Improved Outcomes in Colon and Rectal Surgery part 23 pdf
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (451.18 KB, 10 trang )
20
improved outcomes in colon and rectal surgery
limited versus full internal sphincterotomy. In one study, early
incontinence in patients undergoing full sphincterotomy (10.9%)
was increased compared to patients who underwent limited sphinc-
terotomy (2.2%, p = 0.039). (91) However, this difference did not
persist with long-term follow-up with only 2 patients who under-
went full sphincterotomy reporting persistent incontinence. In the
other study, there was no significant difference in posttreatment
and baseline incontinence scores between the two types of sphinc-
terotomy.(92) Overall, internal sphincterotomy up to the dentate
line has been shown to produce faster healing and pain relief but is
associated with increased rates of early incontinence compared to
sphincterotomy to the fissure apex.
Given the significant variation in incontinence rates after sphinc-
terotomy, several investigators have sought to further characterize
incontinence in patients with chronic anal fissure with respect to
type, frequency, and permanence. In a study of preoperative and
postoperative incontinence in 126 patients with chronic anal fissure,
Anmari and colleagues found that 28% of patients had minor
preoperative disturbances in continence that persisted postopera-
tively.(93) Casillas and colleagues found that patients endorsed a
higher rate of incontinence in response to a questionnaire than was
recorded in their medical record or was reported in a telephone
survey.(94) In other studies, risk factors for incontinence were
identified.(95–96) These include preexisting sphincter injuries, IAS
division >50%, injury to external anal sphincter during the proce-
dure, functional impairment with age, shorter sphincter in females,
and posterior keyhole deformity. While external anal sphincter
injury during anal stretch and a posterior keyhole deformity after
posterior sphincterotomy clearly result in higher rates and more
severe forms of incontinence, the presence of other risk factors in
patients who are undergoing lateral internal sphincterotomy result
in lower rates of minor incontinence which are frequently tempo-
rary. In fact, some incontinence scales are so sensitive to changes
in continence, that one study identified no impairment in quality
of life despite decreases in continence scores.(97) Overall, inconti-
nence after sphincterotomy remains a real complication that must
be considered in patients at higher risk (female, older age, previous
anorectal surgery) with subsequent modification of the surgical
procedure if necessary.
Refractory fissures
Several recent studies have been performed to identify character-
istics associated with fissure persistence despite treatment. In one
study the etiologic and manometric differences between anterior
and posterior anal fissures which failed to heal with nitrate therapy
were examined. When comparing patients with both anterior and
posterior fissure who failed medical therapy, Jenkins and colleagues
found that anterior fissures were more common in younger women
(33 years vs. 44) and were more likely to be associated with obstetric
trauma and an occult external anal sphincter defect.(98) Patients
with anterior fissure were also more likely to have normal or low
anal resting pressures compared to controls. This was significantly
different from the elevated resting pressures measured in patients
with posterior fissure. In addition, the maximum squeeze pressure
was significantly lower than normal controls and patients with
posterior fissure. Corby and colleagues also found that postpartum
females have lower anal resting pressure and squeeze pressures than
they did antepartum.(99) Thus, postpartum females who develop
anal fissure (9% incidence) have reduced anal canal resting pres-
sure and treatments to decrease internal sphincter tone can lead to
incontinence.
Other investigators have sought to identify medical therapy
that can act as rescue treatments for patients with persistent anal
fissure before a surgical treatment is undertaken. In one study
2% diltiazem ointment was used for treatment of chronic anal
fissure refractory to GTN.(100) Fissure healing occurred in
49% of patients with no recurrence over 8 weeks of follow-up.
Healing was not dependent on whether a full course of GTN was
completed with fissure persistence or GTN treatment was discon-
tinued secondary to adverse side-effects. In two other studies BT
was used to treat nitrate resistant fissures (GTN and ISDN). (101,
102) Forty-three to 50% of patients with nitrate resistant fissures
achieved healing with BT treatment. Patients with nitrate resistant
fissures have also been randomized to another course of nitrates
or nitrates plus BT.(103, 104) More patients healed their fissures
with a combination of BT and nitrates (47–67%) compared to
BT alone (20–27%). More studies are needed to determine the
optimal treatment for refractory or persistent fissures.
Alternative surgical therapies are available for chronic anal fis-
sure associated with low anal resting pressure or for those that
persistent after surgical sphincterotomy. These include island
advancement flaps. Nyam and colleagues described advancement
flaps in a series of patients with low anal resting pressure and
maximum squeeze pressure.(105) Some patients had external
sphincter defects and others had previous fissure surgery. Patients
underwent fissurectomy with flap coverage by perianal skin. All 20
patients healed with one contracture at the donor site and mini-
mal donor site discomfort. Leong et al. compared anal advance-
ment flap to lateral internal sphincterotomy for the treatment
of chronic anal fissure.(106) More patients healed with sphinc-
terotomy (100%) compared to anal advancement flap (85%).
A number of flaps, such a V-Y anoplasty, have been described for
chronic anal fissure with good success (Figure 20.2). A key to reduc-
ing complications with flap closure is careful hemostasis, which
reduces the risk of hematoma formation, flap loss, and infection.
Design of the flap with good length-to-width ratio is important
to ensure adequate vascularity and minimal tension. (107) Anal
advancement flaps remain an important surgical alternative for
patients with low pressure fissures and persistent fissure despite
previous anorectal surgery.
CoMPliCationS oF Surgery For anal StenoSiS
Acquircd anal stenosis can be a late sequela of a variety of
anorectal surgical procedures. It has been reported to occur after
5 to 10% of radical hemorrhoidectomies and after fissurectomy,
radiation injury, and Moh’s chemosurgery.(108, 109) The cause
of these strictures is excessive removal of the anodermal lining
of the anal canal: and thus is generally preventable. In cases of
severe, symptomatic anal stenosis, a variety of flaps can be used
to resurface the anal canal and expand its circumference. The
key to success with any of these flaps is that they be carefully
designed to maintain vascularity and that subflap hematoma
formation is averted to minimize the risk for infection and flap
necrosis.
20
surgery and nonoperative therapy of anal fissure
Patients should undergo complete mechanical and antibiotic
bowel preparation before surgery. After surgery, bowel activ-
ity may be restricted with a clear liquid diet for a day or two.
After this period, patients are allowed a regular diet and given
fiber supplements and laxatives to avert constipation One final,
important point is the limitation of patient activity for several
weeks so that flap motion is minimal, to allow neovascularity
to occur
Anal S-plasty (Figure. 20.3) was first proposed by Ferguson
(110) as a method to correct Whitehead deformities in 13
patients. Later Corman et al. (111) modified the procedure for
use in the management of anal stenosis. The key to the success of
this approach is development of ’ large, full-thickness skin flaps
with a base-to-length ratio >1.0. Ferguson recommended a base
of 7 to 10 cm and maintenance of a thin layer of fat globules on
the deep aspect of the flap so that adequate vascularity can be
ensured. He further cautioned against overzealous hemostasis on
the flap itself so as not to impair blood flow. The flaps are then
rotated toward the anal canal so that the anodermal defect can
be resurfaced. The flaps are sutured in place, and the remaining
semilunar defect is sutured to allow its primary healing. It is wise
to use a closed suction drain beneath the flap to avert seroma or
hematoma formation.
For less severe anal strictures that require less skin coverage, the
Y-V anoplasty, is an excellent alternative because it is simple to per-
form and is less traumatic for the patient. This technique was ini-
tially described by Penn (112) in 1948. Again, successfu1 healing of
the flap requires a length-to-base ration <3.0. Gingola and Arvanitis
(113) presented a series of 14 patients. Thirteen healed within 14
days with no episodes of infection or hematoma formation. Five
patients sloughed a small portion of the flap tip but required no
additional treatment. Experiences reported by other authors sup-
port the low rate (10 to 25%) of tip necrosis and the high rate (85
to 92%) of stenosis relief associated with this technique (114, 115).
It must be remembered, however that Y-V advancement flaps limit
how much anal resurfacing can be accomplished.
Other uses for the Y-V advancement flap have been advo-
cated. Rosen (116) used it to treat anal stcnosis and ectropion
(Figure 20.4).The blood supply for this flap is based on perfo-
rating vessels in the subcutaneous fat. The Y-V advancemcnt
flap is well suited for covering the lower anal canal but has lim-
ited application for stenosis above the dentate line.
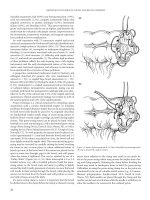
Figure 20.2 V-Y anoplasly. (A) Incisions create
a V-shaped or triangular flap which is advanced
to close defect. (B) Closure of skin behind “V”
pushes the flap into the anal canal, and the flap is
sutured in place.
(A) (B)
Figure 20.3 Ana1 S-plasty. (A) Ectroion is excised
and S-shaped incisions are created. (B) and (C)
flaps are rotated lo close the defects and sutured
in place.
(A)
(B) (C)
20
improved outcomes in colon and rectal surgery
Figure 20.4 Y-V anoplasty. (A) Y shaped incision is made. (B) V-shaped flap is mobilized and advanced to the top of the defect. (C) Flap is and sutured in place.
(A)
(B) (C)
Figure 20.5 House advancement flap. (A) House-shaped flap is created. (B) The flap is advanced into the anal canal and (C) sutured in place.
(A) (B) (C)
Figure 20.6 Diamond flap. (A) Diamond-shaped flap is created. (B) The flap is advanced into the anal canal to fill the defect. (lnsert demonstrates perforating
subcutaneous blood supply). (C) Flap is sutured in place.
(A)
(B)
(C)
Other techniques of flap formation have been suggested.
Christensen et al. (117). proposed the use of “house” advance-
ment pedicle flaps. The editors prefer the house flap because it is
easy to construct, can cover as much as 25% of the anal circumfer-
ence, and permits primary closure of the donor site (Figure 20.5).
If additional coverage is needed, two, three, or four flaps may be
used. Caplin and Kodner (118) recommended the use of the dia-
mond flap for many of the same reasons (Figure 20.6).
ConCluSion
Chronic anal fissure is a common and painful anorectal dis-
order. Many treatments are available for benign idiopathic fis-
sures. The goal of treatment is to reduce the high anal resting
pressure or internal sphincter hypertonia in fissure patients.
First line therapy consists of either topical nitrates or calcium
channel antagonists. If topical therapies fail, a repeat treatment
course can be prescribed. As second line therapy, botulinum
2
surgery and nonoperative therapy of anal fissure
toxin injection or internal sphincterotomy can be performed.
Ideally, patients who are at risk of incontinence after inter-
nal anal sphincter division should attempt medical therapy
(age>50, multiparous female, previous anorectal surgery). If
sphincterotomy is contemplated for high risk patients, preop-
erative anal manometry and ultrasound should be considered.
Alternatively, fissurectomy with anal advancement flap can be
performed. There is scarce data on the ideal treatment for resist-
ant anal fissure. Patients at high risk of fissure persistence may
be considered for internal sphincterotomy as first line therapy
(symptom duration >12 months, presence of a sentinel pile,
persistently elevated mean anal resting pressure).
reFerenCeS
1. Oh C, Divino DM, Steinhagen RM. Anal Fissure:20 year
experience. Dis Colon Rectum 1995; 38: 378–82.
2. Pescatori M, Interisano A. Annual report of the Italian colo-
proctology units. Tech Coloproctol 1995; 2: 29–30.
3. Gibbons CP, Read NW. Anal hypertonia in fissures:cause or
effect? Br J Surg June 1986; 73(6): 443–45.
4. Klosterhalfen B, Vogel P, Rixen H, Mittermayer C. Topography
of the inferior rectal artery: a possible cause of chronic, pri-
mary anal fissure. Dis Colon Rectum 1989; 32(1): 43–52.
5. Schouten WR, Briel JW, Auwerda JJ. Relationship between
anal pressure and anodermal blood flow: the vascular
pathogenesis of anal fissures. Dis Colon Rectum 1994;
37(7): 664–9.
6. Brisinda G, Albanese A, Cadeddu F et al. Botulinum neu-
rotoxin to treat chronic anal fissure:results of a randomized
‘Botox vs. Dysport’ controlled trial. Aliment Pharmacol Ther
2004; 19: 695–701.
7. Nelson R. A systematic review of medical therapy for anal
fissure. Dis Colon Rectum 2004; 47: 422–31.
8. Nelson R. Meta-analysis of operative techniques for fissure-in-
ano. Dis Colon Rectum 1999; 42: 1424–31.
9. Nelson R. Nonsurgical therapy for anal fissure. Cochrane
Database Syst Rev 2006; 4: CD003431.
10. Nelson R. Operative procedures for fissure in ano. Cochrane
Database Syst Rev 2005; 2: CD002199.
11. Gough MJ, Lewis A. The conservative treatment of fissure-in-
ano. Br J Surg 1983; 70: 175–6.
12. Orsay C, Rakinic J, Perry WB et al. Practice parameters
for the management of anal fissures (revised). Dis Colon
Rectum 2004; 47: 2003–7.
13. Jensen SL. Treatment of first episodes of acute anal fissure:
prospective randomized study of lignocaine ointment versus
hydrocortisone ointment or warm sitz baths plus bran.
Br Med J (Clin Res Ed) 1986; 292(6529): 1167–9.
14. Jensen SL. Maintenance therapy with unprocessed bran in
the prevention if acute anal fissure recurrence. J Royal Soc
Med 1987; 80(5): 296–8.
15. Gupta PJ. Randomized, controlled study comparing sitz
bath and no sitz bath treatments in patients with acute anal
fissures. ANZ J Surg 2006; 76: 718–21.
16. McDonald P, Driscoll AM, Nicholls RJ. The anal dilator in
the conservative management of acute anal fissures. Br J Surg
1983; 70: 25–6.
17. Bacher H, Mischinger HJ, Werkgartner G et al. Local nitro-
glycerin for treatment of anal fissures: an alternative to
lateral sphincterotomy? Dis Colon Rectum 1997: 40(7):
840–5.
18. Antropoli C, Perrotti P, Rubino M et al. Nifedipine for local
use in conservative treatment of anal fissures. Dis Colon
Rectum 1999; 42(8): 1011–5.
19. Garrido R, Lagos N, Lattes K et al. Gonyautoxin: New treat-
ment for acute and chronic anal fissures. Dis Colon Rectum
2005; 48(2): 335–43.
20. O’Kelly T. Brading A, Mortensen N. Nerve mediated relax-
ation of the human internal anal sphincter: role of nitric
oxide. Gut 1993; 34: 689–93.
21. Loder PB, Kamm MA, Nicholls RJ, Phillips RKS. ‘Reversiable
chemical sphincterotomy’ by local application of glyceryl
trinitrate. Br J Surg 1994; 81: 1386–9.
22. Schouten WR, Briel JW, Boerma MO et al. Pathophysiological
aspects and clinical outcome of intra-anal application of
isosorbide dinitrate in patients with chronic anal fissure.
Gut 1996; 39: 465–9.
23. Thornton MJ, Kennedy ML, King DW. Manometric effect
on topical glyceryl trinitrate and its impact on chronic anal
fissure healing. Dis Colon Rectum 2005; 48: 1207–12.
24. Lund JN, Scholefield JH. A randomized, prospective, double-
blind, placebo controlled trial of glyceryl trinitrate ointment
in treatment of anal fissure. Lancet 1997; 349: 11–4.
25. Chaudhuri S, Pal AK, Acharya A et al. Treatment of chronic
anal fissure with topical glyceryl trinitrate: a double-blind,
placebo-controlled trial. Indian J Gastroenterol 2001; 20(3):
101–2.
26. Altomare DF, Rinaldi M, Milito G et al. Glyceryl trinitrate
for chronic anal fissure-healing or headache? Dis Colon
Rectum 2000; 43: 174–81.
27. Kennedy ML, Sowter S, Nguyen H, Lubowski DZ. Glyceryl
trinitrate ointment for the treatment of chronic anal
fissure: results of a placebo-controlled trial and long-term
follow-up. Dis Colon Rectum 1999; 42(8): 1000–6.
28. Maan MS, Mishra R, Thomas S, Hadka NS. Randomized,
double-blind trial comparing topical nitroglycerin with
xylocaine and proctosedyl in idiopathic chronic anal fissure.
Indian J Gastroenterol 2004; 23: 91–3.
29. Carapeti EA, Kamm MA, McDonald PJ et al. Randomised
controlled trial shows that glyceryl trinitrate heals anal
fissures, higher doses are not more effective, and there is a
high recurrence rate. Gut 1999; 44: 727–30.
30. Bailey HR, Beck DE, Billingham RP et al. A study to deter-
mine the nitroglycerin ointment dose and dosing interval
that best promote the healing of chronic anal fissures. Dis
Colon Rectum 2002; 45(9): 1192–9.
31. Scholefield JH, Bock JU, Marla B et al. A dose finding study
with 0.1%, 0.2% and 0.4% glyceryl trinitrate ointment
in patients with chronic anal fissures. Gut 2003; 52(2):
264–9.
32. Wierre AJ, Palamba HW, Spillenaar Bilgen EJ, Eggink WF.
Isosorbide dinitrate in the treatment of anal fissure: a ran-
domised, prospective, double blind, placebo-controlled
trial. Eur J Surg 2001; 167: 382–5.
22
improved outcomes in colon and rectal surgery
33. Tankova L, Yoncheva K, Muhtarov M, Kadyan H, Draganov V.
Topical mononitrate treatment in patients with anal fissure.
Aliment Pharmacol Ther 2002; 16: 101–3.
34. Colak T, Ipek T, Urkaya N, Kanik A, Dirlik M. A randomized
study comparing systemic transdermal treatment and local
application of glyceryl trinitrate ointment in the management
of chronic anal fissure. Eur J Surg 2002; Suppl 588: 18–22.
35. Zuberi BF, Rajput MR, Abro H, Shaikh SA. A randomized
trial of glyceryl trinitrate ointment and nitroglycerin patch in
healing of anal fissures. Int J Colorectal Dis 2000; 15: 243–5.
36. Richard CS, Gregoire R, Plewes EA et al. Internal sphinctero-
tomy is superior to topical nitroglycerin in the treatment of
chronic anal fissure. Dis Colon Rectum 2000; 43: 1048–58.
37. Evans J, Luck A, Hewett P. Glyceryl trinitrate vs. lateral
sphincterotomy for chronic anal fissure. Dis Colon Rectum
2001; 44: 93–7.
38. Libertiny G, Knight JS, Farouk R. Randomised trial of topical
0.2% glyceryl trinitrate and lateral internal sphincterotomy
for the treatment of patients with chronic anal fissure: long-
term follow-up. Eur J Surg 2002; 168: 418–21.
39. Parellada C. Randomized, prospective trial comparing
0.2 percent isosorbide dinitrate ointment with sphinc-
terotomy in treatment of chronic anal fissure: a 2 year
follow-up. Dis Colon Rectum 2004; 47: 437–43.
40. Oettle GJ. Glyceryl trinitrate vs. sphincterotomy for treat-
ment of chronic fissure in ano. Dis Colon Rectum 1997;
40(11): 1318–20.
41. Mishra R, Thomas S, Maan MS, Hadke NS. Topical nitro-
glycerin versus lateral internal sphincterotomy for chronic
anal fissure: prospective, randomized trial. ANZ J Surg
2005; 75: 1032–5.
42. Cook TA, Brading AF, McC. Mortensen NJ. Differences in
contractile properties of anorectal smooth muscle and the
effects of calcium channel blockade. Br J Surg 1999; 86: 70–5.
43. Chrysos E, Xynos E, Tzovaras G et al. Effect of nifedepine on
rectoanal motility. Dis Colon Rectum 1996; 39: 212–6.
44. Carapeti EA, Kamm MA, Evans BK, Phillips RK. Topical
diltiazem and bethanechol decrease anal sphincter pressure
without side effects. Gut 1999; 45: 719–22.
45. Perotti P, Bove A, Antropoli C et al. Topical nifedipine with
lidocaine ointment vs. active control for the treatment of
chronic anal fissure: results of a prospective, randomized,
double-blind study. Dis Colon Rectum 2002; 45: 1468–75.
46. Bielecki K, Kolodziejczak M. A prospective randomized trial
of diltiazem and glyceryl trinitrate ointment in the treatment
of chronic anal fissure. Colorectal Disease 2003; 5: 256–7.
47. Kocher HM, Steward M, Leather AJM, Cullen PT. Randomized
clinical trl assessing the side effects of glyceryl trinitrate
and diltiazem hydrochloride in the treatment of chronic anal
fissure. Br J Surg 2002; 89: 413–7.
48. Shrivastava UK, Jain BK, Kumar P, Saifee Y. A comparison
of the effects of diltiazem and glyceryl trinitrate ointment in
the treatment of chronic anal fissure: a randomized clinical
trial. Surg Today 2007; 37: 482–5.
49. Ezri T, Susmallian S. Topical nifedipine versus topical glyc-
eryl trinitrate for treatment of chronic anal fissure. Dis
Colon Rectum 2003; 46: 805–8.
50. Mustafa NA, Cengiz S, Turkyilmaz S, Yucel Y. Comparison
of topical glyceryl trinitrate ointment and oral nifedipine in
the treatment of chronic anal fissure. Acta Chir Belg 2005;
105: 55–8.
51. Ho KS, Ho YH. Randomized clinical trial comparing oral
nifedipine with lateral anal sphincterotomy and tailored
sphincterotomy in the treatment of chronic anal fissure.
Br J Surg 2005; 92: 403–8.
52. Katsinelos P, Papaziogas B, Koutelidakis I et al. Topical 0.5%
nifedipine vs. lateral internal sphincterotomy for the treatment
of chronic anal fissure: long-term follow-up. Int J Colorectal
Dis 2006; 21: 179–83.
53. Jonas M, Neal K, Abercrombie JF, Scholefield JH. A ran-
domized trial of oral vs. topical diltiazem for chronic anal
fissures. Dis Colon Rectum 2001; 44: 1074–8.
54. Jones OM, Brading AF, Mortensen NJ. Mechanism of action
of botulinum toxin on the internal anal sphincter. Br J Surg
2004; 91(2): 224–8.
55. Fernandez LF, Conde Freire R, Rios Rios A et al. Botulinum
toxin for the treatment of anal fissure. Dig Surg 1999; 16(6):
515–8.
56. Espi A, Melo F, Minguez M et al. Therapeutic use of botuli-
num toxin in anal fissure. Int J Colorectal Dis 1998; 12: 163.
57. Maria G, Brisinda G, Bentivoglio AR et al. Botulinum toxin
injections in the internal anal sphincter for the treatment
of chronic anal fissure: long-term results after two different
dosing regimens. Ann Surg 1998; 228(5): 664–9.
58. Brisinda G, Maria G, Sganga G et al. Effectiveness of higher
doses of botulinum toxin to induce healing in patients with
chronic anal fissures. Surgery 2002; 131: 179–84.
59. Minguez M, Melo F, Espi A et al. Therapeutic effects of dif-
ferent doses of botulinum toxin in chronic anal fissure. Dis
Colon Rectum 1999; 42: 1016–21.
60. Maria G, Brisinda G, Bentivoglio AR et al. Influence of
botulinum toxin site of injections on healing rate in patient
with chronic anal fissure. Am J Surg 2000; 179; 46–50.
61. Colak T, Ipek T, Kanik A, Aydin S. A randomized trial of bot-
ulinum toxin vs. lidocain pomade for chronic anal fissure.
Acta Gastroenterol Belg 2002; 65(4): 187–90.
62. Siproudhis L, Sebille V, Pigot F et al. Lack of efficacy of botu-
linum toxin in chronic anal fissure. Aliment Pharmacol Ther
2003; 18: 515–24.
63. Maria G, Cassetta E, Gui D et al. A comparison of botulinum
toxin and saline for the treatment of chronic anal fissure.
N Engl J Med 1998; 338: 217–20.
64. Brisinda G, Maria G, Bentivoglio AR et al. A comparison of
injections of botulinum toxin and topical nitroglycerin oint-
ment for the treatment of chronic anal fissure. N Engl J Med
1999; 341: 65–9.
65. Brisinda G, Cadeddu F, Brandara F, Marniga G, Maria G.
Randomized clinical trial comparing botulinum toxin injec-
tions with 0.2 percent nitroglycerin ointment for chronic
anal fissure. Br J Surg 2007; 94: 162–7.
66. De Nardi P, Ortolano E, Radaelli G, Staudacher C. Comparison
of glycerine trinitrate and botulinum toxin-a for the treat-
ment of chronic anal fissure:long-term results. Dis Colon
Rectum 2006; 49: 427–32.
2
surgery and nonoperative therapy of anal fissure
67. Fruehauf H, Fied M, Wegmueller B, Bauerfeind P, Thumshirn
M. Efficacy and safety of botulinum toxin a injection com-
pared with topical nitroglycerin ointment for the treatment
of chronic anal fissure: a prospective randomized study. Am
J Gastroenterol 2006; 101: 2107–12.
68. Minguez M, Melo F, Epsi A et al. Longterm followup of CAF
after healing with BT. Gastroenterology 2002; 123: 112–7.
69. Arroyo A, Perez F, Serrano P et al. Surgical versus chemi-
cal (botulinum toxin) sphincterotomy for chronic anal
fissure: long-term results of a prospective randomized
clinical and manometric study. Am J Surg 2005; 189:
429–34.
70. Iswariah H, Stephens J, Rieger N, Rodda D, Hewett P.
Randomized prospective controlled trial of lateral internal
sphincterotomy versus injections of botulinum toxin for
the treatment of idiopathic fissure in ano. ANZ J Surg 2005;
75: 553–5.
71. Mentes BB, Irkorucu O, Akin M, Leventoglu S, Tatlicioglu
E.Comparison of botulinum toxin injection and lateral
internal sphincterotomy for the treatment of chronic anal
fissure. Dis Colon Rectum 2003; 46: 232–7.
72. Watts JM, Bennett RC, Goligher JC. Stretching of the anal
sphincters in the treatment of chronic fissure-in-ano. BMJ
1965; 2: 342–3.
73. Eisenhammer S. The surgical correction of chronic anal
(sphincteric) contracture. S Afr Med J 1951; 25: 486–9
74. Chowcat NL, Araujo JGC, Boulos PB. Internal sphinctero-
tomy for chronic anal fissure: long term effects on anal
pressure. Br J Surg 1986; 73: 915–6.
75. Jensen SL, Lund F, Nielson OV, Tange G. Lateral subcutane-
ous sphincterotomy versus anal dilatation in the treatment
of fissure in ano in outpatients: a prospective randomized
study. BMJ 1984; 289: 528–30.
76. Olsen J, Mortensen PE, Krogh Peterson I, Christiansen J. Anal
sphincter function after treatment of fissure-in-ano by lat-
eral subcutaneous sphincterotomy versus anal dilation. Int J
Colorectal Dis 1987; 2: 155–7.
77. Marby M, Alexander-Williams J, Buchman P et al. A ran-
domized controlled trial to compare anal dilation with lat-
eral subcutaneous sphincterotomy for anal fissure. Dis Colon
Rectum 1979; 22: 308–11.
78. Saad AM, Omer A. Surgical treatment of chronic anal fis-
sure-in-ano: a propetive randomised study. East Afr Med J
1992; 69(11): 613–5.
79. Weaver RM, Ambrose NS, Alexander-Williams J, Keighley
RB. Manual dilatation of the anus vs. lateral subcutaneous
sphincterotomy in the treatment of chronic fissure-in-ano:
results of a prospective, randomized clinical trial. Dis Colon
Rectum 1987; 30: 420–3.
80. Gough MJ, Lewis A. The conservative treatment of fissure-in-
ano. Br J Surg 1983; 70: 175–6.
81. Di Visconte MS, Di Bella R, Munegato G. Randomized, pro-
spective trial comparing 0.25 percent glycerin trinitrate oint-
ment and anal cryothermal dilators only with 0.25 percent
glycerin trinitrate ointment and only with anal cryothermal
dilators in the treatment of chronic anal fissure: a two year
follow-up. Dis Colon Rectum 2006; 49: 1822–30.
82. Renzi A, Brusciano L, Pescatori M et al. Pneumatic bal-
lon dilation for chronic anal fissure: a prospective, clini-
cal, endosonographic and manometric study. Dis Colon
Rectum 2005; 48: 121–6.
83. Boschetto S, Giovannone M, Tosoni M, Barberani F. Hydro-
pneumatic anal dilation in conservative treatment of chronic
anal fissure:clinical outcomes and randomized comparison with
topical nitroglycerin. Tech Coloproctol 2004; 8: 89–93.
84. Abcarian H. Surgical Correction of chronic anal fissure:
results of lateral internal sphincterotomy vs. fissurectomy-
midline sphincterotomy. Dis Colon Rectum 1980; 23: 31–6.
85. Parks A. The management of fissure in ano. Hosp Med 1967;
1: 737.
86. Notaras MJ. Lateral subcutaneous sphincterotomy for anal
fissure- a new technique. Proc R Soc Med 1969; 62: 713.
87. Boulos PB, Araujo JGC. Adequate internal sphincterotomy
for chronic anal fissure: subcutaneous or open technique?
Br J Surg 1984; 71: 360–2.
88. Kortbeek JB, Langevin JM, Khoo REH, Heine JA. Chronic
fissure in ano: a randomized study comparing open and
subcutaneous lateral internal sphincterotomy. Dis Colon
Rectum 1992; 35: 835–7.
89. Arroyo A, Perez F, Serrano P, Candela F. Open versus closed
lateral sphincterotomy performed as an outpatient proce-
dure under local anesthesia for chronic anal fissure: prospec-
tive randomized study of clinical and manometric longterm
results. J Am Coll Surg 2004; 199: 361–7.
90. Wiley M, Day P, Rieger N, Stephens J, Moore J. Open vs. closed
lateral internal sphincterotomy for idiopathic fissure-in-
ano: A prospective, randomized, controlled trial. Dis Colon
Rectum 2004; 47: 847–52.
91. Elsebae MMA. A study of fecal incontinence in patients with
chronic anal fissure: prospective, randomized, controlled
trial of the extent of internal anal sphincter division during
lateral sphincterotomy. World J Surg 2007; 31: 2052–7.
92. Bulent Mentes B, Ege B, Leventoglu S, Oguz M, Karadag A.
Extent of lateral internal sphincterotomy: up to the dentate
line or up to the fissure apex? Dis Colon Rectum 2005; 48:
365–70.
93. Ammari FF, Bani-Hani KE. Faecal incontinence in patients
with anal fissure: a consequence of internal sphincterotomy
or a feature of the condition? Surgeon 2004; 2(4): 225–9.
94. Casillas S, Hull TL, Zutshi M et al. Incontinence after
lateral sphincterotomy: Are we underestimating it? Dis
Colon Rectum 2005; 48: 1193–9.
95. Garcia-Aguilar J, Belmonte Montes J, Perez JJ et al. Incontinence
after lateral internal sphincterotomy:anatomic and functional
evaluation. Dis Colon Rectum 1998; 41: 423–7.
96. Rosa G, Lolli R, Piccinelli D et al. Calibrated lateral internal
sphincterotomy for chronic anal fissure. Tech Coloproctol
2005; 9: 127–32.
97. Hyman N. Incontinence after lateral internal sphinctero-
tomy: a prospective study and quality of life assessment. Dis
Colon Rectum 2004; 47: 35–8.
98. Jenkins JT, Urie A Molloy RG. Anterior anal fissures are asso-
ciated with occult sphincter injury and abnormal sphincter
function. Colorectal Dis 2008; 10(3): 280–5.
2
improved outcomes in colon and rectal surgery
99. Corby H, Donnelly VS, O’Herlihy C, O’Connell PR. Anal
canal pressures are low in postpartum anal fissure. B J Surg
1997; 84: 86–8.
100. Jonas M, Speake W, Scholfield JH. Diltiazem heals glyceryl
trinitrate-resistant chronic anal fissures. Dis Colon Rectum
2002; 45: 1091–5.
101. Lindsey I, Jones OM, Cunningham C, George BD, Mortensen
NJM. Botulinum toxin as second line therapy for chronic
anal fissure failing 0.2 glyceryl trinitrate. Dis Colon Rectum
2003; 46: 361–6.
102. Witte ME, Klaase JM. Botulinum toxin A injection in ISDN
ointment-resistant chronic anal fissures. Dig Surg 2007; 24:
197–201.
103. Lysy J, Israeli-Yatzkan Y, Sestiery-Ittah M et al. Topical
nitrates potentiate the effect of botulinum toxin in the
treatment of patients with refractory anal fissure. Gut 2001;
48: 221–4.
104. Jones OM, Merrie A, Cunningham C et al. Randomized
clinical trial of botulinum toxin plus glyceryl trinitrate vs.
botulinum toxin alone for medically resistant chronic anal
fissure: overall poor healing rates. Dis Colon Rectum 2006;
49: 1574–80.
105. Nyam DCNK, Wilson RG, Stewart KJ, Farouk R, Bartolo
DCC. Island advancement flaps in the management of anal
fissures. B J Surg 1995; 82: 326–8.
106 Leong AF, Seow-Choen F. Lateral sphincterotomy compared
with anal advancement flap for chronic anal fissures. Dis
Colon Rectum 1995; 38: 69–71.
107. Fleshman JW. Fissure in ano and anal stenosis. In: Beck DE,
Wexner SD, eds. Fundamentals of Anorectal Surgery,New
York: McGraw-Hill, 1992: 170–82.
108. Leong AFPK, Seow-Choen F. Lateral sphincterotomy com-
pared with anal advancement flap for chronic anal fissure.
Dis Colon Rectum 1995; 38: 69–71.
109. Oh C, Albanese C. S-plasty for various anal lesions. Am J Surg
1992; 163: 606–8.
110. Ferguson JA. Repair of “Whitehead defornllty” of the anus.
Sur’g Gynecol Obstet 1959; 108: 115–6.
111. Corman ML, Veidenheimer MC, Coller JA. Anoplasty for
anal stricture. Surg Clin North Am 1976; 56: 727–31.
112. Penn JA. A case of anal reconstruction by means of local
skin flaps. Br J Plast Surg 1948; 1: 87–8.
113. Gingold BS, Arvanitis M. V-V anoplasty for treatment of
anal stricture. Surg Gynecol Obstet 1986; 162: 241–2.
114. Angelchik PD, Harms BA, Starling JR. Repair of anal stricture
and mucosal ectropion with V-V or pedicle flap anoplasty.
Am J Surg 1993; 166: 55–9.
115. Ramanujan PS, Venkatesh KS, Cohen M. V-V anoplasty for
severe anal stenosis. Contemp Surg 1988; 3: 62–8.
116. Rosen L. V- Y advancement for anal ectropion. Dis Colon
Rectum 1986; 29: 596–8.
117. Christensen MA, Pitsch RM, Cali RL, Blatchford GJ, Thorson
AG. “House” advancement pedicle flap for anal stenosis. Dis
Colon Rectum 1992; 35: 201–3.
118. Caplin DA, Kodner IJ. Repair of anal stricture and mucosal ectro-
pion by simple flap procedures. Ills Colon Rectum 1986; 29: 92.
Surgery for pilonidal disease and hidradenitis suppurativa
Paula I Denoya and Eric G Weiss
CHALLENGING CASE
A 35-year-old healthy male undergoes pilonidal cystectomy by
wide local excision down to sacral fascia. Six months postop-
eratively his wound has failed to heal as manifest by a persistent
4 × 4 cm by 2 cm deep granulation bed.
CASE MANAGEMENT
The patient has a nonhealing pilonidal wound. Options include
surgical reexcision with intensive postop wound management or
some type of excision and flap closure as described in this chapter.
INTRODUCTION
Pilonidal disease and hidradenitis suppurativa are both condi-
tions affecting the perianal area, and therefore are often referred
to the colorectal surgeon for management. The management of
these diseases can be quite challenging. Both conditions may be
complicated by recurrent disease and may result in significant
scarring or large open wounds in the perianal or coccygeal area.
PILONIDAL DISEASE
Pilonidal disease is a chronic suppurative condition which occurs
most commonly in the sacrococcygeal area. It typically presents
as a painless cyst or sinus opening in the gluteal cleft, as an acute
or recurrent abscess, or as chronic draining sinuses. It most com-
monly affects Caucasian males between the ages of 15 and 30
and is essentially not seen after the age of 45. The true incidence
is unknown, but pilonidal disease is responsible for the loss of
significant healthcare resources and workhours.
Historical Perspective
Pilonidal disease is believed to have been first described by Mayo
(1) in 1833. The term “pilonidal,” originating from the Latin
words for “hair” and “nest,” was not coined until 1880 by Hodges.
(2) The disease became more widely known during World War
II, when the number of soldiers developing it put a burden on
the military. At this point, it acquired the name of “jeep disease;”
a term coined by Buie (3) based on the idea that the disease was
caused by trauma to the skin of the lower back from riding in jeeps
for extended periods of time under hot and sweaty conditions.
Early in the documented history of this disease, the etiology
was believed to be congenital. The pilonidal cysts and sinuses
were thought to be embryologic remnants resulting from failed
involution of the neural tube structures. This theory was sup-
ported by studies of fetuses, which identified remnants of midline
structures. It was believed that these structures would normally
involute before birth, but sometimes failed to do so and led to the
development of pilonidal sinuses.(4)
In 1946, Patey (5) introduced a theory of an acquired etiology
for pilonidal disease, suggesting that hair piercing into the sacro-
coccygeal skin caused the sinuses and infected cysts. This acquired
theory was later supported by other studies (6–8) and is now widely
accepted as the etiology of pilonidal disease. Loose hairs from the
head or back fall and accumulate in the gluteal cleft. The hairs are
then drilled into the skin deep in the cleft by friction from the but-
tocks rubbing together while walking. As the person ambulates, the
hairs get pulled into the sinus, creating a cyst containing hair and
debris. This can periodically get infected and drain spontaneously
through lateral sinus tracts, or present acutely as an abscess. Studies
of surgical specimens have found cysts containing hair and debris,
but hair follicles have never been found in the cyst wall itself, sup-
porting the theory that the hair is of external origin.(9) (Figure
21.1) Bascom studied the midline pits and believed that these are
likely enlarged hair follicles which are involved in the etiology of
the disease.(10) He theorized that ingrown hairs originating in
these midline gluteal hair follicles were pushed into the subcutane-
ous fat and resulted in pilonidal abscess. Pilonidal disease has also
rarely been described in other areas of the body, such as the hands
of barbers (11), sheep shearers (12), and others who handle loose
hairs.(13) This further supports the acquired nature of the disease.
Diagnosis
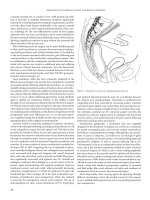
The diagnosis is made by physical examination in a patient who
generally fits the demographics of being hirsute and in the 2nd or
3rd decade of life. Characteristic findings on exam are small mid-
line pits at the superior aspect of the gluteal cleft, approximately
3–5 cm from the anus. (Figure 21.2) There may be only one or mul-
tiple pits present, and there may be tufts of hair or debris in them.
Some patients may also have lateral fistula openings which can
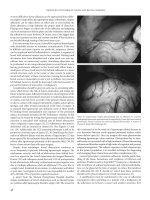
Figure 21.1 Pilonidal cyst opened after excision showing hair inside cyst cavity.
improved outcomes in colon and rectal surgery
undergoing incision and drainage for first presentation of pilo-
nidal abscess, 58% healed the wounds within 10 weeks, and 21%
recurred during the 18 month follow-up period.(17) This recur-
rence rate of approximately 20% is consistent with that found
throughout the literature.
Management of Chronic Disease
Chronic pilonidal disease may present in several forms: a nonheal-
ing wound after initial drainage, chronically draining sinuses, or
recurrent pain and infection. The goals of definitive treatment of
the disease are to remove the diseased tissue in a manner that will
prevent recurrence, to change the local environment of the gluteal
cleft, to decrease the chance of recurrence and allow healing, and
to allow the patient to resume their normal activities and return
to work quickly. There is no one ideal approach to managing this
disease.
Nonsurgical Treatment
There is very little role for purely nonsurgical management of pilo-
nidal disease. In select patients who are found to have asympto-
matic midline pits with no evidence of infection, it is possible to just
observe the patients. Prophylactically, the patient may be instructed
to ensure good hygiene, to keep the area of the gluteal cleft dry,
and to periodically shave the area to keep hairs from accumulating.
Patients who present with acute abscess will require drainage, but
sometimes may be able to be managed nonoperatively afterwards.
Figure 21.2 Chronic pilonidal disease showing midline pit.
Figure 21.3 Acute pilonidal abscess. Note midline opening with abscess slightly
to the right of midline.
periodically drain purulent discharge. In the acute presentation, the
patient may present with an abscess which is usually found just off
the midline, along with the typical finding of midline pits. (Figure
21.3) The differential diagnosis includes perianal abscess or fistula,
hidradenitis suppurativa, and other presacral or spinal lesions such
as chordoma or ependymoma. However, pilonidal disease can usu-
ally be identified by its characteristic location in the gluteal cleft
away from the anus, and by the presence of midline pits. In severe
cases where there is doubt, imaging with CT scan or MRI (14) may
be useful, though usually not necessary.
There have been rare reports of malignancy developing in
chronic pilonidal sinuses. Most commonly these are squamous
cell carcinomas. These tumors are fairly aggressive, with a high
recurrence rate and poor prognosis.(15, 16)
Management of Acute Disease
The treatment for an acute pilonidal abscess is similar to that of
an abscess in any other location. Incision and drainage, leaving
the wound to heal by secondary intention, is the accepted treat-
ment modality. The patient can be positioned either in lateral
decubitus or prone position, though prone is generally preferred.
Incision and drainage may be performed under local, regional,
or general anesthesia, and may be done in an ambulatory setting
such as the office or emergency room. The area should be prepped
in standard fashion and local anesthetic infiltrated over the area
of fluctuance. A vertical incision or an ellipse of skin should be
made over the fluctuant area, 1–2 cm off the midline. Purulent
fluid, along with hair or other debris, may be found in the cyst
cavity. This should be removed and the cavity packed. An alter-
nate technique for patients with a large abscess cavity is to use
catheter drainage, as described in Chapter 19. The patient may
be discharged on antibiotics to treat the overlying cellulitis if
present and instructed in wound care. In a series of 73 patients
surgery for pilonidal disease and hidradenitis suppurativa
Approximately 20% of patients who undergo abscess drainage
will suffer from recurrent disease. There is little information avail-
able regarding the nonoperative treatment of these recurrences.
Armstrong et al. (18) reported faster healing in 101 patients who
were managed with gluteal cleft shaving and good perineal hygiene
following incision and drainage, when compared with 229 patients
who underwent surgical management after drainage. Following
these initial findings, the authors implemented a policy of nonop-
erative management for their patient population. They reported
only 150 hospital admissions for complications of pilonidal disease
during the study period of 17 years, of whom only 23 patients
required surgical management. They did not specifically report how
many total patients were under their care during the study period. It
is likely that the patients who responded well to nonoperative treat-
ment had milder disease than the ones that required further surgery.
This conservative approach can be considered in select patients with
mild disease, or in patients with significant medical contraindica-
tions to surgery. Many surgeons advocate some form of depilation
following surgery to aid in healing. Whether this is shaving, waxing,
chemical depilation, or laser treatments can be left up to individual
patient or surgeon preference.(19)
Other methods that have been described with varying success
are fibrin glue or phenol injections into the sinuses. Greenberg
et al. reported a series of 30 patients treated with fibrin glue
injection with no recurrence or infection after a follow-up of 23
months.(20) Another study of six patients who had injection of
fibrin glue into the sinus after curettage of pits reported no recur-
rences at 1 year.(21) However, this technique has not been tested
in larger case series or randomized trials.
Phenol sclerotherapy has been used for treatment of pilonidal
disease with varying success. Early studies showed potential benefit
in uncomplicated cases. Dogru et al. reported a series of 41 patients
(22) who had crystallized phenol applied to the wounds after
limited excision of midline pits. Most patients required 2–3 appli-
cations, and 95% healed completely. There were two recurrences of
disease. However, another study of 45 patients who had 1–2 mL of
80% phenol solution into the sinus reported 60% healing, and five
patients developed abscess requiring operative drainage.(23)
Surgical Options for Chronic Pilonidal Disease
Operations for chronic pilonidal disease involve excision of the
diseased tissue. This may result in a large defect which is difficult
to close in an area which is subject to significant tension and mois-
ture. This challenge has fueled the development of many different
surgical techniques in an attempt to find the ideal operation. So
far, no technique has proven to be ideal. This section of the chapter
will review the most common operations and give an algorithm
for the surgical approach to the management of chronic disease.
The operations described may be performed in prone or lateral
decubitus position, under regional or general anesthesia. The jack-
knife prone position, with the operating table flexed at the waist
approximately 30 degrees and the buttocks taped apart, provides
the ideal exposure for most operations and is recommended unless
contraindicated by individual patient factors. Standard periopera-
tive antibiotics are given before incision. There is no need to con-
tinue antibiotics postoperatively unless there is overlying cellulitis
from acute infection. Many of these operations may be done on an
outpatient basis. The more complex flaps require the patients to
remain in the hospital on bedrest for approximately 2 days. These
patients may also receive postoperative antibiotics for a few days
until the drains are removed. Sutures are usually removed between
7 and 10 days after surgery.
Wide Local Excision
The most commonly performed operation for pilonidal disease
is wide local excision. An elliptical incision including all sinus
tracks is made and carried down to the sacrococcygeal fascia, so
that the entire cyst is removed. There is debate as to the best way
to manage the large wound that results. The benefits of leaving the
wound open to heal by secondary intention include less chance of
infection or wound breakdown, but this is counterbalanced by the
increased time required to completely heal the wound, the need
for frequent dressing changes, the added discomfort of having an
open wound, and the increased time lost from work. There are few
randomized trials which examined this problem. (Table 21.1) A
series of 120 patients (24) who were randomized to either excision
left open to heal by secondary intention or excision with primary
Table 21.1 Procedures for pilonidal disease: wide excision with healing by secondary intention or primary closure.
Study
Closure
Number of Patients
Hospital Time
(days)
Healing Time (days)
Infection (%)
Recurrence (%)
Follow-up
(months)
Sondenaa et al.
(24) (1996)
Open
60 Outpatient 86 13 5 50
Closed 60 Outpatient 23 30 10 50
Al-Salamah et al.
(27) (2007)
Open 192 4
(-)
3.12 3 35
Closed 188 3.6
(-)
4.2 3.7 36
Fazeli et al. (45)
(2006)
Open 72 1.76 41 13.9 4.2 22
Mentes et al. (25)
(2006)
Closed 493 5.5
(-)
1.2 5.6 18
Tejirian et al. (26)
(2007
Open 26
(-)
147
(-)
35
(-)
Marsupialization 42
(-)
42
(-)
2
(-)
Note: (-) not described.