Improved Outcomes in Colon and Rectal Surgery part 21 pptx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (204.28 KB, 10 trang )
improved outcomes in colon and rectal surgery
more favorable outcomes, Zmora et al. were able to achieve a 53%
healing rate in a prospective study of 60 patients with complex
cryptoglandular fistulas treated with fibrin glue with intraadhe-
sive ceftazidime.(63)
The wide variety of successful healing in studies looking at the
use of fibrin glue in the treatment of fistula-in-ano is multifactorial.
Differences in the trials include patient selection, use of autologous
versus commercially prepared fibrin adhesive, etiology of the fistula
(cryptoglandular vs. Crohn’s disease vs. other causes), complexity
of the fistula, and length of follow-up. While the application of the
tissue adhesive seems fairly straightforward, there are also assuredly
subtle differences in the application techniques of different sur-
geons. The heterogeneity of the published trials makes direct com-
parisons very difficult. While success rates vary over a wide range,
the advantages of attempting to treat high transsphincteric fistulas
with fibrin glue in terms of simplicity of technique, negligible com-
plication rate, and ease of reapplication for failed treatments make
it an attractive option, at least initially. Most surgeons seem willing
to accept a higher than expected failure rate in exchange for a low
complication rate, understanding that treatment failures will need
to be addressed in some other manner.
Anal Fistula Plug
The topic that has perhaps generated the most discussion in recent
years is the use of the Surgisis® Anal Fistula Plug™ (AFP) (Cook
Surgical, Inc., Bloomington, IN). The AFP is a cone shaped bio-
prosthetic fashioned from Surgisis®, a bioabsorbable xenograft
made of lyophilized porcine intestinal submucosal. Surgisis® has
been used extensively in abdominal and inguinal hernia repairs.
(64–66) It is relatively resistant to infection, produces no foreign
body or giant cell reaction, and becomes repopulated with host
cell tissue within 3–6 months, providing mechanical integrity
while acting as a scaffold to guide tissue incorporation. The AFP
is inserted into the fistula tract and secured at the level of the pri-
mary opening. The principal effect is to close the primary fistula
opening, though incorporation of the AFP into the tract itself can
theoretically contribute to fistula closure. The advantages of this
technique include negligible risk of incontinence postoperatively,
relative simplicity in placement of the AFP, less postoperative
patient discomfort, and the ability to repeat the procedure in cases
of failure without major consequences.
Johnson et al. (67) initially reported a small, nonrandomized,
prospective cohort study comparing the efficacy of fibrin glue
versus AFP in the treatment of high transsphincteric fistulas. At a
mean follow-up of 14 weeks, in the fibrin glue cohort, healing was
seen in 40% (4 of 10), whereas in the AFP cohort, 13 of 15 (87%)
had healed (p < 0.05). The main advantage of the plug technique
compared with fibrin glue was felt to be the ability to securely close
the primary opening, which is felt to be a critical step in the suc-
cessful treatment of anal fistulas. The drawback of fibrin glue is its
liquid nature, and its tendency to run out of the fistula tract, even
when both primary and secondary openings are sutured closed.
Champagne et al. (68) went on to report an overall healing
rate of 83% for cryptoglandular fistulas treated with an AFP in
a series of 46 patients followed for a mean of 12 months (range
6–24 months). The same authors reported a similar 80% success
rate for treatment of Crohn’s-related fistulas with an AFP in 20
patients at a median follow-up of 10 months.(69) Ellis (70) also
reported success in a small group patients with transsphincteric
(n = 13) and rectovaginal fistulas (n = 5) with 88% complete fis-
tula closure at a median follow-up of 6 months.
Other studies report inconsistent results. Van Koperen et al.
reported a series of 17 patients treated with an AFP with only 41%
success.(71) Patients with cryptoglandular disease and no history
of previous fistula surgery fared better than those with a history of
previous surgical intervention. In the small subsets of patients with
Crohn’s disease (n = 1) and HIV infection (n = 2), 100% healing
was seen, as opposed to 29% complete healing (4 of 14) in patients
with cryptoglandular disease. Schwandner et al. (72) reported an
overall success rate of 61%. The subset of patients with Crohn’s
fistulas related to Crohn’s disease showed higher closure rates than
those with fistulas of cryptoglandular origin (85.7% vs. 45.5%).
More recent studies have varied widely in their results, reporting
healing rates ranging from 24% to 71.4%.(73–76)
One of the largest prospective studies was reported by Ky et al.
(77) The authors studied 45 patients with simple (n = 24) and
complex (n = 20) anorectal fistulas treated with AFP’s. An early
healing rate of 84% at 3 to 8 weeks postoperatively progressively
declined to 54.6% at a mean follow-up of 6.5 months. Healing
rates were significantly higher in patients with simple rather
than complex fistulas (70.8% vs. 35%, p < 0.02) and in patients
without Crohn’s disease compared to those with Crohn’s disease
(66.7% vs. 26.6%, p < 0.02).
Despite a number of publications attesting to the safety and
efficacy of the AFP, uniformity of opinion was lacking because
of contradictory reports in the literature as well as a lack of Level
I evidence showing any clear benefit. Because of this, a consensus
conference involving 15 surgeons with extensive experience with
the AFP was held in May 2007 to make formal recommendations
regarding inclusion/exclusion criteria, pre-, intra-, and postop-
erative management, and definition of outcome failure.(78)
Some technical notes regarding placement of the plug bear men-
tioning. It is essential that all sources of perineal sepsis are resolved
prior to placement. The use of pre- or postoperative antibiotics and
preoperative bowel cleansing has not been studied in a prospec-
tive, randomized fashion. In most of the studies described herein, a
preoperative dose of intravenous antibiotic was administered, and
varying regimens of postoperative antibiotics were utilized. The
consensus panel did not make specific recommendations regard-
ing preoperative bowel preparation; a single dose of preoperative
systemic antibiotics was recommended without postoperative
continuation.(78) Thorough cleansing of the fistula tract with
hydrogen peroxide is generally recommended. Mechanical cleans-
ing via curetting, debridement, or brushing is not recommended
due to disruption and enlargement of the tract. The technique of
fixation of the plug to the primary opening recommended by the
manufacturer involves a figure of eight absorbable suture through
the mucosa, submucosa, and internal anal sphincter that inverts the
proximal end of the anal fistula plug beneath the mucosa, anchor-
ing it to the tract while closing the primary opening over the plug
(Figure 19.5). Earlier studies as well as the manufacturer’s recom-
mendations suggested fixation of the distal end of the plug to the
secondary fistula opening as an essential step in plug placement.
Most surgeons have abandoned this step, now simply trimming the
9
surgery and nonoperative therapy of perirectal abscesses and anal fistulas
distal end of the plug flush with the skin without fixation, as it has
been suggested that external fixation creates tension on the primary
fixation site with patient movement, predisposing to plug extru-
sion. The consensus panel also recommended not fixing the distal
end of the plug to the secondary opening. The majority of AFP
failures are due to plug extrusion, untreated or persistent source(s)
of perineal sepsis, or postoperative infectious complications.
Ligation of Intersphincteric Fistula Tract
An interesting new concept in the surgical management of fistula-
in-ano has recently been described—ligation of the intersphincteric
fistula tract (LIFT).(79) In this method, intersphincteric dissec-
tion is performed and the fistula tract is identified and ligated in
this plane, leaving the sphincter muscles themselves undisturbed.
The authors reported complete fistula healing in 17 of 18 patients
(94.4%), with a mean healing time of 4 weeks and no disturbances
in anal function. While this study was small and observational in
nature, the simplicity of the technique and its negligible impact on
sphincter function certainly warrant further investigation.
ADDITIONAL ISSUES
Recurrence
Recurrence after incision and drainage of an anorectal abscess
and anal fistula, should be considered as two entities. True recur-
rence after abscess drainage is typically due to inadequate drain-
age or inadequate postoperative care. What is more commonly
seen is actually “persistent” disease as the abscess cavity matures
into a fistula. Vasilevsky and Gordon reported recurrent or per-
sistent disease in 48% of patients (11% recurrent abscess, 37%
fistula-in-ano) after undergoing incision and drainage of ano-
rectal abscesses.(80) Results similar to these have been reported
by several authors, which argue against primary fistulotomy at
the time of initial abscess drainage, as unnecessary fistulotomy
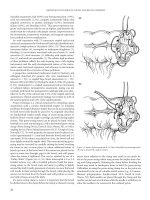
Figure 19.5 AFP product insert.
(A) (C)
(D)(B)
9
improved outcomes in colon and rectal surgery
with potential altered fecal continence can be avoided in approxi-
mately 50% of patients.
Common reasons for recurrent anorectal infection include missed
infection at the time of initial drainage in adjacent anatomic planes,
presence of an undiagnosed fistula at the time of initial abscess drain-
age, and failure to completely drain the abscess initially.(81) In a series
of 500 patients undergoing anorectal abscess drainage, Onaca et al.
reported that 7.6% required reoperation within 10 days of the initial
procedure.(82) Factors leading to reoperation included incomplete
drainage (23%), missed loculations (15%), missed abscesses (4%),
and postoperative bleeding (3%). Horseshoe abscesses were associ-
ated with a 50% rate of operative failure.(82)
Similarly, recurrent fistula-in-ano is often seen after surgi-
cal management due to a failure to identify a primary opening
or recognize secondary extensions of a fistula. Secondary tracts
accounted for early recurrences in 20% of patients studied by
Sangwan. (83) Sygut et al. reported a 14.3% recurrence rate after
surgical management of fistula-in-ano, though recurrence was
much more common after surgery for recurrent fistulas (51.7%)
than primary fistulas (5.4%).(84) In this same study, recurrence
was also more common in multi-tract fistulas (32.4%) than
single-tract fistulas (12%).
Recurrence rates after fistulotomy range from 0–18% (Table 19.1).
Premature closure of the fistulotomy wound is a clear risk factor for
recurrence; this can be prevented by creating an external wound
larger than the anal wound, ensuring that the internal wound will heal
first. Meticulous postoperative care is essential to avoid bridging and
pocketing of the wound.(99, 100) Epithelialization of the tract may
also occur, leading to persistent fistula-in-ano.(101) Garcia-Aguilar
et al. performed a retrospective study that reviewed the records of
624 patients undergoing surgery for fistula-in-ano in an effort to
determine factors associated with recurrence and incontinence.(98)
Recurrence was seen in 8% of patients; univariate and multivariate
regression analysis showed that factors associated with recurrence
included complex fistula type, horseshoe extension, lack of identi-
fication, or lateral location of the primary fistula opening, previous
fistula surgery, and the experience of the surgeon. Recurrence rates
after staged fistula repairs using setons range from 0% to 9% (34, 98,
102–109), though the largest study with a 0% recurrence rate had
only 21 patients.(106)
Interestingly, the success rate of fistula surgery has been shown to
decrease with time. In a study by van der Hagen et al. (110), recur-
rence rates following fistulotomy at 12, 48, and 72 months were 7%,
26%, and 39%, respectively, with 33% of recurrences occurring in
the first 24 months after surgery. A similar trend was seen following
the use of endorectal advancement flaps, with recurrence rates of
22%, 63%, and 63% seen at 12, 48, and 72 months respectively; 69%
of recurrences were seen within the first 24 months. Van Koperen et
al. (111) demonstrated recurrence rates at 3-year follow-up of 7%
for fistulotomy, and 21% for rectal advancement flaps.
Mizrahi et al. (112) described features associated with fistula
recurrence in a series of 106 consecutive endorectal advancement
flaps performed on 94 patients. Recurrence was seen in 40.4% of
patients at a mean follow-up of 40.3 months. Recurrence was
not associated with prior attempt at repair, type of fistula, ori-
gin of fistula, preoperative steroid use, postoperative bowel con-
finement, postoperative antibiotic use, or creation of a diverting
stoma. Recurrence was significantly more common in patients
with Crohn’s disease (p < 0.04). Sonada et al. reported a simi-
lar recurrence rate of 36.4% of patients undergoing endorec-
tal advancement flap for repair of anorectal and rectovaginal
fistulas in a series of 105 patients.(42) Factors that negatively
impacted the healing rate were Crohn’s disease (p = 0.027) and a
diagnosis of rectovaginal as opposed to anorectal fistula (0.002).
Patients on oral corticosteroid therapy showed a trend towards
recurrence, though this did not reach statistical significance; no
patients taking more than 20 mg/day of prednisone achieved
long-term healing.
Cigarette smoking has been shown to negatively impact fistula
closure after endorectal advancement flap. In a series of 105 patients
undergoing endorectal advancement flap for anal fistulas not
related to Crohn’s disease, Zimmerman et al. reported successful
fistula closure in 69%.(113) In patients who did not smoke ciga-
rettes, healing was seen in 79%, compared with 60% in smokers
(p < 0.037). Furthermore, a significant correlation was seen between
the healing rate and the number of cigarettes smoked per day
(p = 0.003). Using intraoperative laser Doppler flowmetry, it has also
been shown that median bloodflow before endorectal advancement
flap in nonsmokers was 35 volts, compared with 18 volts in smokers
(p = 0.018).(114) Thus, it seems likely that impaired wound heal-
ing due to diminished perfusion may be a contributing factor in
the failure of endorectal advancement flaps in smokers. Efforts to
encourage smoking cessation preoperatively should be undertaken
to minimize postoperative morbidity.
Incontinence
Fecal incontinence after abscess drainage should be relatively
infrequent and is typically the result of iatrogenic damage to the
sphincter mechanism. Compromised continence may also be
seen postoperatively if the external sphincter is damaged dur-
ing incision and drainage in patient with borderline preopera-
tive continence. Inadvertent injury to the puborectalis during
drainage of supralevator abscesses has also been reported.(115)
Prolonged packing may prevent granulation tissue formation
and promote generation of excessive scar tissue.(116) Primary
fistulectomy at the time of incision and drainage has also been
reported to cause disturbed fecal continence.(33)
On the other hand, incontinence rates following surgical
management of fistula-in-ano vary widely. The incidence of
incontinence is related to the complexity of the fistula and the
level of the primary fistula opening, with complex fistulas and
those with posterior and high openings and fistula extensions at
a higher risk.(97) Posterior fistulotomy has a higher incidence
of recurrence due to a more circuitous route of the tract, result-
ing in division of more sphincter muscle. Drainage of extensions
may damage small nerves and create scar tissue around the ano-
rectum.(97) The incidence of incontinence is also related to the
patient’s preoperative sphincter function and their would-heal-
ing ability. The incidence of impaired continence also increases
with age and is more common in females.(98) Fecal seepage
without true sphincter compromise can occur if the edges of a
fistulotomy wound do not heal completely, preventing complete
closure of the anus and allowing for leakage of fecal contents,
and flatus.
9
surgery and nonoperative therapy of perirectal abscesses and anal fistulas
In a large review of 844 patients undergoing surgery for anal
fistulas, Rosa et al. (117) demonstrated a 6.9% incidence of altered
postoperative sphincter function. Incontinence to flatus was seen
in 4.0%, liquid stool in 2.6%, and solid fecal material in 0.3%.
The majority of patients in this series underwent fistulotomy or a
combined fistulotomy-fistulectomy method. Sygut et al. reported
postoperative gas and/or stool incontinence in 10.7% of patients
undergoing surgical management of anal fistulas, mainly in the
form of fistulotomy and cutting setons.(84) In this study, rates
of incontinence were higher following surgery for recurrent vs.
primary fistulas (39.7% vs. 3.7%) and after surgery for multitract
as opposed to singletract fistulas (29.4% vs. 8.3%). In a review of
624 patients undergoing anal fistula surgery, Garcia-Aguilar et al.
(98) showed that 45% of patients complained of some degree of
altered continence. Factors associated with postoperative inconti-
nence included female sex, high fistula type, type of surgery, and
previous fistula surgery. Incontinence after staged fistulotomy
using a seton ranges from 0% to 64%.(34, 97, 98, 102–109) Again,
all of the studies showing no recurrences were small, with the
largest being only 20 patients.(105)
In a study looking at long-term functional outcome, Van
Koperen et al. (111) reported that after fistulotomy for low cryp-
toglandular fistulas, fecal soiling was seen in 41% of patients and
fecal incontinence was seen in 2.8% of patients at 3 year follow-up.
Following rectal advancement flaps, soiling was seen in 43% and
incontinence was seen in 2.9% at 3 year follow-up. None of poten-
tial risk factors examined (sex, age, prior fistula surgery, smok-
ing) were significant in both univariate and multivariate analysis.
Schouten et al. (39) showed that 35% of patients had deteriorated
continence postoperatively after endorectal advancement flap. The
number of previous attempts at fistula repair did not adversely
affect continence.
Zimmerman et al. (46) reported deteriorated continence after
anocutaneous advancement flap in 30% of patients. Aguilar et al.
reported disturbances in continence to flatus in 7% and liquid
stool in 6% in a series of 189 patients undergoing fistulectomy
with endorectal advancement flap.(118) Kodner et al. reported
unchanged or improved continence in 98% of patients undergo-
ing endorectal advancement flap for anorectal fistulas.(40) Other
series have reported no alteration in postoperative continence
after rectal advancement flaps.(45, 119)
Toyonaga et al. performed an interesting study looking at pre-
and postfistulotomy manometry studies.(120) They found that
fistulotomy significantly decreased maximum resting pressure
(85.9 to 60.2 mmHg, p < 0.0001) and length of the high pressure
zone (3.92 to 3.82 cm, p = 0.035), but did not affect voluntary
contraction pressure (164.7 to 160.3 mmHg, p = 0.2792). Anal
sphincter dysfunction, in the form of soiling, incontinence to fla-
tus, or incontinence to liquid stool, occurred in 20.3% of patients.
Multivariate analysis showed that while fistulotomy did not affect
voluntary contraction pressure, those with lower preoperative
voluntary contraction pressures were more likely to suffer from
altered continence postoperatively, as were those who had under-
gone multiple drainage procedures. Age, sex, previous fistula sur-
gery, duration of symptoms, and location and level of the primary
opening did not significantly influence continence postoperatively.
The authors concluded that preoperative anal manometry may
be helpful in choosing the proper surgical procedure for patients
with fistula-in-ano.
Manometry studies following endorectal advancement flaps
performed by Uribe et al. (121) also showed significant reduc-
tion in maximum resting pressure 3 months after surgery (83.6
vs. 45.6 mmHg, p < 0.001), as well as significant reduction in
maximum squeeze pressure (208.8 vs. 169.5 mmHg, p < 0.001).
Disturbed anal continence was seen 21.4% of patients. None of
the variables looked at (age, sex, previous fistula surgery, Crohn’s
disease) were predictive of postoperative incontinence. In con-
trast, manometry studies following endorectal advancement
Table 19.1 Results of fistula surgery.
Author Year No. Patients Recurrence % Incontinence %
Bennett (85) 1962 108 2.0 36.0
Hill (86) 1967 626 1.0 4.0
Lilius (87) 1968 150 5.5 13.5
Mazier (88) 1971 1000 3.9 0.001
Ani & Solanke (89) 1976 82 17.0 1.0
Marks & Ritchie (90) 1977 793 – 3, 17, 25*
Ewerth et al. (91) 1978 143 2.8 3.5
Adams & Kovalcik (92) 1981 133 3.8 0.8
Kuijpers (93) 1982 51 4.0 10.0
Sainio & Husa (94) 1985 199 11.0 34.0
Vasilevsky & Gordon (95) 1985 160 6.3 0.7, 2.0, 3.3**
Fucini (96) 1991
99 3.0 0, 0.2, 0.5***
Van Tets (97) 1994 19 – 33.0
Sangwan (83) 1994 461 6.5 2.8
Garcia-Aguilar et al. (98) 1996 293 7.0 42.0
* 3% solid stool, 17% liquid stool, 25% flatus.
** 0.7% solid stool, 2.0% liquid stool, 3.3% flatus.
*** 0 solid stool, 0.2% liquid stool, 0.5% flatus.
9
improved outcomes in colon and rectal surgery
flaps were performed by Kreis et al. (122), showing no difference
in preoperative and postoperative maximum squeeze pressure
(100.0 vs. 118.0 mmHg), maximum resting pressure (56.6 vs.
52.8 mmHg), rectal compliance (4.4 vs. 3.5 ml/mmHg), or any
other anorectal manometry parameter.
Other studies evaluating preoperative manometric parameters
differ somewhat. Chan and Lin (123) examined 45 patients with
intersphincteric fistulas and showed low preoperative resting pres-
sure to be the only independent factor predicting postoperative
incontinence. In a prospective study by Perez et al. (124) looking
at combined fistulotomy with primary sphincter reconstruction,
there were significant preoperative differences seen on manom-
etry between continent and incontinent patients that disappeared
after operation. There were neither clinical nor manometric dif-
ferences between pre- and postoperative values in fully continent
patients, although three patients (12.5%) reported minor altera-
tions of continence.
Crohn’s Disease
The overall incidence of anorectal fistulas associated with Crohn’s dis-
ease limited to the ileocecum is 20–25%; this rises to approximately
60% when Crohn’s disease affects the rectum.(125) Disease isolated
to the anorectum is seen in only 5% of patients.(126) Fistulizing
anorectal Crohn’s disease can be among the most frustrating con-
ditions surgeons are called upon to manage. Surgical treatment is
fraught with the problems of poor wound healing, delayed wound
healing, and sphincter injury. It is widely held that incontinence in
patients with anorectal Crohn’s disease is usually the result of aggres-
sive surgeons and not aggressive disease.(127) Thus, a conservative
approach is practiced in most instances, taking extreme care to pro-
tect the sphincter. When in doubt, one cannot be faulted for simply
draining the suppurative process by placing a draining seton.
Any acute infectious process must be drained appropriately and
medical management of the disease should be optimized before
even considering surgical treatment. For low-lying posterior fistu-
las, fistulotomy may be considered, especially if there is not rectal
disease. Anterior fistulotomies in females should be avoided because
of the risk of postoperative incontinence. Endorectal advancement
flaps are also a viable option, especially when there is no rectal
disease. Joo et al. (128) described 31 endorectal advancement flaps
performed in 26 patients, resulting in fistula eradication in 71% of
cases. Success was more likely in the absence of concomitant small
bowel Crohn’s disease than in patients with concomitant small
bowel Crohn’s disease (87% vs. 25%, p < 0.05). Other series have
shown that the presence of Crohn’s disease predisposes endorectal
advancement flaps to failure.(42, 112)
Data regarding the efficacy of the Surgisis© AFP is mixed. As
mentioned earlier, O’Connor et al. reported the AFP to be effective
in 80% of patients and 83% of fistula tracts in a series of 20 patients
with 36 fistula tracts. Patients with single fistulas were more likely
to have success and success was not correlated with antitumor
necrosis factor therapy.(69) Schwander et al. actually showed better
healing rates with AFP’s in patients with anal fistulas and Crohn’s
disease than in patients without Crohn’s (85.7% vs. 45.5%). On the
other hand, Ky et al. (77) reported complete fistula healing with an
AFP in 26.6% of patients with Crohn’s disease compared to 66.7%
of patients without Crohn’s (p < 0.02).
For patients with fulminant perineal sepsis due to fistulizing
perineal Crohn’s, a low threshold for a diverting stoma must be
entertained, especially since a large number of these patients will
go on to require proctectomy.
Nonsurgical Management
For the most part, there is no role for nonoperative management
of anorectal abscesses. Occasionally, an early inflammatory pro-
cess, marked by pain and erythema or induration without fluc-
tuance, may be prevented from progressing to an abscess with
early initiation of antibiotic therapy. However, once an abscess
has formed, antibiotics alone are insufficient. Failure to appropri-
ately drain an anorectal abscess in a timely manner subjects the
patient to the risk of progressive perineal sepsis, including opera-
tive risks associated with surgery in the septic patient, technical
complications associated with anorectal surgery in the face of
severe inflammation (unclear anatomy, bleeding, risk of inadver-
tent sphincter injury), and necrotizing perineal soft tissue infec-
tion (Fournier’s gangrene) with associated mortality rates as high
as 67% (129–132), as described below.
Nonoperative management of anal fistulas falls into two main
categories – those related to cryptoglandular disease and those
related to Crohn’s disease. There is very little in the literature
regarding nonoperative management of chronic cryptoglandular
fistulas. Obviously, acute suppurative processes must be drained,
typically with a seton. Draining setons may be left in place indef-
initely, with little consequence other than patient discomfort. As
discussed later, in exceedingly rare cases, invasive carcinoma may
develop in the setting of a chronic fistula.
Conservative (nonoperative) therapy for anal fistulas in the set-
ting of Crohn’s disease is the standard approach typically followed.
(331) Initial drainage of the acute suppurative process without
division of the fistula tract is typically performed by placing drain-
ing setons. Long-term indwelling draining setons may be used as
an effective management modality for complex perianal Crohn’s
fistulas, without a negative impact on continence.(134)
A number of medical therapies are utilized for the treatment
of anal fistulas related to Crohn’s disease. Ciprofloxacin has been
reported to improve symptoms in two small, uncontrolled trials.
(135, 136) Metronidazole had also been studied in a number of
uncontrolled trials with varying rates of symptom relief and fistula
healing.(137–140) Metronidazole must be used for maintenance
to be effective, as high recurrence rates are seen on discontinua-
tion.(133) The combination of ciprofloxacin and metronidazole
has also been shown to be effective in a small retrospective study
at reducing symptoms and healing fistulas; most patients in this
series also regressed with cessation of treatment.(141)
A number of immunomodulators are also employed in the
medical management of perianal Crohn’s fistulas. Azathioprine
and 6-mercaptopurine have been shown to induce complete
fistula closure in 31–39% of patients, with even higher rates of
symptom reduction without complete closure.(142–144) Again,
recurrence occurred frequently with discontinuation of treat-
ment. Methotrexate and cyclosporine A have each been shown
to be efficacious in inducing remission on patients with Crohn’s
disease (145, 146), though data regarding their effect specifically
on anal fistulas resulting from Crohn’s disease has been lacking.
9
surgery and nonoperative therapy of perirectal abscesses and anal fistulas
Infliximab, a chimeric monoclonal antibody against tumor
necrosis factor-alpha (TNF-α), has revolutionized the medical
management of Crohn’s disease. In mucosal biopsies of patients
with Crohn’s disease, enhanced secretion of TNF-α with failure
to release enhanced quantities of soluble TNF-α receptors is
seen. Infliximab reduces disease activity by blocking the effects
of TNF-α and has been shown to be an effective maintenance
therapy in patients with Crohn’s disease with fistulas (147) and
without fistulas.(148) Despite a lack of convincing Level 1 data
proving the efficacy of infliximab specifically in the setting of
perianal Crohn’s fistulas, its use in this setting is becoming more
widespread.
Long-term data regarding the efficacy of infliximab in effect-
ing perianal fistula closure is lacking. The combination of seton
drainage and infliximab infusion has been shown to be effective
as well, with healing rates ranging from 47–100%.(149–151) The
timing of seton removal in these patients is not clear. If removed
too early, the patient is at risk of developing recurrent perianal
abscesses, and if they are not removed, complete fistula healing
will not occur. Poritz et al. (152) reported 44% complete anal
fistula healing when the seton(s) were removed between the
second and third infliximab infusions.
As the use of infliximab escalates, patients who have failed treat-
ment are undergoing subsequent surgical intervention for anorec-
tal fistulas, raising concerns over whether preoperative infliximab
treatment has an adverse effect on anal fistula surgery. Gaertner
et al. (153) showed that patients with Crohn’s disease and anal
fistulas who were treated initially with infliximab and underwent
subsequent surgical treatment showed similar healing rates com-
pared with patients who did not undergo previous infliximab
treatment (60% vs. 59%). Kraemer et al. (154) reported that 9 of
11 patients with Crohn’s disease and anal fistulas who underwent
preoperative infliximab treatment followed by advancement flaps
demonstrated complete healing. Thus, it seems feasible to proceed
with anal fistula surgery after failed infliximab treatment, expect-
ing to acceptable rates of wound healing.
HIV-positive patients
Patients with anorectal abscesses who are HIV-positive require
timely incision and drainage, as presentation is often delayed. In
this population, the use of adjunct antibiotics is strongly recom-
mended. Because these patients are at increased risk if of poor
wound healing (155), care should be taken to minimize the size
of surgical wounds while ensuring adequate drainage. In one
study (155), serious septic complications or uncommon pre-
sentations of anorectal sepsis were seen in 13% of HIV-positive
patients who initially presented with anorectal suppuration. In
another study (156), perianal sepsis in HIV-positive patients
was frequently associated with in situ neoplasia. Interestingly,
immunosuppressive disease has not been found to contribute
to the need for early reoperation following initial abscess drain-
age.(82)
In a review by Munoz-Villasmil et al. (157) of 83 immunocom-
promised patients with perianal sepsis, 28% were HIV-positive. In
this population, 91% of surgical wounds were healed in 8 weeks.
Incontinence was seen in 6% of patients postoperatively, and
recurrence was seen in 7%.
Carcinoma Associated with Fistula-In-Ano
In rare instances, patients with long-standing anal fistulas may
go on to develop invasive carcinoma. Although this occurs more
commonly in the setting of Crohn’s disease, carcinomas arising
from anal fistulas have been reported in patients without Crohn’s
disease.(158, 159) While Crohn’s disease is associated overall with
an approximately 6-fold increase in colorectal cancer compared
to the general population (160), the incidence of anal cancer aris-
ing from an anal fistula in the setting a Crohn’s disease is signifi-
cantly lower.
In a series of over 1000 patients with anorectal manifestations of
Crohn’s disease, Ky et al. (125) reported seven patients (0.7%) who
developed invasive carcinoma related to anorectal fistulas. Four
patients developed squamous cell carcinoma and three developed
adenocarcinoma. The average duration of Crohn’s disease before
cancer diagnosis was 14 years and average age at diagnosis was
47 years. Presenting symptoms included pain (n = 5), persistent
fistula (n = 2), persistent anal ulcer (n = 1), and rectovaginal
fistula (n = 1). In four patients, the diagnosis of carcinoma was
overlooked at initial examination, resulting in significant delay
in diagnosis. All four patients with squamous cell carcinoma
were treated with chemoradiation. Two of these were success-
fully treated with no evidence of residual disease. One died of
carcinoma 6 months after treatment. The fourth patient required
abdominoperineal resection due to persistent disease and died 1.5
years later. One of the patients treated successfully with chemo-
radiation developed a second primary squamous cell carcinoma
11 years later, which was successfully treated with wide local exci-
sion. All three patients with adenocarcinoma were treated with
abdominoperineal resection. One received preoperative chemo-
radiation; this patient died 3.5 years later. Of the remaining
2 patients, one died in the early postoperative period, and the
second died of unrelated causes 5 years later.
A number of other case reports in the literature describe patients
with carcinoma arising from chronic fistulas and unhealed wounds
in a setting of Crohn’s disease.(161, 162) The take home message is
that one must maintain a high degree of suspicion for carcinoma
in patients with persistent or complex anal fistulas, especially in
the setting of long-standing Crohn’s disease. In this setting, com-
plex fistulas with associated anorectal strictures and/or severe
anorectal pain mandate a thorough examination. In cases where
anorectal examination is limited or unequivocal, exam under
anesthesia with biopsy or curettage of the fistula tract is essential.
Because lesions are typically diagnosed at a later stage of disease,
prognosis is poor. Timely diagnosis and institution of appropriate
therapy is essential to improve survival rates.
REFERENCES
1. Parks AG. Pathogenesis and treatment of fistula-in-ano.
Br Med J 1961; 1: 463–9.
2. Parks AG, Gordon PH, Hardcastle JD. A classification of
fistula-in-ano. Br J Surg 1976; 63: 1–12.
3. Cirocco WC, Reilly JC. Challenging the predictive accuracy
of Goodsall’s rule for anal fistulas. Dis Colon Rectum 1992;
35: 537–42.
4. Halligan S, Stoker J. Imaging of fistula in ano. Radiology
2006; 239: 18–33.
9
improved outcomes in colon and rectal surgery
5. Berman L, IsraelGM, McCarthy SM et al. Utility of mag-
netic resonance imaging in anorectal disease. World
J Gastroenterol 2007; 13: 3153–8.
6. Kuijpers HC, Schulpen T. Fistulography for fistula-in-ano:
is it useful? Dis Colon Rectum 1985; 28: 103–4.
7. Weisman RI, Orsay CP, Pearl RK, Abcarian H. The role of
fistulography in fistula-in-ano. Report of five cases. Dis
Colon Rectum 1991; 34: 181–4.
8. Law PJ, Talbot RW, Bartram CI, Northover JMA. Anal endo-
sonography in the evaluation of perianal sepsis and fistula-in-
ano. Br J Surg 1989; 76: 752–2.
9. Cataldo PA, Senagore A, Lutchtefeld MA. Intrarectal
ultrasound in the evaluation of perirectal abscesses. Dis
Colon Rectum 1993; 36: 554–8.
10. Lengyel AJ, Hurst NG, Williams JG. Pre-operative assessment
of anal fistulas using endoanal ultrasound. Colorectal Dis
2002; 4: 436–40.
11. Seow-Choen F, Burnett S, Bartram CI, Nicholls RJ.
Comparison between anal endosonography and digital
examination in the evaluation of anal fistulas. Br J Surg 1991;
78: 445–7.
12. Cheong DMO, Nogueras JJ, Wexner SD, Jagelman DG. Anal
endosonography for recurrent anal fistulas: image enhance-
ment with hydrogen peroxide. Dis Colon Rectum 1993; 36:
1158–60.
13. Poen AC, Felt-Bersma RJ, Eijsbouts QA et al. Hydrogen
peroxide-enhanced transanal ultrasound in the assessment
of fistula-in-ano. Dis Colon Rectum 1998; 41: 1147–52.
14. Moscowitz I, Baig MK, Nogueras JJ et al. Accuracy of hydro-
gen peroxide enhanced endoanal ultrasonography in assess-
ment of the internal opening of an anal fistula complex.
Tech Coloproctol 2003; 7: 133–7.
15. Navarro-Luna A, Garcia-Domingo MI, Rius-Macias
J, Marco-Molina C. Ultrasound study of anal fistulas with
hydrogen peroxide enhancement. Dis Colon Rectum 2004;
47: 108–14.
16. Buchanan GN, Bartram CI, Williams AB et al. Value of
hydrogen peroxide enhancement of three-dimensional
endoanal ultrasound in fistula-in-ano. Dis Colon Rectum
2005; 48: 141–7.
17. Gravante G, Giordano P. The role of three-dimensional
endoluminal ultrasound imaging in the evaluation of ano-
rectal diseases: a review. Surg Endosc 2008; 22: 1570–8.
18. Giordano P, Grondona P, Hetzer H et al. Three-dimensional
endoanal ultrasonography is better than conventional anal
endosonography in the assessment of fistula in ano. Dis
Colon Rectum 2004; 47: 607–8.
19. Ratto C, Grillo E, Parello A et al. Endoanal ultrasound-
guided surgery for anal fistula. Endoscopy 2005; 37: 722–8.
20. West RL, Dwarkasing S, Felt-Bersma RJ et al. Hydrogen
peroxide-enhanced three-dimensional endoanal ultrasonog-
raphy and endoanal magnetic resonance imaging in evaluat-
ing perianal fistulas: agreement and patient preference. Eur
J Gastroenterol Hepatol 2004; 16: 1319–24.
21. West RL, Zimmerman DD, Dwarkasing S et al. Prospective
comparison of hydrogen peroxide-enhanced three-dimen-
sional endoanal ultrasonography and endoanal magnetic
resonance imaging of perianal fistulas. Dis Colon Rectum
2003; 46: 1407–15.
22. Buchanan GN, Halligan S, Bartram CI et al. Clinical
examination, endosonography, and MR imaging in pre-
operative assessment of fistula in ano: comparison with
outcome-based reference standard. Radiology 2004; 233:
674–81.
23. Barker PG, Lunniss PJ, Armstrong P et al. Magnetic reso-
nance imaging of fistula-in-ano: technique, interpretation,
and accuracy. Clin Radiol 1994; 49: 7–13.
24. Beckingham IJ, Spencer JA, Ward J et al. Prospective evaluation
of dynamic contrast enhanced magnetic resonance imaging in
the evaluation of fistula in ano. Br J Surg 1996; 83: 1396–8.
25. Beets-Tan RG, Beets GL, van der Hoop AG et al. Preoperative
MR imaging of anal fistulas: Does it really help the surgeon?
Radiology 2001; 218: 75–84.
26. Schaefer O, Lohrmann C, Langer M. Assessment of anal fis-
tulas with high-resolution subtraction MR-fistulography:
comparison with surgical findings. J Magn Reson Imaging
2004; 19: 91–8.
27. Beck DE, Fazio VW, Lavery IC et al. Catheter drainage of
ischiorectal abscesses. South Med J 1988; 81: 444–6.
28. Hanley PH, Ray JE, Pennington EE, Grablowsky OM. A ten
year follow up study of horseshoe-abscess fistula-in-ano.
Dis Colon Rectum 1976; 19: 507–15.
29. Rosen SA, Colquhoun P, Efron J et al. Horseshoe abscesses
and fistulas: how are we doing? Surg Innov 2006; 13:
17–21.
30. Vasilevsky C. Fistula-in-ano and abscess. In: Beck DE,
Wexner SD, eds. Fundamentals of Anorectal Surgery, 2nd
Edition. Philadelphia: W.B. Saunders, 1998: 153–73.
31. Fucini C. One stage treatment of anal abscesses and fistulas.
A clinical appraisal on the basis of two different classifica-
tions. Int J Colorectal Dis 1991; 6: 12–6.
32. Tang C-L, Chew S-P, Seow-Choen F. Prospective random-
ized trial of drainage alone vs drainage and fistulotomy for
acute perianal abscesses with proven internal opening. Dis
Colon Rectum 1996; 39: 1415–17.
33. Schouten WR, van Vroonhoven TMJV. Treatment of anorec-
tal abscesses with or without primary fistulectomy: results
of a prospective randomized trial. Dis Colon Rectum 1991;
34: 60–3.
34. Pearl RK, Andrews JR, Orsay CP et al. Role of the seton in
the management of anorectal fistulas. Dis Colon Rectum
1993; 36: 573–9.
35. Parks AG, Stitz RW. The treatment of high fistula-in-ano.
Dis Colon Rectum 1976; 19: 487–99.
36. Kennedy HL, Zegarra JP. Fistulotomy without external sphinc-
ter division for high anal fistulae. Br J Surg 1990; 77: 898–90.
37. Fazio VW. Complex anal fistulae. Gastroenterol Clin North
Amer 1987; 16: 93–114.
38. Gordon PH. Management of anorectal abscess and fistulous
disease. In Kodner IJ, Fry RD, Roe JP, eds. Colon, Rectal and
Anal Surgery. St. Louis: CV Mosby, 1985: 91–107.
39. Schouten WR, Zimmerman DD, Briel JW. Transanal
advancement flap repair of transsphincteric fistulas. Dis
Colon Rectum 1999; 42: 1419–22.
9
surgery and nonoperative therapy of perirectal abscesses and anal fistulas
40. Kodner IJ, Mazor A, Shemesh EI et al. Endorectal advance-
ment flap repair of rectovaginal and other complicated
anorectal fistulas. Surgery 1993; 114: 682–9.
41. Dixon M, Root J, Grant S, Stamos MJ. Endorectal
flap advancement repair is an effective treatment for
selected patients with anorectal fistulas. Am Surg 2004;
70: 925–7.
42. Sonada T, Hull T, Piedmonte MR, Fazio VW. Outcomes of
primary repair of anorectal and rectovaginal fistulas using
the endorectal advancement flap. Dis Colon Rectum 2002;
45: 1622–8.
43. Hyman N. Endoanal advancement flap repair for complex
anorectal fistulas. Am J Surg 1999; 178: 337–40.
44. Chew SSB, Adams WJ. Anal sphincter advancement flap for
low transsphincteric anal fistula. Dis Colon Rectum 2007;
50: 1090–3.
45. Amin SN, Tierney GM, Lund JN, Armitage NC. V-Y
advancement flap for treatment of fistula-in-ano. Dis Colon
Rectum 2003; 46: 540–3.
46. Zimmerman DD, Briel JW, Gosselink MP, Schouten WR.
Anocutaneous advancement flap repair of transsphincteric
fistulas. Dis Colon Rectum 2001; 44: 1474–80.
47. Cintron JR, Park JJ, Orsay CP et al. Repair of fistulas-in-ano
using autologous fibrin tissue adhesive. Dis Colon Rectum
1999; 42: 607–13.
48. Park JJ, Cintron JR, Orsay CP et al. Repair of chronic anorec-
tal fistulae using commercial fibrin sealant. Arch Surg 2000;
135: 166–9.
49. Cintron JR, Park JJ, Orsay CP et al. Repair of fistulas-in-
ano using fibrin adhesive: long-term follow-up. Dis Colon
Rectum 2000; 43: 944–9.
50. Lindsey I, Smilgin-Humphreys MM, Cunningham C et al.
A randomized, controlled trial of fibrin glue vs. conven-
tional treatment for anal fistula. Dis Colon Rectum 2002;
45: 1608–15.
51. Sentovich SM. Fibrin glue for all anal fistulas. J Gastrointest
Surg 2001; 5: 158–61.
52. Buchanan GN, Bartram CI, Phillips RK. Efficacy of fibrin
sealant in the management of complex anal fistula. Dis
Colon Rectum 2003; 46: 1167–74.
53. Loungnarath R, Dietz DW, Mutch MG et al. Fibrin glue
treatment of complex anal fistulas has low success rate. Dis
Colon Rectum 2004; 47: 432–6.
54. Gisbertz SS, Sosef MN, Festen S, Gerhards MF. Treatment of
fistulas in ano with fibrin glue. Dig Surg 2005; 22: 91–4.
55. Maralcan G, Baskonus I, Aybasti N, Gokalp A. The use of
fibrin glue in the treatment of fistula-in-ano: a prospective
study. Surg Today 2006; 36: 166–70.
56. Adams T, Yang J, Kondylis LA, Kondylis PD. Long-term out-
look after successful fibrin glue ablation of cryptoglandular
transsphincteric fistula-in-ano. Dis Colon Rectum 2008; 51:
1488–90.
57. Ellis CN, Clark S. Fibrin glue as an adjunct to flap repair
of anal fistulas: a randomized, controlled study. Dis Colon
Rectum 2006; 49: 1736–40.
58. van Koperen PJ, Wind J, Bemelman WA, Slors JF. Fibrin glue
and transanal rectal advancement flap for high transsphincteric
perianal fistulas: is there any advantage? Int J Colorectal Dis
2008; 23: 697–701.
59. Jain SK, Kaza RCM, Pahwa M, Bansal S. Role of cyanoacry-
late in the management of low fistula in ano: a prospective
study. Int J Colorectal Dis 2008; 23: 355–8.
60. Barillari P, Basso L, Larcinese A et al. Cyanoacrylate glue
in the treatment of ano-rectal fistulas. Int J Colorectal Dis
2006; 21: 791–4.
61. Singer M, Cintron J, Nelson R et al. Treatment of fistulas-in-
ano with fibrin sealant in combination with intra-adhesive
antibiotics and/or surgical closure of the internal fistula
opening. Dis Colon Rectum 2005; 48: 799–808.
62. Gustafsson UM, Graf W. Randomized clinical trial of local
gentamycin-collagen treatment in advancement flap repair
for anal fistula. Br J Surg 2006; 93: 1202–7.
63. Zmora O, Neufeld D, Ziv Y et al. Prospective, multicenter
evaluation of highly concentrated fibrin blue in the treat-
ment of complex cryptogenic perianal fistulas. Dis Colon
Rectum 2005; 48: 2167–72.
64. Franklin ME Jr, Gonzalez JJ Jr, Michaelson RP et al. Preliminary
experience with new bioactive prosthetic material for repair
of hernias in infected fields. Hernia 2002; 6: 171–4.
65. Franklin ME Jr, Gonzalez JJ Jr, Glass JL. Use of porcine small
intestine submucosa as a prosthetic device for laparoscopic
repair of hernias in contaminated fields: 2-year follow-up.
Hernia 2004; 8: 186–9.
66. Franklin ME Jr, Trevino JM, Portillo G et al. The use of small
intestinal submucosa as a prosthetic material for laparoscopic
hernia repair in infected and potentially contaminated fields:
long-term follow-up. Surg Endosc 2008; 22: 1941–6.
67. Johnson EK, Gaw JU, Armstrong DN. Efficacy of anal fistula
plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon
Rectum 2006; 49: 371–6.
68. Champagne BJ, O’Connor LM, Ferguson M et al. Efficacy of
anal fistula plug in closure of cryptoglandular fistulas: long-
term follow-up. Dis Colon Rectum 2006;4 9: 1817–21.
69. O’Connor L, Champagne BJ, Ferguson MA et al. Efficacy of
anal fistula plug in closure of Crohn’s anorectal fistulas. Dis
Colon Rectum 2006: 49: 1569–73.
70. Ellis CN. Bioprosthetic plugs for complex anal fistulas: an
early experience. J Surg Educ 2007; 64: 36–40.
71. van Koperen PJ, D’Hoore A, Wolthuis AM et al. Anal fistula
plug for closure of difficult anorectal fistula: a prospective
study. Dis Colon Rectum 2007; 50: 2168–72.
72. Schwandner O, Stadler F, Dietl O et al. Initial experience on
efficacy in closure of cryptoglandular and Crohn’s trans-
sphincteric fistulas by the use of the anal fistula plug. Int
J Colorectal Dis 2008; 23: 319–24.
73. Lawes DA, Efron JE, Abbas M et al. Early experience with
the bioabsorbable anal fistula plug. World J Surg 2008; 32:
1157–9.
74. Christoforidis D, Etzioni DA, Goldberg SM et al. Treatment
of complex anal fistulas with the collagen fistula plug. Dis
Colon Rectum 2008; 51: 1482–7.
75. Thekkinkattil D, Botterill I, Ambrose S et al. Efficacy of the
anal fistula plug in complex anorectal fistulae. Colorectal
Dis 2008 Jul 15 [Epub ahead of print].
9
improved outcomes in colon and rectal surgery
76. Garg P. To determine the efficacy of anal fistula plug in the
treatment of high fistula-in-ano – an initial experience.
Colorectal Dis 2008 Jul 15 [Epub ahead of print].
77. Ky AJ, Sylla P, Steinhagen R et al. Collagen fistula plug for the
treatment of anal fistulas. Dis Colon Rectum 2008; 51: 838–43.
78. The Surgisis® AFP™ anal fistula plug: report of a consensus
conference. Colorectal Dis 2008; 10: 17–20.
79. Rojanasakul A, Pattanaarun J, Sahakitrungruang C,
Tantiphlachiva K. Total anal sphincter saving technique for
fistula-in-ano: the ligation of intersphincteric fistula tract.
J Med Assoc Thai 2007; 90: 581–6.
80. Vasilevsky CA, Gordon PH. The incidence of recurrent
abscesses or fistula-in-ano following anorectal suppuration.
Dis Colon Rectum 1984; 27: 126–30.
81. Abcarian H. Surgical management of recurrent anorectal
abscess. Contemp Surg 1982; 21: 85–91.
82. Onaca N, Hirshberg A, Adar R. Early reoperation for perirectal
abscess: a presentable complication. Dis Colon Rectum 2001;
44: 1469–73.
83. Sangwan YP, Rosen L, Riether RD et al. Is simple fistula-in-
ano simple? Dis Colon Rectum 1994; 37: 885–9.
84. Sygut A, Zajdel R, Kedzia-Budziewska R et al. Late results of
treatment of anal fistulas. Colorectal Dis 2007; 9: 151–8.
85. Bennet RC. A review of orthodox treatment for anal fistula.
Proc R Soc Med 1962; 55: 756–7.
86. Hill JR. Fistulas and fistulous abscesses in the anorectal
region: personal experience in management. Dis Colon
Rectum 1967; 10: 421–34.
87. Lilius HG. Fistula-in-ano, an investigation of human foetal
anal ducts and intramuscular glands and a clinical study of
150 patients. Acta Chir Scand 1968; 383(Suppl): 1–88.
88. Mazier WP. The treatment and care of anal fistulas: a study
of 1000 patients. Dis Colon Rectum 1971; 14: 134–44.
89. Ani AN, Solanke TF. Anal fistula: A review of 82 cases. Dis
Colon Rectum 1976; 19: 51–5.
90. Marks CG, Ritchie JK. Anal fistulas at St. Mark’s Hospital.
Br J Surg 1977; 64: 84–91.
91. Ewerth S, Ahlberg J, Collste G, Holmstrom B. Fistula-in-
ano: a six year follow-up study of 143 operated patients.
Acta Chir Scand 1978; 482(suppl): 53–5.
92. Adams D, Kovalcik PJ. Fistula-in-ano. Surg Gynecol Obstet
1981; 153: 731–3.
93. Kuijpers JHC. Diagnosis and treatment of fistula-in-ano.
Neth J Surg 1982; 34: 147–52.
94. Sainio P, Husa A. A prospective manometric study of the
effect of anal fistula surgery on anorectal function. Acta
Chir Scand 1985; 151: 279–88.
95. Vasilevsky CA, Gordon PH. Results of treatment of fistula-in-
ano. Dis Colon Rectum 1985: 28: 225–31.
96. Fucini C. One stage treatment of anal abscesses and fistulas.
A clinical appraisal on the basis of two different classificaions.
Int J Colorectal Dis 1991; 6: 12–6.
97. Van Tets WF, Kuijpers HC. Continence disorders after anal
fisulotomy. Dis Colon Rectum 1994; 37: 1194–7.
98. Garcia-Aguilar JC, Belmonte C, Wong WD, Goldberg SM,
Madoff RD. Surgical treatment of fistula-in-ano. Factors
associated with recurrence and incontinence. Dis Colon
Rectum 1996; 39: 723–9.
99. McElwain JM, Maclean D, Alexander RM et al. Anorectal
problems: experience with primary fistulotomy for anorectal
abscess, a report of 1000 cases. Dis Colon Rectum 1975; 27:
593–4.
100. Seow-Choen F, Phillips RKS. Insights gained from the
management of problematic anal fistulae at St. Mark’s
Hospital, 1984–88. Br J Surg 1991; 78: 539–41.
101. Lunniss PJ, Sheffield JP, Talbot IC et al. Persistence of
idiopathic anal fistula may be related to epithelialization.
Br J Surg 1995; 82: 32–3.
102. Williams JG, Macleod CAH, Goldberg SM. Seton treatment
of high anal fistulas. Br J Surg 1991; 78: 1159–61.
103. Ramanujam PS, Prasad ML, Abcarian H. The role of seton
in fistulotomy of the anus. Surg Gynecol Obstet 1983; 157:
419–22.
104. Kuijpers HC. Use of the seton in the treatment of extras-
phincteric anal fistula. Dis Colon Rectum 1984; 27: 109–10.
105. Culp CE. Use of penrose drains to treat certain anal fistu-
las: a primary operative seton. Mayo Clin Proc 1984; 59:
613–17.
106. Christensen A, Miles J, Christiansen J. Treatment of trans-
sphincteric anal fistulas by the seton technique. Dis Colon
Rectum 1986; 29: 454–5.
107. Held D, Khubchandani I, Sheets J et al. Management of
anorectal horseshoe abscess and fistula. Dis Colon Rectum
1986; 29: 793–7.
108. Fasth SB, Nordgren S, Hulten L. Clinical course and manage-
ment of suprasphincteric and extrasphincteric fistula-in-ano.
Acta Chir Scand 1990; 156: 397–402.
109. Graf W, Pahlman L, Egerbald S. Functional results after
seton treatment of high transsphincteric anal fistulas. Eur
J Surg 1995; 161: 289–91.
110. van der Hagen SJ, Baeten CG, Soeters PB, van Gemert WG.
Long-term outcome following mucosal advancement flap for
high perianal fistulas and fistulotomy for low perianal fistu-
las: recurrent perianal fistulas: failure of treatment or recur-
rent patient disease? Int J Colorectal Dis 2006; 21: 784–90.
111. van Koperen PJ, Wind J, Bemelman WA et al. Long-
term functional outcome and risk factors for recurrence
after surgical treatment for low and high perianal fistu-
las of cryptoglandular origin. Dis Colon Rectum 2008; 51:
1475–81.
112. Mizrahi N, Wexner SD, Zmora O et al. Endorectal advance-
ment flap: are there predictors of failure? Dis Colon Rectum
2002; 45: 1616–21.
113. Zimmerman DD, Delamarre JB, Gosselink MP et al. Smoking
affects the outcome of transanal mucosal advancement flap
repair of trans-sphincteric fistulas. Br J Surg 2003; 90: 351–4.
114. Zimmerman DD, Gosselink MP, Mitalas LE et al. Smoking
impairs rectal mucosal bloodflow – a pilot study: possible
implications for transanal advancement flap repair. Dis
Colon Rectum 2005; 48: 1228–32.
115. Seow-Choen F, Nicholls RJ. Anal fistula. Br J Surg 1992; 79:
197–205.
9
surgery and nonoperative therapy of perirectal abscesses and anal fistulas
116. Mazier WP. The treatment and care of anal fistulas: a study
of 1000 patients. Dis Colon Rectum 1971; 14: 134–44.
117. Rosa G, Lolli P, Piccinelli D et al. Fistula-in-ano: anatomo-
clinical aspects, surgical therapy and results in 844 patients.
Tech Coloproctol 2006; 10: 215–21.
118. Aguilar PS, Plasencia G, Hardy TJ et al. Mucosal advance-
ment in the treatment of anal fistula. Dis Colon Rectum
1985; 28: 496–8.
119. Dixon M, Root J, Grant S, Stamos MJ. Endorectal flap
advancement repair is an effective treatment for selected
patients with anorectal fistulas. Am Surg 2004; 70: 925–7.
120. Toyonaga T, Matsushima M, Kiriu T et al. Factors affecting
continence after fistulotomy for intersphincteric fistula-in-
ano. Int J Colorectal Dis 2007; 22: 1071–5.
121. Uribe N, Millan M, Minguez M et al. Clinical and mano-
metric results of endorectal advancement flaps for complex
anal fistula. Int J Colorectal Dis 2007; 22: 259–64.
122. Kreis ME, Jehle EC, Ohlemann M et al. Functional results
after transanal rectal advancement flap repair of trans-
sphincteric fistula. Br J Surg 1998; 85: 240–2.
123. Chang SC, Lin LK. Change in anal continence after surgery
for intersphincteral anal fistula: a functional and manomet-
ric study. Int J Colorectal Dis 2003; 18: 111–5.
124. Perez F, Arroyo A, Serrano P et al. Fistulotomy with primary
sphincter reconstruction in the management of complex
fistula-in-ano: prospective study of clinical and manomet-
ric results. J Am Coll Surg 2005; 200: 897–903.
125. Ky A, Sohn N, Weinstein MA, Korelitz BI. Carcinoma arising
in anorectal fistulas of Crohn’s disease. Dis Colon Rectum
1998; 41: 992–6.
126. Lockhart-Mummery HE. Symposium. Crohn’s disease: anal
lesions. Dis Colon Rectum 1975; 18: 200–2.
127. Alexander-Williams J, Buchmann P. Perianal Crohn’s dis-
ease. World J Surg 1980; 4: 203–8.
128. Joo JS, Weiss EG, Nogueras JJ, Wexner SD. Endorectal
advancement flap in perianal Crohn’s disease. Am Surg
1998; 64: 147–50.
129. Huber P Jr, Kissack AS, Simonton ST. Necrotizing soft tissue
infection from rectal abscess. Dis Colon Rectum 1983; 26:
507–11.
130. Bubrick MP, Hitchcock CR. Necrotizing anorectal and
perineal infections. Surgery 1979; 86: 655–62.
131. Lauks SS. Fournier’s gangrene in anorectal surgery. Surg
Clin North Am 1994; 74: 1339–52.
132. Kovalcik P, Jones J. Necrotizing perineal infections. Am Surg
1983; 49: 163–6.
133. Lichtenstein GR. Treatment of fistulizing Crohn’s disease.
Gastroenterology 2000; 119: 1132–47.
134. Thornton M, Solomon MJ. Long-term indwelling seton for
complex anal fistulas in Crohn’s disease. Dis Colon Rectum
2005; 48: 459–63.
135. Turunen U, Farkkila M, Seppala K. Long-term treatment
of perianl or fistulous Crohn’s disease with Ciprofloxacin
(suppl 148). Scand J Gastroenterol 1989; 24: 144.
136. Wolf J. Ciprofloxacin may be useful in Crohn’s disease.
Gastroenterology 1990; 98: A212.
137. Bernstein LH, Frank MS, Brandt LJ, Riley SJ. Healing of peri-
anal Crohn’s disease with metronidazole. Gastroenterology
1980; 79: 357–65.
138. Brandt L, Bernstein L, Boley S, Frank M. Metronidazole
therapy for perianal Crohn’s disease: a follow-up study.
Gastroenterology 1982; 83: 383–7.
139. Jakobovits J, Schuster MM. Metronidazole therapy for
Crohn’s disease and associated fistulae. Am J Gastroenterol
1984; 79: 533–40.
140. Schneider MU, Laudage G, Guggenmoos-Holzman I,
Riemann JF. Metronidazol in der behandlung des morbus
Crohn. Dtsch Med Wochenschr 1985; 110: 1724–30.
141. Solomon M, McLeod R, O’Connor B et al. Combination
ciprofloxacin and metronidazole in severe perianal Crohn’s
disease. Can J Gastroenterol 1993; 7: 571–3.
142. Present DH, Korelitz BI, Wisch N et al. Treatment of Crohn’s
disease with 6-mercaptopurine: a long-term randomized
double blind study. N Engl J Med 1980; 302: 981–7.
143. Korelitz BI, Present DH. Favorable effect of 6-mercaptopurine
on fistulae of Crohn’s disease. Dig Dis Sci 1985; 30: 58–64.
144. O’Brien JJ, Bayless TM, Bayless JA. Use of azathioprine
or 6-mercaptopurine in the treatment of Crohn’s disease.
Gastroenterology 1991; 101: 39–46.
145. Feegan BC, Rochon J, Fedorak RN et al. Methotrexate for
the treatment of Crohn’s disease. N Eng J Med 1995; 332:
292–7.
146. Stein RB, Hanaper SB. Medical therapy for infammatory
bowel disease. Gastroenterol Clin North Am 1999; 28:
297–321.
147. Sands BE, Anderson FH, Bernstein CN et al. Infliximab
maintenance therapy for fistulizing Crohn’s disease. N Eng
J Med 2004; 350: 876–85.
148. Hanauer SB, Feagan BG, Lichtenstein GR et al. Maintenance
infliximab for Crohn’s disease: the ACCENT I randomised
trial. Lancet 2002; 359: 1541–9.
149. Regueiro M, Mardini H. Treatment of perianal fistulizing
Crohn’s disease with infliximab alone or as an adjunct to
exam under anesthesia with seton placement. Inflamm
Bowel Dis 2003: 9: 98–103.
150. Topstad DR, Panaccione R, Heine JA et al. Combined seton
placement, infliximab infusion, and maintenance immu-
nosuppressives improve healing rate in fistulizing anorec-
tal Crohn’s disease: a single center experience. Dis Colon
Rectum 2003; 46: 577–83.
151. Talbot C, Sagar PM, Johnston MJ et al. Infliximab in the
surgical management of complex fistulating anal Crohn’s
disease. Colorectal Dis 2005; 7: 164–8.
152. Poritz LS, Rowe WA, Koltun WA. Remicade does not abol-
ish the need for surgery in fistulizing Crohn’s disease. Dis
Colon Rectum 2002; 45: 771–5.
153. Gaertner WB, Decanini A, Mellgren A et al. Does infliximab
infusion impact results of operative treatment for Crohn’s
perianal fistulas? Dis Colon Rectum 2007; 50: 1754–60.
154. Kraemer M, Kirschmeier A, Marth T. Perioperative adjuvant
therapy with infliximab in complicated anal Crohn’s disease.
Int J Colorectal Dis 2008; 23: 965–9.