Tsang WW, Li MK. Double-blind,
randomized trial comparing Harmonic Scalpel hemorrhoid-
ectomy, bipolar scissors hemorrhoidectomy, and scissors exci-
sion: ligation technique. Dis Colon Rectum 2002; 45: 789–94.
41. Khan S, Pawlak SE, Eggenberger JC et al. Surgical treatment
of hemorrhoids: prospective, randomized trial comparing
closed excisional hemorrhoidectomy and the Harmonic
Scalpel technique of excisional hemorrhoidectomy. Dis
Colon Rectum 2001; 44: 845–9.
42. Armstrong DN, Ambroze WL, Schertzer ME, Orangio GR.
Harmonic Scalpel vs. electrocautery hemorrhoidectomy: a
prospective evaluation. Dis Colon Rectum 2001; 44: 558–64.
43. Tan JJ, Seow-Choen F. Prospective, randomized trial com-
paring diathermy and Harmonic Scalpel hemorrhoidectomy.
Dis Colon Rectum 2001; 44: 677–9.
44. Bessa SS. Ligasure vs. conventional diathermy in excisional
hemorrhoidectomy: a prospective, randomized study. Dis
Colon Rectum 2008; 51: 940–4.
45. Wang JY, Lu CY, Tsai HL et al. Randomized controlled trial of
LigaSure with submucosal dissection versus Ferguson hem-
orrhoidectomy for prolapsed hemorrhoids. World J Surg
2006; 30: 462–6.
46. Chung YC, Wu HJ. Clinical experience of sutureless closed
hemorrhoidectomy with LigaSure. Dis Colon Rectum 2003;
46: 87–92.
47. Franklin EJ, Seetharam S, Lowney J, Horgan PG. Randomized,
clinical trial of Ligasure vs conventional diathermy in hem-
orrhoidectomy. Dis Colon Rectum 2003; 46: 1380–3.
48. Altomare DF, Milito G, Andreoli R et al. Ligasure Precise vs.
conventional diathermy for Milligan-Morgan hemorrhoid-
ectomy: a prospective, randomized, multicenter trial. Dis
Colon Rectum 2008; 51: 514–9.
49. Palazzo FF, Francis DL, Clifton MA. Randomized clinical
trial of Ligasure versus open haemorrhoidectomy. Br J Surg
2002; 89: 154–7.
50. Jayne DG, Botterill I, Ambrose NS et al. Randomized clinical
trial of Ligasure versus conventional diathermy for day-case
haemorrhoidectomy. Br J Surg 2002; 89: 428–32.
51. O’Donovan S, Ferrara A, Larach S, Williamson P. Intraoperative
use of Toradol® Facilitates Outpatient Hemorrhoidectomy.
Dis Colon Rectum 1994; 37: 793–9.
52. Kuo RJ. Epidural Morphine for Post-hemorrhoidectomy
Analgesia. Dis Colon Rectum 1984; 27: 529–30.
53. Kilbride MJ, Senagore AJ, Morse M. Improving patient
safety with transdermal fentanyl for post-hemorrhoidec-
tomy pain. Dis Colon Rectum 1994. [Letter to the Editor,
p. 104].
54. Kilbride MJ, Morse M, Senagore AJ. Transdermal Fentanyl
Improves Management of Postoperative Hemorrhoidectomy
Pain. Dis Colon Rectum 1994; 37: 1070–2.
55. Goldstein ET, Williamson PR, Larach SW. Subcutaneous
morphine pump for postoperative hemorrhoidectomy pain
management. Dis Colon Rectum 1993; 36: 439–46.
56. Hoff SD, Bailey HR, Butts DR et al. Ambulatory surgical
hemorrhoidectomy - a solution to postoperative urinary
retension? Dis Colon Rectum 1994; 37: 1242–4.
57. Petros JG, Bradley TM. Factors influencing postoperative
urinary retention in patients undergoing surgery for benign
anorectal disease. Am J Surg 1990; 159: 374–6.
58. Tammela T, Kontturi M. Lukkarinen O. Postoperative uri-Postoperative uri-
nary retention. I. Incidence and predisposing factors. Scand J
Urol Nephrol 1986; 20: 197–201.
59. Leventhal A, Pfau A. Pharmacologic management of postop-
erative over-distension of the bladder. Surg Gynecol Obstet
1976; 146: 347–8.
60. Shafik A. Role of warm water bath in inducing micturition
in postoperative urinary retention after anorectal operations.
Urol Int 1993; 50: 213–7.
61. Corman ML. Complications of hemorrhoid and fissure sur-
gery. In: Ferrari BT, Ray JE, Gathright JB, eds. Complications
of colon and rectal surgery - prevention and management.
Philadelphia: WB Saunders, 1985: 91–100.
62. Kilbourne NJ. Internal hemorrhoids: comparative value of
treatment by operative and by injection methods - a survey
of 62,910 cases. Ann Surg 1934; 99: 600–8.
77
hemorrhoidal surgery
63. Salvati EP, Eisenstat TE. Hemorrhoidal disease. In: Zuidema
GD, Condon RE, eds. Shackelford’s surgery of the alimentary
tract. 3rd Ed. Philadelphia: WB Saunders 1991: 294–307.
64. Milsom JW. Hemorrhoidal disease. In: Wexner SD, Beck DE,
eds. Fundamentals of anorectal surgery. New York: McGraw
Hill, 1992: 192–214.
65. Gabriel WB. Haemorrhoids. In: The principles and prac-
tice of rectal surgery, 5th ed. Springfield, Illinois: Charles C.
Thomas, 1963: 110–64.
66. Rosen L, Sipe P, Stasik JJ, Riether RD, Trimpi HD. Outcome
of delayed hemorrhage following surgical hemorrhoidec-
tomy. Dis Colon Rectum 1993; 36: 743–6.
67. Cirocco WC, Golub RW. Local epinephrine injection as
treatment for delayed hemorrhage after hemorrhoidectomy.
Surgery 1995; 117: 235–7.
68. Basso L, Pescatori M. Outcome of delayed hemorrhage fol-
lowing surgical hemorrhoidectomy. [Letter to the Editor].
Dis Colon Rectum 1994; 37: 288–9.
69. Corman ML. management of postoperative constipation in
anorectal surgery. Dis Colon Rectum 1979; 22: 149.
70. Araghizadeb F. Fecal impaction. Clin Colorectal Surg 2005;
18: 116–9.
71. Parks AG. The surgical treatment of Haemorrhoids. Br J Surg
1956; 43: 337–51.
72. Anal continence after haemorrhoidectomy. Lancet 1982; 11:
696.
73. Corman ML. Complications of hemorrhoidal and fissure sur-
gery. In Ferrari BT, Ray JE, Gathright JB, eds. Complications
of Colon and Rectal Surgery. Philadelphia: WB Saunders,
1985: 91–100.