Improved Outcomes in Colon and Rectal Surgery part 25 pdf
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (216.05 KB, 10 trang )
improved outcomes in colon and rectal surgery
will result in urge incontinence as well with stool clustering and
increased frequency. Secondly, the history should identify concomi-
tant symptoms, such as urinary incontinence and pelvic organ pro-
lapse since FI is frequently only part of a more general pelvic floor
dysfunction. Thirdly, questions should elicit underlying risk factors,
particularly those that are readily corrected (Table 22.1). Emphasis
must be given to a detailed obstetric history to identify surrogate
markers of a traumatic childbirth (instrumental delivery, prolonged
second stage of labor, birth weight greater than 4 kg, episiotomy)
(8) and to evaluate the presence of FI symptoms in the postpartum
period. A careful assessment of stool consistency and defecation
habits will help determine the potential benefits of a bowel regulat-
ing treatment. Finally, a detailed history of FI will guide selection of
appropriate investigations.
Physical examination should identify possible causes, effects
and coexisting conditions of FI. Perineal scarring, diminished
perineal body, or palpable sphincter defects will suggest obstet-
ric trauma. A patulous anus is a sign of sphincter denervation.
Dermatitis and excoriation result from prolonged exposure to
feces and poor hygiene. Furthermore the clinician should actively
look for any anorectal conditions causing “pseudo-incontinence”
such as rectal prolapse or prolapsing hemorrhoids, skin tags,
mucosal ectropion, or fistula in-ano. Digital rectal examination
provides gross information on sphincter bulk, anal canal tone,
anal stenosis, and presence of masses. A vaginal exam is essential
to assess for coexisting conditions such as rectocele, enterocele,
uterine or vaginal apex prolapse and cystocele.
Additional studies
The aim of additional studies is to identify the cause of FI and
risk factors amenable to treatment. Endoscopic examination, at
least a flexible sigmoidoscopy if not a full colonoscopy, should be
performed to rule out conditions that may contribute to FI such
as polyps, malignant lesions, or inflammatory bowel disease.
A variety of anorectal physiology tests (ARP) are available
to further clarify the etiology of FI. Sphincter anatomy can be
assessed by endoanal ultrasound or MRI; resting and squeeze
anal canal pressures can be measured by manometry; anorectal
sensation and reflexes (minimal volume to elicit sensation, maxi-
mal tolerated volume, presence of rectoanal inhibitory reflex) can
be estimated by balloon inflation manometry or with techniques
that measure thermal change sensitivity or mucosal electrosen-
sitivity; integrity of sphincter innervation can be evaluated by
pudendal nerve terminal motor latency and electromyography;
defecation dynamics can be assessed by barium cinedefecogra-
phy, balloon expulsion tests, and more recently by dynamic MRI.
However, the need for ARP testing outside of research cent-
ers is still debated (15–17) for several reasons. There is relative
absence of standardization of test techniques and norm values
established from large cohorts of healthy individuals. Few stud-
ies have shown clear correlations between baseline ARP and
treatment outcome. Studies evaluating the clinical utility of ARP
have all too often included only small numbers of patients and
contained important design flaws. Having said that, in these
studies ARP testing appeared to improve the understanding of
etiology and change treatment strategy of FI in approximately
15%.(18–20) Most changes in treatment strategy were due to
more accurate assessment of sphincter defects with endoanal
ultrasound (EAUS). Since the decision between medical or sur-
gical management (sphincter repair) of FI is largely based on
the extent of sphincter injury, imaging of the sphincter makes
sense. Both EAUS and MRI (with endorectal coil) appear to be
highly accurate in identifying sphincter defects (21), especially
of the distal part of the anal canal.(22) EAUS has the advantage
of being inexpensive and readily available. Three-dimensional
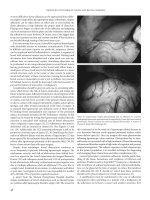
EAUS (Figure 22.1a and 22.1b) and transperineal ultrasound
may further increase accuracy.(23, 24) MRI depicts the external
Figures 22.1a and 22.1b (a)EAUS image of a normal anal canal with complete internal
sphincter (IS) hypoechogenic ring and external sphincter (ES) hyperechogenic ring.
(b)EAUS image of anal canal with a wide internal (IS) and external sphincter (ES)
defect. Note the thickening and retraction of the internal sphincter and the anterior
scar (S) replacing the external sphincter.
(a)
(b)
s
IS
ES
ES
IS
surgical treatment of fecal incontinence
sphincter clearly because of the contrast between fat and striated
muscle and accurately visualizes external sphincter atrophy.
External sphincter atrophy can also be accurately diagnosed with
3D-EAS (25); its significance is not fully understood but it may
adversely affect outcome after sphincterplasty.(26) The value of
sphincter imaging has also been demonstrated in men with FI.
The presence of a sphincter defect –an internal sphincter defect
secondary to anal surgery in the vast majority of men – was a
clear predictor of failure of conservative management.(27)
In conclusion, the assessment of a patient with FI must include
a directed, detailed history and examination. After excluding cases
of “pseudo-incontinence” or minor seepage additional work-up
must include endoscopy and sphincter imaging, especially if treat-
ment with sphincteroplasty is considered. Defecography is helpful
to confirm suspected rectal procidentia. The utility of further ARP
studies will depend on local availability and expertise.
TREATMENT
Medical therapy
Initial treatment of FI should be conservative even if there is little evi-
dence to guide clinicians in the selection of drug therapies.(28) The
main targets of medical treatment of FI are intestinal transit and stool
consistency. A thorough work-up is mandatory in patients with FI
related to chronic diarrhea to identify and treat the underlying cause
of diarrhea. Dietary changes and prescription of either fiber supple-
mentation or fiber restriction must be individualized to each patient
as the change in bowel transit can be very variable.(29) In a placebo
controlled trial, psyllium and gum arabic, two natural soluble fibers,
were shown to reduce by 50% the proportion of incontinent stools in
39 patients with FI of loose or liquid stools.(30) Antidiarrheal medi-
cation (loperamide, diphenoxylate plus atropine, bile-acid binders,
codeine) is the next step in medical management of FI. Loperamide
has been shown to be more effective than diphenoxylate plus atropine,
and have fewer side effects than both diphenoxylate plus atropine and
codeine.(31) In addition to its effect on intestinal motility, loperamide
may improve sphincter tone and rectal sensation.(32) In an open label
trial in 18 patients with idiopathic FI, amitriptyline, a tricyclic antide-
pressant agent, was shown to improve FI scores in 89% of patients after
4 weeks of treatment; the proposed mechanism is a decrease in rectal
motor complexes and stool frequency.(33) Further studies are needed
to evaluate the true efficacy of this drug.
A different approach of medical treatment is to enhance anal
sphincter function by application of topical agents, such as
phenylephrine gel, an α1-adrenergic agonist. Three small dou-
ble blind placebo trials from the St. Mark’s hospital in the UK
showed significant improvement in sphincter tone (34) and FI
symptoms in half of ileal pouch patients (35) and in one third of
FI patients with anatomically intact sphincters.(36) Conversely,
Park et al. (37) in a double blind trial on 35 patients with FI after
low anterior resection found no improvement in FI or quality of
life scores with 30% topical phenylephrine compared to placebo.
Limited efficacy combined with frequent allergic reactions, limits
wider acceptance of this treatment.
Constipation and impaction can lead to overflow incontinence.
Such patients will benefit from routine tap-water enemas or laxa-
tives to empty the rectum regularly. Patients with postdefecation
seepage may also benefit from routine enemas as well as appli-
cation of cotton wicks at the anus and barrier creams to avoid
excoriation and pruritus.
Anal plugs for the management of FI is a different approach that
appears intuitive to many patients. A recent Cochrane review of pub-
lished randomized trials suggested that anal plugs seem to be difficult
to tolerate but if they are tolerated, they can be a useful tool in FI pre-
vention either as substitute or adjuvant treatment option.(38) Anal
plug models exist in a variety of forms, sizes, material, and function.
Devices with intrarectal sensors alerting the patient of an imminent
bowel movement with a beep have also been described.(39, 40)
Biofeedback
The goal of biofeedback is to improve external sphincter contrac-
tion (strength and duration) in response to rectal distention by
providing the patient with feedback information on perform-
ance and progress. In general, three different protocols are used:
(1) coordination training, which teaches patients to contract
their external sphincter muscle in response to rectal distention
counteracting the reflex internal sphincter relaxation; (2) sen-
sory training, which teaches patients to recognize progressively
smaller volumes of rectal distention enabling them to contract
the sphincter in time; and (3) strength training, which teaches
patients to isolate and exercise their sphincter muscle without
using rectal distention. In most centers, either manometry equip-
ment or an EMG probe is used to provide “feedback” information
to patients. The three training methods are sometimes combined;
the length and number of sessions varies widely.
Biofeedback is widely used and often included as first line option
in treatment algorithms for FI. No obvious clinical or physiologic
predictors of success have been identified. Patient age, etiology of
FI, duration, and severity of symptoms do not appear to predict
outcome; biofeedback has been used successfully in a variety of
situations including presence of external sphincter defects (41) or
in patients with poor functional outcome after sphincteroplasty
for obstetric injury.(42)
A systematic review on biofeedback through 2000 (43) found 46
original studies, only 8 of which employed some form of control
arm. All but one study (44), which included patients with neurogenic
FI, reported improvement of symptoms in a range of 53–100% of
patients. Overall, 617 of 861 (72%) reported to be cured or improved.
The same author performed a Cochrane review (45), including only
randomized or quasi randomized trials and concluded that the cur-
rent literature provides no evidence that biofeedback or anal sphinc-
ter and pelvic floor exercises improve outcome compared to other
conservative management methods. Training to enhance rectal
discrimination of sensation seemed to be helpful in reducing FI in
one short follow-up randomized study.(46) In absence of high level
evidence, interpreting the literature on biofeedback is problematic.
Some patients seem to benefit and there has been no morbidity
reported. High motivation both from the patient’s and therapist’s
side are crucial prerequisites for a successful outcome.
Sphincteroplasty
Anal sphincteroplasty is an appropriate therapy for patients with
significant FI, unresponsive to medical therapy and a documented
anal sphincter defect.
improved outcomes in colon and rectal surgery
Overlapping sphincteroplasty is usually performed under
general anesthesia, in the prone jack knife position after prior
mechanical bowel preparation and prophylactic antibiotics. A
curvilinear incision is made in the perineal skin closer to the vagi-
nal introitus than the anus to preserve tissue on the anal side. A
Lone Star® retractor is used for exposure and a needle tip electro-
cautery is preferred for more precise dissection with less char.
The external sphincter, en bloc with the internal sphincter and
anterior scar tissue is mobilized and dissected free from the skin
and ischiorectal fat laterally, from the posterior vaginal wall ante-
riorly and from the anoderm and rectal wall posteriorly. Careful
dissection, occasionally aided by inserting a finger in the vaginal
or rectal side, avoids buttonholing, especially on the rectal side.
Any injured venous sinuses on the posterior vaginal wall should
be suture ligated to avoid delayed hemorrhage. Care must be
taken with the posterolateral portions of the dissection to avoid
injury to branches of the pudendal nerve. Dissection in the mid-
line continues until soft, pliable tissue is reached on both the
vaginal and rectal sides and laterally until the two ends of the
external sphincter can be overlapped several centimeters without
tension. If the midline tissue is entirely scar tissue, it is divided to
perform an overlapping repair. If muscle is encountered in the
midline it is left intact and an imbricating repair rather than over-
lapping repair is performed. The overlapping repair is done with
absorbable 2–0 monofilament mattress sutures creating a snug
anal opening without excess tension on the mobilized tissue. The
wound is closed in a vertical or “T” fashion to decrease tension on
the skin. The center of the incision is left open and a short ¼ inch
Penrose or closed suction drain is placed through the opening
to facilitate drainage. The drain is removed before the patient’s
discharge. Vaginal packing may be placed to help with hemostasis
and if used is typically removed the next day. If planned, anterior
levatoroplasty is performed before the overlap. Proponents argue
that the levatoroplasty adds essential bulk to the perineal body and
lengthens the anal canal. Opponents believe that a levatoroplasty
increases the incidence of postoperative dyspaurenia. Diversion
of the fecal stream did not improve healing or functional results
of the repair in a randomized trial.(47)
As with any perineal wound, healing after overlapping sphinc-
teroplasty is slow with frequent separation of the skin edges.
Postoperative care includes the avoidance of impaction with the
use of bulk agents and tap water enemas and protection of the
surrounding skin with barrier ointments. Vaginal tampons and
intercourse are proscribed for 6 weeks.
One variation is the approximation of the ends of the sphincter
muscle rather than overlapping them. This technique is particu-
larly appropriate when a portion of the muscle is intact. In a ran-
domized study by Tjandra et al. (48) of 23 women with anterior
sphincter defects on EAUS, no functional difference was found
between patients repaired with the approximation technique and
those undergoing an overlapping repair.
Functional results after overlapping sphincteroplasty are good
or excellent approximately in two-thirds of patients in studies with
a follow-up under 4 years (Table 22.2a) and approximately in one
half of patients in studies with a longer follow-up (Table 22.2b).
Bravo-Gutierrez et al. reviewed functional outcome a median of
10 years after sphincteroplasty in 130 women and found that 58%
reported some incontinence of solid stool compared to 36% at
a 3 years follow-up.(49) Similarly, Barisic et al. found increased
failure rates with time as poor results were reported by 39% at 80
months compared to 9% at 3 months.(50) Malouf et al. reviewed
the results of sphincter repair in 46 patients a median (range)
of 77 (60–96) months.(51) Excluding 8 immediate failures 85%
of the others reported improvement at 15 months but only
50% at 77 months. Only 4 patients were completely continent
of stool but the median subjective rating of satisfaction with the
Table 22.2a Functional results of sphincteroplasty – short and
midterm follow-up.
Months
Follow-up
Median Excellent
Author Year n (range) or Good Fair Poor
Nikiteas (106) 1996 42 38 (12–66) 60% 17% 23%
Oliveira (107) 1996 55 29* 71% 9% 20%
Young (108) 1998 57 18 86% — 14%
Gilliland (109) 1998 77 24 (2–96) 55% 14% 31%
Karoui (110) 2000 86 40
a
81% — 19%
Buie (111) 2001 158 43
a
(6–120) 62% 26% 12%
Morren (112) 2001 55 40 (5–137) 56% 24% 20%
Pinta (113) 2003 39 22 (2–99) 31% 38% 31%
Evans (114) 2006 66 45* 77% — —
a. Mean.
Table 22.2b Functional results of sphincteroplasty – long term follow-up.
Author
Year
n included/n initial
Years Follow-up
Median (range)
Excellent or Good
Fair
Poor
Londono-Schimmer (115) 1994 94/128 4.9 (1–8.2) 50% 25% 25%
Malouf (51) 2000 46/55 6.4 (5–8) 50% 9% 41%
Halverson (116) 2002 49/71 5.3 (2–11.8) 49% — 51%
Zorcolo (117) 2005 62/93 5.8
a
(2–9.3) 54% 16% 30%
Barisic (50) 2006 56/65 6.7
a
48% 13% 39%
Bravo-Gutierrez (49) 2004 130/182 10 (7–16) 41% — 57%
Maleskar (52) 2007 64/72 at 7 62% 24% 15%
Grey (118) 2007 47/85 5–12 60% 36% 4%
a. Mean.
surgical treatment of fecal incontinence
long term results was 8 out of 10. Other studies document more
optimistic results. Maleskar et al. reported on 64 of 72 patients
responding to a questionnaire after a median of 7 years.(52) The
median CCF-FI score dropped from 16 preoperatively to 5 at 12
months and to 7 at a median follow-up of 7 years. Ninety five
percent of patients were satisfied with the results and 62% were
fully continent or incontinent to gas only. Interestingly, Vaizey
et al. found no difference in incontinence scores, patient rating of
improvement or satisfaction between the findings at 20 months
and 60 months in a group of patients who underwent a repeat
sphincter repair following a failed repair.(53)
If the initial repair fails and a persistent defect is demon-
strated by ultrasound, repeat sphincteroplasty can still provide
satisfactory results (54, 55) even with long-term follow-up.(53)
Breakdown of the wrap is not the only cause of failure. Progressive
neuropathy and the aging process in general are thought to con-
tribute to some deterioration of symptoms over time.
Patients with poor results may be candidates for biofeedback,
artificial bowel sphincter, or sacral nerve stimulation.
The role of sphincteroplasty in patients with incontinence and
sphincter defects is evolving with the addition of new modali-
ties of therapy. Further research is necessary to determine which
patients are appropriate candidates and whether adjunct therapies
such as biofeedback or sacral nerve stimulation would improve
the functional results.
Artificial Bowel Sphincter
The artificial bowel sphincter (ABS) is a treatment modality for
urinary incontinence which was adapted for FI. In 1996, the man-
ufacturing company (American Medical Systems, Minnetonka,
MN, USA) adapted the original device for its use in FI as the
Acticon
TM
Neosphincter device. Although other models have
been recently developed (56, 57), this device is the most widely
employed and reported in the literature.
The ABS consists of three components: an inflatable cuff,
placed around the deficient sphincter, a pressure-regulating bal-
loon placed in the retropubic space, and a control pump placed in
the scrotum or labia. The three components and the connecting
tubing are filled with saline. In the neutral state, the fluid fills the
cuff occluding the anal canal. When the patient desires to defecate,
he empties the cuff by manually compressing the pump, which
pushes the fluid into the pressure regulating balloon. The cuff
refills spontaneously in approximately 45 seconds.
The ABS is an invasive procedure with significant morbidity.
Candidates include patients who have failed all medical treatment
and are not candidates for a sphincter repair. Sufficient perineal
tissue without excessive scarring or prior radiation and a normal
rectal reservoir are required to minimize risk for late erosion and
dysfunction.(58)
Mundy et al. (59) performed a systematic review of the litera-
ture published through 2002 on safety and effectiveness of ABS in
FI. They included 13 case series involving 1 to 112 patients with
a mean follow-up time of up to 60 months. No study included
a control group or reported intention to treat results prevent-
ing judgment of the true effectiveness of ABS. Approximately a
third to half of patients needed surgical revision of the ABS and
one quarter required explantation, most commonly because of
infection or erosion. Wound healing problems, material breakage
or migration, fecal impaction, chronic pain, and dissatisfaction
also occurred. In patients with successful implantation, all stud-
ies reported clinically significant improvements in FI severity and
quality of life.
O’Brien et al. (60) performed a randomized trial on 14 patients
with severe FI comparing ABS to optimal medical therapy. In
the ABS group one out of seven patients had explantation of
the device after failed wound healing and two had prolonged
hospitalization for repeated fecal impaction or wound healing
problems. At 6 months, the Cleveland Continence Score showed
a 75% improvement in the ABS group with significantly better
quality of life scores. No significant changes were observed in the
medical treatment group.
Long term follow-up studies on ABS report higher rates of
reintervention and explantation with a functional ABS (61–63)
remaining in approximately 50 to 60% of patients. Patients who
retained their ABS seemed to have sustained improvement of FI
and quality of life over time (63) but a significant number expe-
rience evacuation difficulties.(58, 61) Michot et al. (58) found a
reduction of the explantation rate from 50% to 20% when com-
paring their early and late experience. The authors related this
improvement to better patient selection and liberal use of divert-
ing colostomy. Parker et al. (63) found no difference in failure
rates over time. A convened “best practice group” of colorectal
surgeons, whose infection rate was 9% and long term functional
device rate 82% have recently introduced a protocol to minimize
infection.(64)
ABS provides good continence in those patients who retain
their device at the expense of significant surgical morbidity and
possible chronic evacuation difficulties. Recent guidelines for
intraoperative prevention of infection may help improve out-
comes by decreasing morbidity.
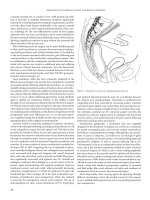
Dynamic graciloplasty
The concept behind dynamic graciloplasty (DGP) is to create a
sphincter with an autologous striated muscle wrap. The muscle
is then stimulated with a constant low-frequency electric current
by an implantable pulse generator with the goal of inducing the
fast-twitch, readily fatigued (Type II) muscle fibers to change to
slow-twitch, fatigue resistant (Type I) muscle fibers, similar to the
normal external sphincter. A pedicled gracilis flap is harvested on
one side, transposed, wrapped around the anus and anchored
with its distal tendon to the contralateral ischial tuberosity. The
electrode is implanted in the muscle or close to the obturator
nerve and the stimulator is implanted in the lower abdomen,
subcutaneously or beneath the rectus sheath. Increasing levels
of neurostimulation are used to condition the muscle during the
first 2 months. Thereafter, the patient can regulate defecation
with the aid of an external magnet by turning the stimulator off
to relax the muscle allowing emptying of the rectum and turning
the stimulator back on to maintain continence.
Similar to ABS, DGP is reserved as an alternative to colostomy
for patients suffering severe FI unresponsive to simpler treat-
ment. As opposed to the ABS, DPG involves transposition of
healthy tissue and can be applied even to patients with severe loss
of perineal tissue.
improved outcomes in colon and rectal surgery
A systematic review of the literature through 1999 on DGP by
the Australian Safety and Efficacy Register of New Interventional
Procedures-Surgical found that DGP was effective at restoring
continence in 42 to 85% of patients but was associated with an
average risk of complications of 1.12 per patient and reopera-
tion of 0.14 to 1.07 per patient (65); none of the included studies
provided a high level of evidence. Overall DGP related mortal-
ity was 1% and the most common complications were infection
(28%), hardware dysfunction, or displacement (15%) and leg
pain (13%). The Dynamic Graciloplasty Therapy Study Group
undertook a large international multicenter prospective trial
including 115 eligible patients, 27 of whom had a preexisting
functioning stoma.(66–68) The success rate, defined as 50% or
more reduction in incontinent episodes, was 62% at 12 months
and 56% at 24 months for nonstoma patients and 37.5% and 43%
in patients with preexisting stoma at 12 and 24 months respec-
tively. Significant improvement in quality of life subscales was
noted. One patient died postoperatively and major complications
requiring hospitalization or surgical intervention occurred 89
times in 61 (50%) patients; 90% resolved completely. Rongen et al.
(69) from the Maastricht group reported the largest single center
experience with DGP on 200 consecutive patients with a median
follow-up of 5 years. The success rate (continent to solid and liq-
uid stool) was 72%, ranging from 52% in patients with congenital
FI to 82% in patients with traumatic FI. The success seemed to
persist over time as complications decreased and technical suc-
cess improved. Chronic evacuation problems persisted in 16% of
the patients. The indications for sphincter replacement surgery
either with ABS or DGP are decreasing in favor of SNS (70) given
the significant difference in morbidity. DGP is not available in the
USA as the producer of the stimulator (Medtronic Corporation,
Minneapolis, MN) decided not to pursue FDA approval.
Sacral Nerve Stimulation
Sacral Nerve Stimulation (SNS) is an innovative and rapidly
expanding treatment modality. It has been used for urinary
incontinence since 1981 and was approved by the FDA for that
indication in 1997. The observation that bowel symptoms simul-
taneously improved in many patients led to the first implantation
of a sacral nerve stimulator to treat FI in 1994.(71) In the USA,
a multicenter study completed enrollment of 120 patients in 2006
and the manufacturer (Medtronic Corporation, Minneapolis,
MN) is expected to pursue FDA approval in 2008.
The goal of placing a stimulating electrode into the sacral foramina
was to recruit residual function of the striated pelvic floor and exter-
nal sphincter muscles. Initial selection criteria for SNS stipulated
reduced or absent voluntary sphincter function, intact nerve-muscle
connection and an intact sphincter muscle.(72) It became apparent
that the effect of SNS was not limited to an increase of voluntary
squeeze pressure. Somewhat inconclusive and often contradictory,
studies suggest that SNS may decrease urge thresholds, reduce spon-
taneous rectal motility, reduce spontaneous sphincter relaxation,
and improve anal and perianal skin sensitivity.(72) Sheldon et al.
(73) showed in a crossover study in 10 women with FI that SNS also
affects the central nervous system; they documented a decrease in
corticoanal excitability. More recently, in a cohort of patients with FI
successfully treated with SNS, Gooneratne et al. (74) demonstrated
a normalization of elevated levels of rectal mucosal substance P, a
substance known to play a role in contractility and afferent signaling
in visceral sensation.
While the understanding of the physiology of SNS still remains
unclear, patient selection has become more pragmatic. The efficacy
of SNS can be tested on an individual patient temporarily with
minimal consequences and a high predictive value of permanent
therapeutic effect. The screening procedure consists of a percutane-
ous stimulation of the S2–S4 roots on both sides. The testing is done
under local or general anesthesia by insertion of a needle electrode
into the dorsal sacral foramina. The site providing the most effective
bellows-like motion of the pelvic floor along with plantar flexion of
the first and second toes (typically S3 root) is selected for tempo-
rary stimulation. Continuous stimulation is applied for a minimum
of 1 week. If the stimulation is well tolerated and successful (50%
or greater reduction in incontinent episodes per week or days with
incontinence per week), a permanent pulse generator is connected
to the electrode and implanted. Surgical replacement of the battery
is necessary after 7–10 years for Interstim I and 5–7 years for the
newer and smaller model Interstim II.
More than 75% of patients tested with temporary stimulation
will have a 50% or more improvement in symptoms, which is
required to justify permanent implantation (Table 22.3). The
therapeutic benefit seems to persist in studies with follow-up
over 2 years.(75–77) SNS has been shown not only to decrease
the frequency of FI but also to improve the ability to postpone
defecation (76), improve sexual activity (78) and quality of life.
(72, 79) A Swiss group performed a cost analysis on a cohort of
36 patients including expenses generated by failures and compli-
cations and found that SNS is more cost efficient than colostomy
or dynamic graciloplasty but obviously more expensive than
conservative treatment alone.(80)
The indications for SNS have progressively expanded. Accepted
contraindications include conditions where implantation is impos-
sible or too risky (e.g., spina bifida, pilonidal sinus, pyoderma
gangrenosum), chronic diarrhea, irritable bowel syndrome, rec-
tal prolapse, mental or physical inability to adhere to treatment,
severe bleeding diathesis, pregnancy, and the presence of cardiac
pacemaker or implantable defibrillator.(81) Earlier contraindica-
tions such as previous rectal surgery, multiple sclerosis, Parkinson’s
disease, and spinal cord injury have been recently challenged.
(82) The most interesting controversial issue is the use of SNS in
patients with FI and sphincter defects as these patients are tra-
ditionally treated with sphincteroplasty. Initial studies did not
include patients with sphincter defects except very minor ones.
Dudding et al. (83) analyzed the 10 year experience with SNS at St.
Mark’s hospital in the UK in an effort to identify predictive factors
of success. Patients with evidence of sphincter trauma had a greater
risk of failure compared to patients with intact sphincters (7/29 vs.
0/16, p=0.04). Conversely, in a retrospective study, Melenhorst et
al. (84) compared a group of women with a functionally failed
but anatomically intact previous sphincter repair to a group of
women with an external sphincter defect of 17–30%. They found
no significant difference in baseline characteristics and a similar
outcome after a 2-year follow-up. In a controlled randomized
study Tjandra et al. compared SNS to optimal medical treatment.
Close to half of patients in the SNS arm had evidence of external
surgical treatment of fecal incontinence
sphincter defect (120° or less) and more than half had a previous
sphincter repair. Despite that, excellent results were achieved in the
SNS arm as 66% of patients had a 75–100% reduction of inconti-
nent episodes per week. On the contrary, patients in the medical
treatment arm experienced no change in FI severity or FI-related
quality of life scores. In absence of a randomized study that spe-
cifically addresses the question, there is currently no evidence to
support the idea that SNS should replace sphincteroplasty as avail-
able studies are subject to important patient selection bias.
Complications with SNS are rare and include wound problems
(dehiscence, seroma, infection, bleeding), electrode dislodgment
or fracture, pain at the site of the electrode or pulse generator,
excessive tingling in the vaginal region, loss of effect, or deteriora-
tion of bowel symptoms. Complications leading to explantation
of the stimulator occur in approximately 5%.(72, 85)
The role of SNS in the treatment of FI is expected to grow.
Further understanding of the physiology involved may improve
patient selection and stimulation modes. Peripheral nerve stimu-
lation may render the technique simpler and applicable to patients
with sacral abnormalities. Transcutaneous intermittent stimula-
tion of the posterior tibial nerve has been reported to improve
urinary continence and has been tried more recently in FI with
encouraging preliminary results.(86, 87) Direct stimulation of
the pudendal nerve is another field of investigation.
Injectable bulking agents
The goal of injectable bulking agents (IBA) is to restore a normal
contour of the anal canal and add bulk to provide a better seal. IBA
are usually injected under local anesthesia as an office or outpa-
tient procedure; the injections may be into the submucosa or in the
intersphincteric space and in all quadrants of the anal canal or at
the site of a sphincter defect. Injection under ultrasound guidance
in the intersphincteric space yielded better results than digitally
guided injections in a randomized study.(88) Small studies have
reported the use of Polytetrafluoroethylene (Teflon®), autologous
fat, glutaraldehyde cross-linked collagen (Contigen®), textured
silicon particles (Bioplastique®, renamed PTQ implants
TM
), and
pyrolytic carbon coated zirconium oxide beads (Durasphere®),
but their true efficacy remains to be determined. The autologous
materials are short lived and adipose tissue injections carry the
risk of fat embolism. Cost as well as migration, ulceration, leak-
age, infection, pain and local, or distal inflammatory reactions
are concerns with the synthetic materials. The two most popular
agents are PTQ implants
TM
and Durasphere®; both were shown
to be safe and to attenuate severity of FI in a majority of patients.
(88, 89) Quality of life improvements are less pronounced. Results
after long-term follow-up are mixed and reinjection is necessary
in some patients.(90, 91) An ongoing multicenter randomized
placebo controlled study on Durasphere ® injection will hopefully
help determine the place of IBA in the treatment of FI.(92, 93) The
FDA has not yet approved any IBA for the use in FI.
Antegrade colonic enema
In 1990, Malone described the creation of a continent stoma using
the appendix; this stoma was catheterized to perform ante grade
colonic enemas (ACE) in five patients with intractable FI.(94)
Modifications of the technique for patients in whom the appendix
cannot be used due to previous appendectomy or fibrosis include
construction of the stoma with a cecal flap or an “ileal neo-appen-
dix”. ACE is used frequently in pediatric surgery for children with
severe defecation disorders following anorectal malformations,
spina bifida, sacrococcygeal teratomas and other abnormalities.
Several small studies report its use in adults and even in patients
undergoing abdomino-perineal resection in combination with a
perineal colostomy.(95) Lefevre et al. (96) recently reported 25
adult patients with intractable FI treated with an ACE procedure.
After a median follow-up of 21 months, 22 patients were avail-
able: 4 had stopped performing enemas but 17 reported perfect
cleanliness. They performed enemas once every 2–3 days spend-
ing an average of 40 minutes. Stenosis of the mucocutaneous
junction occurred in 20%; the majority responded to dilatation.
It occurred more often in patients with native appendicostomy
Table 22.3 Functional results of SNS in large studies.
Author
Year
Study
Design
Temporary
Stimulation
(n)
Permanent
Stimulation
(n %)
f-up
time
months
Baseline FI
Episodes
(n)
Final FI
Episodes (n)
Fully
Continent
(n %)
Matzel (76) 2004 prosp MC 37 34 (92) 24 8.3
b
0.75 12 (37)
Jarrett (119) 2004 prosp MC
59 46 (78) 12 7.5
b
1 19 (41)
Leroi (120) 2005 RCT MC
DB cross
34 27 (79) 6–8
a
7
b
1 5 (26)
Melenhorst (77) 2006 observ SC 134 100 (75) 26 31.3
c
4.4 nr
Holzer (121) 2007 observ SC 36 29 (81) 35 7
c
2 nr
Hetzer (79) 2007 observ SC
44 37 (84) 13 14
b
5 nr
Tjandra (85) 2008 RCT MC 60 54 (90) 12° 9.5
b
3.1 25 (47)
a. timepoint of evaluation.
b. median number of FI episodes per week.
c. median or mean number of FI episodes per 3 weeks; all differences statistically significant.
prosp MC: prospective multicenter.
observ SC: observational single center.
RCT: randomized controlled trial.
DB cross: double blind cross over.
improved outcomes in colon and rectal surgery
than in those with an ileal neo-appendicostomy. Quality of life
measures showed significant improvement for physical health but
persistent low scores in psychological distress (96).(96) Others
have reported similar success and complication rates.(97–99)
As an option before an end colostomy, ACE may be appropriate
in some patients with intractable FI, particularly those in whom
incontinence is combined with constipation and who have failed
other therapies.
Postanal repair
Sir Allan Parks developed the postanal repair in the 1970s relying
on the theory that restoration of an obtuse anorectal angle would
improve continence by recreating a flap valve mechanism. The pro-
cedure was designed to treat neurogenic FI in patients with intact
sphincters. Through a V-shaped incision posterior to the anus, the
intersphincteric space is dissected proximal to the puborectalis; each
muscle layer is plicated in the midline. If an anterior levatorplasty
is added, the procedure is called total pelvic floor repair. Browning
and Parks (100) reported good to excellent results in over 80% but
others have failed to reproduce those findings.(101) Orrom et al.
(102) demonstrated that there were no significant changes in the
anorectal angle in patients after postanal repair questioning the
concept of the operation. Results tend to deteriorate over time (103)
which is an additional reason this treatment has fallen out of favor.
Colostomy
The colostomy is traditionally the end-stage treatment of intrac-
table FI. When it becomes an option, the patient has usually failed
several medical and surgical treatments and experienced years
of misery and socially debilitating symptoms. The patient must
accept a body image change and a whole new type of personal
care. Having said that, a colostomy offers undeniable advantages.
The patient can remain clean and be socially active. Perineal skin
irritation is cured. Special diets or transit regulatory medicine can
be stopped. On the other hand, there is a well-known morbidity
related to colostomy including immediate postoperative but also
longer term complications such as peristomal hernia, skin irrita-
tion, and appliance problems.
A systematic review compared DGP to colostomy in terms of
mortality, morbidity, efficacy, and cost-effectiveness.(65) While it
is intuitive that DGP resulted in better continence, mortality was
similar and morbidity favored colostomy. Colostomy was more
expensive than DGP in the long run provided that DGP does not
fail resulting in a late colostomy. There are no good quality stud-
ies comparing the quality of life of a patient with severe FI prior
and after colostomy. A cross-sectional study comparing patients
with rather moderate FI to patients with a well functioning colos-
tomy (the majority secondary to rectal cancer) found no signifi-
cant difference other than better average adjusted social function
scores for ostomates.(104) The two groups however were differ-
ent; patients with FI were younger and more often female, adding
further bias to this study. Data from rectal cancer surgery indi-
cate that an end colostomy after abdominoperineal resection does
not always equate to a lower quality of life than sphincter sparing
surgery.(105) The option of colostomy should be discussed early
enough and adequate counseling should be offered to the patient
to allow a mature decision to be taken.
CONCLUSION
Fecal incontinence is a common, underreported, devastating
condition. The pathophysiology is complex and variable; the
currently available instruments to measure the degree of dys-
function of the different components are often imprecise and not
standardized. The integration of quality of life scores and diary-
based results has substantially improved the reporting of severity
of FI in the literature. The initial step in management of patients
with FI is to diagnose and treat appropriately underlying condi-
tions of “pseudoincontinence” such as hemorrhoids, anal fistula,
mucosal prolapse, rectal prolapse and diarrheal conditions. The
first line treatment for true FI is medical and aims to regulate
bowel frequency and consistency. Biofeedback may provide some
relief to motivated patients. Those patients who fail medical treat-
ment and are physically fit enough for surgery should undergo
pelvic floor testing with sphincter imaging. Sphincteroplasty is
appropriate for patients with significant sphincter defects and
low anal canal pressures. For those who are not candidates for
sphincteroplasty, SNS seems to be the most promising solution.
ABS, DGP, and ACE are second line therapies that should be con-
sidered before end colostomy and may lead to good functional
outcomes especially in experienced centers.
The surgical treatment of FI has evolved significantly over the past
2 decades. Postanal repair and anal encirclement have been practi-
cally abandoned. While sphincteroplasty remains central, indications
for ABS and DGP are decreasing in favor of SNS. New treatment
modalities have been proposed, some were short lived (SECCA pro-
cedure), and others are still under investigation (IBA). Despite the
multitude of treatment options, the end colostomy may still be the
best compromise solution for many severely incontinent patients.
REFERENCES
1. Johanson JF, Lafferty J. Epidemiology of fecal incontinence:
the silent affliction. Am J Gastroenterol 1996; 91: 33–6.
2. Borrie MJ, Davidson HA. Incontinence in institutions: costs
and contributing factors. CMAJ 1992; 147: 322–8.
3. Nelson R, Furner S, Jesudason V. Fecal incontinence in
Wisconsin nursing homes: prevalence and associations. Dis
Colon Rectum 1998; 41: 1226–9.
4. Nelson R, Norton N, Cautley E, Furner S. Community-
based prevalence of anal incontinence. JAMA 1995; 274:
559–61.
5. Perry S, Shaw C, McGrother C et al. Prevalence of faecal
incontinence in adults aged 40 years or more living in the
community. Gut 2002; 50: 480–4.
6. NIH State-of-the-Science Conference Statement on Prevention
of Fecal and Urinary Incontinence in Adults. NIH Consens
State Sci Statements 2007; 24.
7. Mellgren A, Jensen LL, Zetterstrom JP et al. Long-term cost
of fecal incontinence secondary to obstetric injuries. Dis
Colon Rectum 1999; 42: 857–65.
8. Dudding TC, Vaizey CJ, Kamm MA. Obstetric anal sphinc-
ter injury: incidence, risk factors, and management. Ann
Surg 2008; 247: 224–37.
9. Oberwalder M, Connor J, Wexner SD. Meta-analysis to
determine the incidence of obstetric anal sphincter damage.
Br J Surg 2003; 90: 1333–7.
surgical treatment of fecal incontinence
10. Williams AB, Bartram CI, Halligan S et al. Anal sphinc-
ter damage after vaginal delivery using three-dimensional
endosonography. Obstet Gynecol 2001; 97: 770–5.
11. Snooks SJ, Setchell M, Swash M, Henry MM. Injury to
innervation of pelvic floor sphincter musculature in child-
birth. Lancet 1984; 2: 546–50.
12. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal
incontinence. Dis Colon Rectum 2003; 46: 1591–605.
13. Rockwood TH, Church JM, Fleshman JW et al. Patient
and surgeon ranking of the severity of symptoms associ-
ated with fecal incontinence: the fecal incontinence severity
index. Dis Colon Rectum 1999; 42: 1525–32.
14. Rockwood TH, Church JM, Fleshman JW et al. Fecal
Incontinence Quality of Life Scale: quality of life instrument
for patients with fecal incontinence. Dis Colon Rectum
2000; 43: 9–16.
15. Bharucha AE. Pro: anorectal testing is useful in fecal incon-
tinence. Am J Gastroenterol 2006; 101: 2679–81.
16. Rao SS. A balancing view: Fecal incontinence: test or treat
empirically which strategy is best? Am J Gastroenterol
2006; 101: 2683–4.
17. Wald A. Con: Anorectal manometry and imaging are
not necessary in patients with fecal incontinence. Am J
Gastroenterol 2006; 101: 2681–3.
18. Keating JP, Stewart PJ, Eyers AA, Warner D, Bokey EL.
Are special investigations of value in the management of
patients with fecal incontinence? Dis Colon Rectum 1997;
40: 896–901.
19. Liberman H, Faria J, Ternent CA et al. A prospective evalua-
tion of the value of anorectal physiology in the management
of fecal incontinence. Dis Colon Rectum 2001; 44: 1567–74.
20. Vaizey CJ, Kamm MA. Prospective assessment of the clini-
cal value of anorectal investigations. Digestion 2000; 61:
207–14.
21. Terra MP, Stoker J. The current role of imaging techniques
in faecal incontinence. Eur Radiol 2006; 16: 1727–36.
22. Sentovich SM, Blatchford GJ, Rivela LJ et al. Diagnosing anal
sphincter injury with transanal ultrasound and manometry.
Dis Colon Rectum 1997; 40: 1430–4.
23. Gold DM, Bartram CI, Halligan S et al. Three-dimensional
endoanal sonography in assessing anal canal injury. Br J Surg
1999; 86: 365–70.
24. Lee JH, Pretorius DH, Weinstein M et al. Transperineal three-
dimensional ultrasound in evaluating anal sphincter muscles.
Ultrasound Obstet Gynecol 2007; 30: 201–9.
25. Cazemier M, Terra MP, Stoker J et al. Atrophy and defects
detection of the external anal sphincter: comparison
between three-dimensional anal endosonography and
endoanal magnetic resonance imaging. Dis Colon Rectum
2006; 49: 20–7.
26. Briel JW, Stoker J, Rociu E et al. External anal sphincter
atrophy on endoanal magnetic resonance imaging adversely
affects continence after sphincteroplasty. Br J Surg 1999; 86:
1322–7.
27. Chen H, Humphreys MS, Kettlewell MG et al. Anal ultra-
sound predicts the response to nonoperative treatment of
fecal incontinence in men. Ann Surg 1999; 229: 739–43.
28. Cheetham M, Brazzelli M, Norton C, Glazener CM. Drug
treatment for faecal incontinence in adults. Cochrane
Database Syst Rev 2003: Issue 3: CD002116.
29. Lauti M, Scott D, Thompson-Fawcett MW. Fibre supple-
mentation in addition to loperamide for faecal incontinence
in adults: a randomized trial. Colorectal Dis 2008; 10(6):
553–62.
30. Bliss DZ, Jung HJ, Savik K et al. Supplementation with
dietary fiber improves fecal incontinence. Nurs Res 2001;
50: 203–13.
31. Palmer KR, Corbett CL, Holdsworth CD. Double-blind cross-
over study comparing loperamide, codeine and diphenoxy-
late in the treatment of chronic diarrhea. Gastroenterology
1980; 79: 1272–5.
32. Hallgren T, Fasth S, Delbro DS et al. Loperamide improves
anal sphincter function and continence after restorative
proctocolectomy. Dig Dis Sci 1994; 39: 2612–8.
33. Santoro GA, Eitan BZ, Pryde A, Bartolo DC. Open study
of low-dose amitriptyline in the treatment of patients with
idiopathic fecal incontinence. Dis Colon Rectum 2000; 43:
1676–81.
34. Cheetham MJ, Kamm MA, Phillips RK. Topical phenyleph-
rine increases anal canal resting pressure in patients with
faecal incontinence. Gut 2001; 48: 356–9.
35. Carapeti EA, Kamm MA, Nicholls RJ, Phillips RK.
Randomized, controlled trial of topical phenylephrine for
fecal incontinence in patients after ileoanal pouch construc-
tion. Dis Colon Rectum 2000; 43: 1059–63.
36. Carapeti EA, Kamm MA, Phillips RK. Randomized con-
trolled trial of topical phenylephrine in the treatment of
faecal incontinence. Br J Surg 2000; 87: 38–42.
37. Park JS, Kang SB, Kim DW, Namgung HW, Kim HL. The
efficacy and adverse effects of topical phenylephrine for
anal incontinence after low anterior resection in patients
with rectal cancer. Int J Colorectal Dis 2007; 22: 1319–24.
38. Deutekom M, Dobben A. Plugs for containing faecal
incontinence. Cochrane Database Syst Rev 2005; Issue 3:
CD005086.
39. Giamundo P, Altomare DF, Rinaldi M et al. The ProTect
device in the treatment of severe fecal incontinence: prelim-
inary results of a multicenter trial. Tech Coloproctol 2007;
11: 310–4.
40. Giamundo P, Welber A, Weiss EG et al. The procon incon-
tinence device: a new nonsurgical approach to preventing
episodes of fecal incontinence. Am J Gastroenterol 2002; 97:
2328–32.
41. Leroi AM, Dorival MP, Lecouturier MF et al. Pudendal neu-
ropathy and severity of incontinence but not presence of an
anal sphincter defect may determine the response to bio-
feedback therapy in fecal incontinence. Dis Colon Rectum
1999; 42: 762–9.
42. Jensen LL, Lowry AC. Biofeedback improves functional out-
come after sphincteroplasty. Dis Colon Rectum 1997; 40:
197–200.
43. Norton C, Kamm MA. Anal sphincter biofeedback and pelvic
floor exercises for faecal incontinence in adults a systematic
review. Aliment Pharmacol Ther 2001; 15: 1147–54.
improved outcomes in colon and rectal surgery
44. van Tets WF, Kuijpers JH, Bleijenberg G. Biofeedback treat-
ment is ineffective in neurogenic fecal incontinence. Dis
Colon Rectum 1996; 39: 992–4.
45. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter
exercises for the treatment of faecal incontinence in adults.
Cochrane Database Syst Rev 2006; 3: CD002111.
46. Miner PB, Donnelly TC, Read NW. Investigation of mode
of action of biofeedback in treatment of fecal incontinence.
Dig Dis Sci 1990; 35: 1291–8.
47. Hasegawa H, Yoshioka K, Keighley MR. Randomized trial of
fecal diversion for sphincter repair. Dis Colon Rectum 2000;
43: 961–4.
48. Tjandra JJ, Han WR, Goh J, Carey M, Dwyer P. Direct repair
vs. overlapping sphincter repair: a randomized, controlled
trial. Dis Colon Rectum 2003; 46: 937–42.
49. Bravo Gutierrez A, Madoff RD, Lowry AC et al. Long-term
results of anterior sphincteroplasty. Dis Colon Rectum
2004; 47: 727–31.
50. Barisic GI, Krivokapic ZV, Markovic VA, Popovic MA. Outcome
of overlapping anal sphincter repair after 3 months and after a
mean of 80 months. Int J Colorectal Dis 2006; 21: 52–6.
51. Malouf AJ, Norton CS, Engel AF, Nicholls RJ, Kamm MA.
Long-term results of overlapping anterior anal-sphincter
repair for obstetric trauma. Lancet 2000; 355: 260–5.
52. Maslekar S, Gardiner AB, Duthie GS. Anterior anal sphinc-
ter repair for fecal incontinence: Good longterm results are
possible. J Am Coll Surg 2007; 204: 40–6.
53. Vaizey CJ, Norton C, Thornton MJ, Nicholls RJ, Kamm MA.
Long-term results of repeat anterior anal sphincter repair.
Dis Colon Rectum 2004; 47: 858–63.
54. Giordano P, Renzi A, Efron J et al. Previous sphincter repair
does not affect the outcome of repeat repair. Dis Colon
Rectum 2002; 45: 635–40.
55. Pinedo G, Vaizey CJ, Nicholls RJ et al. Results of repeat anal
sphincter repair. Br J Surg 1999; 86: 66–9.
56. Finlay IG, Richardson W, Hajivassiliou CA. Outcome after
implantation of a novel prosthetic anal sphincter in humans.
Br J Surg 2004; 91: 1485–92.
57. Schrag HJ, Padilla FF, Goldschmidtboing F et al. German
artificial sphincter system: first report of a novel and highly
integrated sphincter prosthesis for therapy of major fecal
incontinence. Dis Colon Rectum 2004; 47: 2215–7.
58. Michot F, Costaglioli B, Leroi AM, Denis P. Artificial anal
sphincter in severe fecal incontinence: outcome of prospec-
tive experience with 37 patients in one institution. Ann Surg
2003; 237: 52–6.
59. Mundy L, Merlin TL, Maddern GJ, Hiller JE. Systematic
review of safety and effectiveness of an artificial bowel
sphincter for faecal incontinence. Br J Surg 2004; 91:
665–72.
60. O’Brien PE, Dixon JB, Skinner S et al. A prospective, ran-
domized, controlled clinical trial of placement of the artifi-
cial bowel sphincter (Acticon Neosphincter) for the control
of fecal incontinence. Dis Colon Rectum 2004; 47: 1852–60.
61. Altomare DF, Binda GA, Dodi G et al. Disappointing long-
term results of the artificial anal sphincter for faecal incon-
tinence. Br J Surg 2004; 91: 1352–3.
62. Christiansen J, Rasmussen OO, Lindorff-Larsen K. Long-term
results of artificial anal sphincter implantation for severe anal
incontinence. Ann Surg 1999; 230: 45–8.
63. Parker SC, Spencer MP, Madoff RD et al. Artificial bowel
sphincter: long-term experience at a single institution. Dis
Colon Rectum 2003; 46: 722–9.
64. Gregorcyk S. The Current Status of the Acticon Neosphincter.
Clin Colon Rectal Surg 2005; 18: 6.
65. Chapman AE, Geerdes B, Hewett P et al. Systematic review
of dynamic graciloplasty in the treatment of faecal inconti-
nence. Br J Surg 2002; 89: 138–53.
66. Baeten CG, Bailey HR, Bakka A et al. Safety and efficacy of
dynamic graciloplasty for fecal incontinence: report of a pro-
spective, multicenter trial. Dynamic Graciloplasty Therapy
Study Group. Dis Colon Rectum 2000; 43: 743–51.
67. Matzel KE, Madoff RD, LaFontaine LJ et al. Complications
of dynamic graciloplasty: incidence, management, and
impact on outcome. Dis Colon Rectum 2001; 44: 1427–35.
68. Wexner SD, Baeten C, Bailey R et al. Long-term efficacy of
dynamic graciloplasty for fecal incontinence. Dis Colon
Rectum 2002; 45: 809–18.
69. Rongen MJ, Uludag O, El Naggar K et al. Long-term follow-up
of dynamic graciloplasty for fecal incontinence. Dis Colon
Rectum 2003; 46: 716–21.
70. Melenhorst J, Koch SM, van Gemert WG, Baeten CG. The
artificial bowel sphincter for faecal incontinence: a single
centre study. Int J Colorectal Dis 2008; 23: 107–11.
71. Matzel KE, Stadelmaier U, Hohenfellner M, Gall FP.
Electrical stimulation of sacral spinal nerves for treatment
of faecal incontinence. Lancet 1995; 346: 1124–7.
72. Matzel KE. Sacral nerve stimulation for fecal disorders: evo-
lution, current status, and future directions. Acta Neurochir
Suppl 2007; 97: 351–7.
73. Sheldon R, Kiff ES, Clarke A, Harris ML, Hamdy S. Sacral
nerve stimulation reduces corticoanal excitability in patients
with faecal incontinence. Br J Surg 2005; 92: 1423–31.
74. Gooneratne ML, Facer P, Knowles CH et al. Normalization
of substance P levels in rectal mucosa of patients with faecal
incontinence treated successfully by sacral nerve stimula-
tion. Br J Surg 2008; 95: 477–83.
75. Holzer B, Rosen HR, Novi G et al. Sacral nerve stimula-
tion for neurogenic faecal incontinence. Br J Surg 2007; 94:
749–53.
76. Matzel KE, Kamm MA, Stosser M et al. Sacral spinal nerve
stimulation for faecal incontinence: multicentre study.
Lancet 2004; 363: 1270–6.
77. Melenhorst J, Koch SM, Uludag O, van Gemert WG, Baeten
CG. Sacral neuromodulation in patients with faecal incon-
tinence: results of the first 100 permanent implantations.
Colorectal Dis 2007; 9: 725–30.
78. Jarrett ME, Nicholls RJ, Kamm MA. Effect of sacral neu-
romodulation for faecal incontinence on sexual activity.
Colorectal Dis 2005; 7: 523–5.
79. Hetzer FH, Hahnloser D, Clavien PA, Demartines N.
Quality of life and morbidity after permanent sacral nerve
stimulation for fecal incontinence. Arch Surg 2007; 142:
8–13.
surgical treatment of fecal incontinence
80. Hetzer FH, Bieler A, Hahnloser D et al. Outcome and cost
analysis of sacral nerve stimulation for faecal incontinence.
Br J Surg 2006; 93: 1411–7.
81. Matzel KE, Stadelmaier U, Hohenberger W. Innovations
in fecal incontinence: sacral nerve stimulation. Dis Colon
Rectum 2004; 47: 1720–8.
82. Matzel KE. Sacral Nerve Stimulation for Fecal Incontinence:
An Update. In: European Society of Coloproctology 2nd
Scientific and Annual General Meeting. Malta; 2007.
83. Dudding TC, Pares D, Vaizey CJ, Kamm MA. Predictive fac-
tors for successful sacral nerve stimulation in the treatment
of faecal incontinence: a 10–year cohort analysis. Colorectal
Dis 2008; 10: 249–56.
84. Melenhorst J, Koch SM, Uludag O, van Gemert WG, Baeten
CG. Is a morphologically intact anal sphincter necessary for
success with sacral nerve modulation in patients with faecal
incontinence? Colorectal Dis 2008; 10: 257–62.
85. Tjandra JJ, Chan MK, Yeh CH, Murray-Green C. Sacral Nerve
Stimulation is more Effective than Optimal Medical Therapy
for Severe Fecal Incontinence: A Randomized, Controlled
Study. Dis Colon Rectum 2008; 51(5): 494–502.
86. Mentes BB, Yuksel O, Aydin A et al. Posterior tibial nerve
stimulation for faecal incontinence after partial spinal injury:
preliminary report. Tech Coloproctol 2007; 11: 115–9.
87. Queralto M, Portier G, Cabarrot PH et al. Preliminary results
of peripheral transcutaneous neuromodulation in the treat-
ment of idiopathic fecal incontinence. Int J Colorectal Dis
2006; 21: 670–2.
88. Tjandra JJ, Lim JF, Hiscock R, Rajendra P. Injectable silicone
biomaterial for fecal incontinence caused by internal anal
sphincter dysfunction is effective. Dis Colon Rectum 2004;
47: 2138–46.
89. Davis K, Kumar D, Poloniecki J. Preliminary evaluation of
an injectable anal sphincter bulking agent (Durasphere) in
the management of faecal incontinence. Aliment Pharmacol
Ther 2003; 18: 237–43.
90. Altomare DF, La Torre F, Rinaldi M, Binda GA, Pescatori M.
Carbon-Coated Microbeads Anal Injection in Outpatient
Treatment of Minor Fecal Incontinence. Dis Colon Rectum
2008; 51(4): 432–5.
91. Maeda Y, Vaizey CJ, Kamm MA. Long-term results of peria-
nal silicone injection for faecal incontinence. Colorectal Dis
2007; 9: 357–61.
92. Chan MK, Tjandra JJ. Injectable silicone biomaterial (PTQ)
to treat fecal incontinence after hemorrhoidectomy. Dis
Colon Rectum 2006; 49: 433–9.
93. van der Hagen SJ, van Gemert WG, Baeten CG. PTQ
Implants in the treatment of faecal soiling. Br J Surg 2007;
94: 222–3.
94. Malone PS, Ransley PG, Kiely EM. Preliminary report: the
antegrade continence enema. Lancet 1990; 336: 1217–8.
95. Portier G, Bonhomme N, Platonoff I, Lazorthes F. Use
of Malone antegrade continence enema in patients with
perineal colostomy after rectal resection. Dis Colon Rectum
2005; 48: 499–503.
96. Lefevre JH, Parc Y, Giraudo G et al. Outcome of antegrade
continence enema procedures for faecal incontinence in
adults. Br J Surg 2006; 93: 1265–9.
97. Gerharz EW, Vik V, Webb G et al. The value of the MACE
(Malone antegrade colonic enema) procedure in adult
patients. J Am Coll Surg 1997; 185: 544–7.
98. Krogh K, Laurberg S. Malone antegrade continence enema
for faecal incontinence and constipation in adults. Br J Surg
1998; 85: 974–7.
99. Portier G, Ghouti L, Kirzin S, Chauffour M, Lazorthes
F. Malone antegrade colonic irrigation: ileal neoappendicos-
tomy is the preferred procedure in adults. Int J Colorectal
Dis 2006; 21: 458–60.
100. Browning GG, Parks AG. Postanal repair for neuropathic
faecal incontinence: correlation of clinical result and anal
canal pressures. Br J Surg 1983; 70: 101–4.
101. Tan JJ, Chan M, Tjandra JJ. Evolving therapy for fecal incon-
tinence. Dis Colon Rectum 2007; 50: 1950–67.
102. Orrom WJ, Miller R, Cornes H et al. Comparison of ante-
rior sphincteroplasty and postanal repair in the treatment
of idiopathic fecal incontinence. Dis Colon Rectum 1991;
34: 305–10.
103. Setti Carraro P, Kamm MA, Nicholls RJ. Long-term results of
postanal repair for neurogenic faecal incontinence. Br J Surg
1994; 81: 140–4.
104. Colquhoun P, Kaiser R Jr, Efron J et al. Is the quality of life
better in patients with colostomy than patients with fecal
incontience? World J Surg 2006; 30: 1925–8.
105. Pachler J, Wille-Jorgensen P. Quality of life after rectal
resection for cancer, with or without permanent colostomy.
Cochrane Database Syst Rev 2005; 2: CD004323.
106. Nikiteas N, Korsgen S, Kumar D, Keighley MR. Audit of
sphincter repair. Factors associated with poor outcome. Dis
Colon Rectum 1996; 39: 1164–70.
107. Oliveira L, Pfeifer J, Wexner SD. Physiological and clinical
outcome of anterior sphincteroplasty. Br J Surg 1996; 83:
502–5.
108. Young CJ, Mathur MN, Eyers AA, Solomon MJ. Successful
overlapping anal sphincter repair: relationship to patient
age, neuropathy, and colostomy formation. Dis Colon
Rectum 1998; 41: 344–9.
109. Gilliland R, Altomare DF, Moreira H Jr et al. Pudendal
neuropathy is predictive of failure following anterior over-
lapping sphincteroplasty. Dis Colon Rectum 1998; 41:
1516–22.
110. Karoui S, Leroi AM, Koning E et al. Results of sphinctero-
plasty in 86 patients with anal incontinence. Dis Colon
Rectum 2000; 43: 813–20.
111. Buie WD, Lowry AC, Rothenberger DA, Madoff RD. Clinical
rather than laboratory assessment predicts continence after
anterior sphincteroplasty. Dis Colon Rectum 2001; 44:
1255–60.
112. Morren GL, Hallbook O, Nystrom PO, Baeten CG, Sjodahl
R. Audit of anal-sphincter repair. Colorectal Dis 2001; 3:
17–22.