Improved Outcomes in Colon and Rectal Surgery part 30 pdf
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (291.91 KB, 10 trang )
Abdominoperineal resection
W Brian Perry, Fia Yi, Clarence Clark, and Danny Kim
CHALLENGING CASE
A 64-year-old woman is 7 days s/p an abdominopernneal resec-
tion for a T2N1 rectal adenocarcinoma. She had received preop-
erative. Her perineal wound has developed increased tenderness,
is swollen, and is draining pus.
CASE MANAGEMENT
The patient’s wound is opened and the patient is started on three
times a day dressing changes. After 2 days the wound is clean and
a vacuum assisted closure (VAC) dressing is placed.
INTRODUCTION
Abdominoperineal resection (APR) completely removes the dis-
tal colon, rectum, and anal sphincter complex using both ante-
rior abdominal and perineal incisions, resulting in a permanent
colostomy. Developed more than 100 years ago, it remains an
important tool in the treatment of rectal cancer despite advances
in sphincter-sparing procedures. We will examine a brief history
of this procedure, current operative techniques and complica-
tions, expected results, (both oncologic and with regard to quality
of life), and what the future may hold for this procedure.
Several recent reports have noted the increase in the use of
sphincter-sparing options for patients diagnosed with rectal can-
cer. Abraham and colleagues found a 10% decrease (60.1–49.9%)
in the rate of APR from 1989 to 2001 as compared with low ante-
rior resection (LAR) using national administrative data.(1) When
controlled for several variables, including patient demographics
and hospital volume, patients were 28% more likely to have an
LAR later in the study period. Schoetz notes that LAR outnum-
bers APR three to one in the submitted case logs of recent color-
ectal fellows.(2) This ratio is similar to that found in the Swedish
rectal cancer registry, where approximately 25% of over 12,000
patients with rectal cancer underwent APR from 1995–2002.(3)
In no study or registry, however, has APR been eliminated.
HISTORY
Early in the twentieth century, most patients with rectal cancer
underwent perineal procedures to address typically advanced,
symptomatic disease. These included the transcoccygeal Kraske
approach and the transsphincteric approach developed by Bevan
in America, later attributed to A. York Mason. Patients were typi-
cally left with profound sphincter dysfunction or fistulae follow-
ing a protracted recovery. A two-staged operation, consisting of
an initial laparotomy and colostomy followed by perineal excision,
was used until the 1930’s with reasonable results.
The operation we now know as APR was first described by
Miles in 1908, but initial reports showed a high operative mortal-
ity, up to 42%. Improvements in perioperative care that came later
reduced this considerably. Refinements in technique continued
through the first half of the twentieth century. Gabriel described
the operation in one stage, with the abdominal portion done
supine and the perineal portion done in the left lateral position.
Lloyd-Davies’ synchronous approach to the abdomen and peri-
neum with the patient in the lithotomy position eliminated the
cumbersome and sometimes dangerous need to reposition the
patient while under anesthesia.(4) Recent advances have included
total mesorectal excision in patients undergoing APR and the
addition of methods to enhance perineal wound healing, espe-
cially in patients who have received neoadjuvant chemoradiation.
Minimally invasive techniques are also being applied to APR, with
good initial results.
PATIENT PREPARATION AND POSITIONING
Preparation for abdominoperineal resection starts with marking
the ideal placement of the colostomy by the primary surgeon or
enterostomal nurse.(5) Patients are instructed to take a mechani-
cal bowel preparation the day before surgery consisting of sodium
phosphate solution or polyethylene glycol. Placement of an epidural
catheter may be considered to improve postoperative analgesia
and to reduce postoperative ileus.(6) Before induction of general
anesthesia, intermittent pneumatic compression devices are placed
on the lower extremities to reduce the risk of venous thromboem-
bolism.(7) Intravenous antibiotics with efficacy against enteric flora
are administered 60 minutes before incision to decrease rate of
surgical site infection.(8) The abdomen and perineum are prepped
and appropriate monitoring is placed.
After induction of anesthesia, a urinary catheter is inserted; ure-
teral stents should be considered if the patient has had prior pelvic
surgery, tumor extension into the urinary tract, or prior pelvic radi-
ation. The patient is placed in the lithotomy position using Allen
stirrups with padding to prevent lower limb acute compartment
syndrome.(9) Positioning also includes symmetric hip extension,
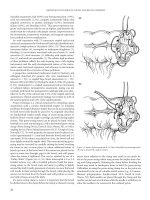
knee flexion, and thigh abduction (Figure 27.1). Ultimately the legs
are balanced in the stirrups, such that the weight is resting on the
feet and the ankle and knee are in line with the opposite shoulder.
A rectal exam is performed under anesthesia followed by irrigation
with dilute betadine solution to remove any residual stool.
OPERATIVE TECHNIQUE
The operative technique used today varies little from Ernest Miles’
description in 1908.(10) Unlike Miles’ method we prefer the two-
team approach with the patient in lithotomy position rather than
lateral semi-prone position. A nonabsorbable purse-string suture is
placed around the anus. The abdomen and perineum are prepared
with antiseptic solution and draped with openings for the abdomi-
nal and perineal dissections. The abdomen and pelvis are accessed
through a midline hypogastric incision that extends to the right
of or through the umbilicus. The abdomen is explored for meta-
static disease and synchronous colon lesions. After confirmation of
resectability, a self-retaining retractor is placed.
abdominoperineal resection
Figure 27.1 Leg positioning for abdominoperineal resection.
The small bowel is packed into the upper abdomen with a moist
towel. The sigmoid and descending colons are then mobilized at
the white line of Toldt in the left lateral gutter. After confirming
adequate mobilization of the descending colon for an end colos-
tomy, the left ureter is identified and preserved. The peritoneum
incision is carried anterior followed by incision of the right lateral
peritoneum. The right ureter is identified and preserved and the
peritoneal incisions are connected anteriorly at the base of the
bladder. For convenience, the proximal sigmoid can be divided
with a linear stapling device and the cut end used as a handle
to aid with the dissection. A finger is passed below the inferior
mesenteric vessels with the plan to leave the sigmoid branches.
This helps minimize vascular compromise of the stoma. It is
unnecessary to ligate the inferior mesenteric artery at its origin as
this has not been shown to increase survival.(11)
The superior hemorrhoidal vessels are transected. The presacral
space is entered without breaching the endopelvic fascia and with
preservation of the mesorectum consistent with Heald’s descrip-
tion of total mesorectal excision.(12) After identifying this avas-
cular plane, the dissection is aided by using a lighted St. Mark’s
retractor to hold the mesorectum anteriorly. As the dissection
continues distally, Waldeyer’s fascia is divided with electrocautery
or sharply to avoid injuring the presacral venous plexus. Staying in
the avascular plane posteriorly and laterally minimizes bleeding.
The lateral ligaments are cauterized or suture-ligated close to the
pelvic side wall to maximize the radial margins. Denonvillier’s fas-
cia in males is dissected down to the pelvic floor anteriorly. Unless
the tumor is anterior, it is not necessary to expose the seminal vesi-
cles in males thus avoiding injury to the nervi erigentes. In females,
the presence of an anteriorly based tumor may require perform-
ance of a posterior vaginectomy. When the pelvic floor is reached
circumferentially around the rectum, the abdominal portion of the
dissection is completed. Once the pelvic dissection is completed,
the colostomy is created and the abdomen is closed.
improved outcomes in colon and rectal surgery
When the abdominal operator has determined that the lesion
is resectable the perineal dissection begins simultaneously with
the abdominal portion of the case. The perineal dissection
begins with an elliptical incision from the perineal body in males
or the posterior vaginal introitus in females to a point midway
between the anus and coccyx. The incision should include the
entirety of the external sphincter muscle, but does not need
to extend laterally to the ischial tuberosities. Dissection is car-
ried down to the levator ani muscles with cautery to minimize
bleeding. The inferior hemorrhoidal arteries located posterior-
laterally are ligated. Using a finger on the tip of the coccyx as a
guide, the posterior dissection is directed anterior to the coccyx
and the anococcygeal raphe is divided. When all that remains
are the anterior attachments, the specimen is drawn through the
opening and used to provide traction to continue the remain-
ing dissection. The specimen is then removed and the pelvis is
irrigated. If sufficient levator muscle remains, the pelvic floor
is reapproximated to reduce the risk for perineal herniation.
Drains are placed and secured followed by closure of the skin
with interrupted permanent or absorbable monofilament suture
in a vertical mattress fashion.
PRESERVATION OF SEXUAL AND URINARY FUNCTION
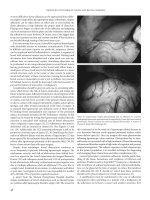
As described by Kyo et al. the neuroanatomy begins with the sym-
pathetic nerve fibers that travel through the lumbar splanchnic
nerves to the superior hypogastric plexus and then divide into
two hypogastric nerves. Parasympathetic fibers emerge from
the second, third, and fourth sacral spinal nerves as the pelvic
splanchnic nerves and join the hypogastric nerves to form the
inferior hypogastric (pelvic) plexus. The pelvic plexus is rectan-
gular and its midpoint is located at the tips of the seminal vesicles
on either side of the rectum (Figure 27.2). The most caudal por-
tion of the pelvic plexus travels at the posterolateral border of the
prostate, lateral to the prostatic capsular arteries and veins and
reaches the hilum of the penis.(13)
The rate of urinary dysfunction and impotence after rectal sur-
gery ranges from 33% to 70% and 20% to 46%, respectively, while
20–60% of potent patients are unable to ejaculate.(14) A surprisingly
large proportion of patients suffer various urinary tract problems
and sexual problems due to extended lymphadenectomy involving
the hypogastric nerve plexus. Therefore, preservation of the pelvic
autonomic nerves lowers the incidence of sexual and urinary mor-
bidity. With preservation of the superior hypogastric nerve plexus,
ejaculation is maintained in 90% of the patients.(15)
Utilizing precise dissection with preservation of autonomic nerves
Kim et al. noted an erection rate of 80%, penetration ability rate of
75% with only 5.5% of patients in their study reporting complete
inability for erection and intercourse. Study by Shirouzu et al. showed
oncologic equivalence between previously described extensive resec-
tion pre-1984 and plexus preserving low rectal surgery post-1985
with local recurrence rates 9.1 and 3.9%, respectively and 10-year,
disease-free survival rate of 77% and 81.5%, respectively. No signifi-
cant difference was noted among the groups.(16)
METHODS OF CLOSURE
The perineal wound can be packed open, partially closed, or
com pletely closed. The peritoneal defect above the pelvic space
can also be sutured closed or left open. Adjunctive procedures
such as drainage of the pelvic space, with or without continuous
irrigation, and omental plugging may also be considered.
Rates of primary healing after perineal wounds are closed range
from 4% to 92%.(10, 17, 19) Open packing relegates all wounds
to secondary healing, is inconvenient, and often painful but may
result in a lower rate of chronic perineal sinus formation.(19)
Closure of the pelvic peritoneum has been advocated to prevent
perineal evisceration and postoperative small bowel obstruction.
However, it may prevent obliteration of the pelvic cavity, lead-
ing to formation of a persistent perineal sinus.(20) Loops of
small bowel may also become incarcerated in small defects in the
peritoneal closure, resulting in postoperative bowel obstruction.
Two studies compared various methods of peritoneal and peri-
neal closure. Irvin and Goligher (19) prospectively randomized
106 patients undergoing proctectomy to one of three methods of
perineal closure: open packing of the perineal wound; primary
closure of the perineal wound without closure of the pelvic peri-
toneum with suction drainage of the pelvis; and primary closure
of the peritoneal and perineal wounds. The overall complication
rate was high: repeated surgery was necessary in 21% of patients
in the open packing group, most often because of hemorrhage,
and in 25% and 19% of the two closed groups, most commonly
for drainage of abscesses. Primary healing occurred in 45% of the
patients with primary closure of both the perineum and perito-
neum and in 43% of patients with open peritoneal and closed
perineal wounds.
In a prospective study part of a multicentre trial in Germany,
Meyer et al. published a standardized technique of perineal closure
that reduced wound complication rates from 17% to 5.4%. The
principle of their approach was to close the perineal wound tightly
in multiple layers (specifically the muscle and ischiorectal as well
as subcutaneous fat) which help to avoid the accumulation of fluid
Figure 27.2 Nerve supply to the rectum.
abdominoperineal resection
within the wound cavity. The residual amount of fluid is then
removed by closed suction drainage. Additionally, it is thought
that the addition of antibiotic carriers provides local infectious
prophylaxis leading to lower rates of perineal wound infection.
(21) This has also been demonstrated in two other prospective
randomized studies and can be considered an adjunct in decreas-
ing the overall morbidity of the perineal wound.(22, 23)
Myocutaneous flaps have been increasingly utilized in the initial
repair of the perineal defect, especially in patients who have
had preoperative radiation therapy. Chessin et al. at Memorial
Sloan Kettering reviewed their experience with rectus abdominis
myocutaneous (RAM) flap closures of the perineal defect.
Comparing the RAM flap group to a historical control, they found
that the incidence of perineal wound complications was 15.8% in
the RAM flap group compared to the 44.1% in the control.(24)
Butler et al. also looked at vertical rectus abdominis myocutaneous
flaps in previously irradiated patients undergoing APR. There was
a significantly lower incidence of perineal abscess (9% vs. 37%),
major perineal wound dehiscence (9% vs. 30%) and drainage
procedures required for perineal or pelvic fluid collections (3%
vs. 25%).(25)
In an effort to fill the pelvic space after rectal resection, Page
et al. advocates an omental plug. They describe mobilization of
the omentum on the left gastroepiploic arterial pedicle, with sub-
sequent placement in the pelvis. Advantages include increased
local blood flow and lymphatic drainage, and obliteration of the
pelvic space. The omental plug also has the advantage of keeping
the small bowel out of the pelvis, thereby decreasing the chance of
radiation enteritis in patients who require postoperative radiation
therapy. The authors report primary healing in 26 of 34 patients
(77%).(26) A recent publication by PJ Nilsson reviewed all avail-
able English language publications on the use of omentoplasty
in APR wound closure. Primary wound healing was the primary
outcome measure. Most authors reported positive results after
omentoplasty and one study showed significant improvement in
perineal healing rate at 6 months. Significant reduction in sinus
formation and wound dehiscence also was reported.(27) Despite
these promising results, there needs to be randomized trials with
well-described patient categories, end points and follow up to
firmly assess whether omentoplasty should be a standard part of
the wound closure.
COMPLICATIONS
Abscess
Abscess formation, intraperitoneal or of the perineal wound, is
the most common major complication after APR.(17) Incidence
of abscess formation ranges from 11% to 16% (17, 18, 28). In
some small series, the incidence of perineal wound infection is
100%.(19) This can be attributed to the large dead space remain-
ing after resection of the rectum and from fecal contamina-
tion. In a retrospective review of patients who had neoadjuvant
chemoradiation followed by APR, Butler observed that there
was a significant decrease of perineal abscess formation (3% vs.
37%) after the placement of a vertical rectus abdominis myocu-
taneous (VRAM) flap to the perineum. The well-vascularized
flap eliminates the dead space in the pelvis, reducing the risk of
fluid collection. The use of a VRAM flap should be considered in
patients who are at high risk for postoperative perineal wound
complications.(25) Alternatively, an omental pedicle flap sutured
to the perineal wound has been observed to decrease the rate of
abscess formation.(29)
Incision and drainage with local wound care is the treatment
of choice for local perineal wound abscesses. There is a small
increased risk of developing a perineal sinus after opening the
skin of a subcutaneous abscess.(30) Thus if the incision is heal-
ing well, the abscess may be amenable to percutaneous drainage.
In addition, percutaneous drainage is the preferred treatment of
presacral and pelvic abscesses.(31)
Intraoperative Hemorrhage
Hemorrhage during surgery can usually be attributed to an error
in technique, but when faced with a pelvis that had previously
received radiation therapy, hemorrhage may be unavoidable.
Bleeding may occur when dissection begins at the sigmoid. This
is usually easily identified and controlled. In the previously irradi-
ated pelvis, planes become distorted making it difficult to identify
vital structures. It is easy to stray laterally, which may result in iliac
vessel injury. These must be repaired immediately to avoid pro-
longed hemorrhage. In a pelvis that has not received radiation, or
if there is minimal fibrosis, meticulous dissection in the proper
plane down to the lateral stalks usually yields minimal bleeding.
The most troublesome bleeding in the pelvis comes from the
posterior dissection along the sacrum. Very rarely, there will be a
prominent medial sacral artery that may be injured. More com-
monly, the bleeding from the sacrum will come from the venous
plexus. If present, the basivertebral vein, which connects the inter-
nal vertebral venous system to the presacral system, can bleed
profusely and be difficult to control. Ideally, by taking sharp dis-
section down the presacral plane, there should be little to no bleed-
ing.(32, 33) Unfortunately this space may be nonexistent in certain
patients or obliterated in an irradiated field. Bleeding from the sac-
rum can be controlled by packing, suture ligation, electrocautery,
finger compression, or thumbtack compression.
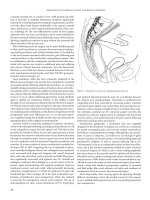
Thumbtack compression is a quick, safe, and effective method of
controlling sacral bleeding. There are several commercial applica-
tion devices available; however, using a clamp or forceps with finger
applications works equally as well (Figure 27.3). Thumbtacks also
prevent damage to the surround venous plexus that may occur
when using the other methods of attempting hemostasis, such as
direct suture ligation or excessive cauterization.(33, 34)
Postoperative Hemorrhage
Bleeding after the completion of the surgery is uncommon (<4%)
and is most commonly associated with perineal wounds that are
packed open.(35) When the perineal wound is packed open, it
is hemostatic until the first dressing change when the tampon-
ade is released. As the packing is removed, it may pull away
clot from surrounding tissues that can result in more bleeding.
Conservative treatment can be attempted with adequate resusci-
tation if needed, a reapplication of packing, and placement of the
patient on strict bed rest. If the patient remains stable, the pack-
ing may be removed in 48–72 hours.(36) Occasionally, reopera-
tion is necessary to control postoperative perineal hemorrhage.
improved outcomes in colon and rectal surgery
Given that nearly all APR wounds are currently closed primarily,
this complication is rare.(37, 38)
Perineal Wound Complications
When comparing abdominoperineal resection with other abdom-
inal and pelvic procedures, the most striking difference is the peri-
neal dissection and ensuing perineal wound. Treatment of this
wound has long been the center of debate and controversy. Miles
in his original description in 1908, recommended open packing,
and his technique is still used by some surgeons. Over the following
75 years, many techniques to treat the perineal wound have been
developed, including partial closure, primary closure, and closure
with continuous irrigation or omental plugging. For purposes of
discussion, perineal wound complications of abdominoperineal
resection can be divided into four categories: hemorrhage, abscess,
perineal sinus, and perineal hernia.
Non Healing Wound and Perineal Sinus
Perineal sinus is defined as a perineal wound that remains unhealed
for a minimum of 6 months. Characteristics include a fixed fibrotic
pelvic cavity, a long, narrow track lined with a thick unyielding peel,
and a small external opening.(39)
Silen and Glotzer compared the pelvic space after APR with
the fixed pleural space after pneumonectomy. The pelvic space
is bound posteriorly and laterally by the rigid bony pelvis, ante-
riorly by the relatively unyielding genitourinary structures, infe-
riorly by the slightly mobile perineal floor (if surgically closed),
and superiorly by the peritoneal contents. Of all these borders,
certainly the peritoneal structures are the most mobile. They
contend that the pelvic space after APR is filled not with gran-
ulation tissue but with a combination of upward migration of
the perineal soft tissues and descent of the peritoneal contents
and argue that any forces (either iatrogenic, such as closure of
the peritoneum or prolonged packing of the pelvis, or second-
ary to complications, such as pelvic abscess or hematoma) that
produce a fixed fibrotic cavity are likely to result in a nonheal-
ing perineal wound.(30) Artioukh et al. reviewed their series of
APR non healing wounds and found several possible contrib-
uting factors, including distant metastases, excessive alcohol
consumption, cigarette smoking, transfusion requirement and
chemoradiation.
Other studies have also observed the increased risk in peri-
neal wound infection and nonhealing in those who have been
exposed to radiotherapy. The Swedish Rectal Cancer trial showed
an increase in wound infection from 10% to 20% and the Dutch
Colorectal Cancer Group had a 31% perineal complication rate
even in those exposed to short-course radiation.(40, 41)
Silen and Glotzer recommended that the peritoneal contents
be allowed to descend into the pelvis, the space be kept irrigated
and well drained to prevent fluid accumulation, and any packing
used in the perineal wound be removed early to prevent develop-
ment of fibrotic wound edges. Despite the excellent description
of perineal healing by Silen and Glotzer and the development
of multiple techniques for perineal closure, nonhealing perineal
wounds remain a common problem. Bacon and Nuguid noted a
40% incidence of persistent perineal sinus in 1042 patients after
rectal resection.(42) In almost 500 patients who underwent APR
at the Lahey and Mayo Clinics, 14–24% had unhealed perineal
wounds at 6 months.
RISK FACTORS
Inflammatory bowel disease versus carcinoma. Rectal resec-
tion is most commonly performed to treat low rectal cancer
or inflammatory bowel disease. Often the extent of soft tissue
resection is much greater in the treatment of rectal cancer with
complete removal of the levator musculature or posterior vagi-
nectomy advocated by some versus the intersphincteric proctec-
tomy (sparing the external anal sphincter and the levator ani)
often used in surgical treatment of inflammatory bowel disease.
An increase in perineal wound complications might be expected
after APR to treat cancer, but Irvin and Goligher found a 9% inci-
dence of unhealed perineal wounds in the treatment of cancer,
compared with a 33% incidence in proctectomies performed for
inflammatory bowel disease.(19) A more contemporary review
of the risk factors for perineal wound complications undertaken
by Christian et al. determined that higher rates of major wound
complications occurred in patients who had APR performed for
anal cancer (50%) as compared to rectal cancer (10%) or inflam-
matory bowel disease (8%). The reasons are unclear although the
extensive tissue dissection involved in a cancer operation with
larger soft tissue loss may be a possibility.(43) There is some evi-
dence to support this in studies that have shown that tumor size
can be a risk factor for poor wound healing.
Radiation Therapy. Radiation therapy is often used in the
treatment of rectal and anal neoplasia both preoperatively and
postoperatively. Christian et al. found that preoperative radiation
therapy for anal cancer patients appeared to be a risk factor for
poor wound healing. Artioukh et al. also found that patients who
had received preoperative radiotherapy were prone to wound
complications (39% vs. 6.7% who did not have radiotherapy).
Fecal Contamination. Fecal contamination during proctectomy
significantly decreases primary healing and may increase the risk
Figure 27.3 Thumbtack occlusion of bleeding basivertebral vein.
abdominoperineal resection
of chronic perineal sinus formation. This complication is presum-
ably related to the development of pelvic infection with secondary
development of a fixed abscess cavity that makes obliteration of the
pelvic space more difficult.(30) Fecal contamination may also lead
to a higher incidence of perineal wound tumor recurrence.
TREATMENT
Nonhealing perineal wounds develop in 8% to 69% of patients
undergoing APR.(10, 18, 19, 28) Because of the scope of the prob-
lem, many techniques have been developed to ensure complete
healing. Early efforts included operative debridement with wide
drainage, including coccygectomy and even partial sacral resection.
(20) These measures were designed to eliminate the rigid fibrotic
space that always accompanies a nonhealing perineal wound. Often
these measures resulted in eventual healing but required exten-
sive wound care for many months. Despite this treatment, some
wounds failed to heal.
Alternative methods to improve healing and decrease wound
care have been developed. Oomen et al. published a set of guidelines
in treating persistent perineal sinuses or complex perineal wounds
with an overall 80% success rate in healing. Their algorithm
consisted of VAC therapy for large defects before placing muscle
flaps in order to decrease the size of the defect. Depending on sinus
length, they either placed a transposition of rectus abdominal
muscle (for sinuses > 10 cm) or a gracilis muscle/gluteal thigh flap
(sinus < 8 cm). Initially success rate was 57%, but after second-
ary surgery in some of the patients, their success rate increased to
80%. Ultimately, the best outcomes were in patients who received
the gracilis or gluteal thigh flap.(44)
The VAC® closure system has also been used more to assist in
dealing with complex perineal wounds that result after extensive
operative debridement’s for persistent perineal sinuses. Pemberton
at the Mayo Clinic (45) published a review of their results with
various techniques in dealing with perineal sinuses. In patients
with difficult perineal sinuses requiring debridement and removal
of the coccyx and caudal part of the sacrum, the VAC® system
had complete resolution of the sinus in nearly all of their patients.
While their evidence is anecdotal, there are documented reports
with healing rates up to 95%.(46, 47)
Omentoplasty is another technique that has been evaluated in
both the primary repair of the perineal wound as well as in com-
plex perineal sinus disease. Yamamoto et al. reported six patients
with persistent perineal sinuses who underwent omentoplasty.
The perineal sinus tract was completely excised and communica-
tion with the pelvis attained. The left or right gastroepiploic vessels
were then ligated and the omentum brought down to the peri-
neum where it was lightly sutured to the skin. After a 28-month
follow-up period, 83% of the patients had completely healed
wounds without any complications.(48)
PERINEAL HERNIA AND EVISCERATION
Perineal hernias are fortunately very rare and often troublesome
to diagnose. Perineal hernia after abdominoperineal resection is
defined as bulging of peritoneal contents through an intact perineal
wound, and perineal evisceration describes extrusion of small
or large bowel through an open perineal wound. However, other
unusual contents have been described, including a leiomyoma,
an aggressive angiomyoma and a large bladder diverticulum.(49)
Evisceration typically occurs immediately after surgery and neces-
sitates repeat surgery with reduction of intestines and repeat pack-
ing. Perineal hernias are a rare complication and occur in about 1%
of patients after APR. This figure increases to 3% after pelvic exen-
teration. Initial symptoms include perineal bulging, often associ-
ated with fullness or pain on sitting.(50, 51) Occasionally, patients
complain of voiding problems if herniated bowel compressed the
bladder.(52) Rarely, skin breakdown occurs, resulting in exposed
bowel in the perineum. Perineal hernias, like parastomal and inci-
sional hernias, do not always require repair. Indications for surgery
are similar for all three postoperative hernias: patient discomfort
refractory to conservative therapy, bowel obstruction, incarcera-
tion, and impending skin loss. Cosmesis alone should rarely merit
surgical repair.
Risk factors that predispose patients to developing perineal her-
nias are not entirely clear. Coccygectomy, previous hysterectomy,
pelvic irradiation, excessive length of the small-bowel mesentery,
the larger size of the female pelvis, and possibly the failure to close
the peritoneal defect have been implicated as possible causes.(53,
54, 55) So et al. described 80% of their patients having perineal
wounds that were laid open or had multiple large drains inserted
through the wound which they postulate may have weaken the
wound and allow hernia formation.(56)
Diagnosis of perineal hernias can be difficult as traditional
fluoroscopic imaging techniques often do not identify them.
Other modalities have been used to include herniography, CT,
and dynamic MRI. A comparative study of dynamic MRI and
dynamic cystocolpoproctography showed that MRI was the only
modality that identified levator ani hernias.(49)
There is a paucity in large published series to describe which
technique of perineal defect closure is superior. Various case
reports and retrospective reviews provide much of the literature
in this respect. In a review of the literature, closure techniques
have ranged from the use of simple suture closure, prosthetic
mesh, human dura mater allograft (57), gracilis myocutaneous
flap (58), gluteus flap and retroflexion of the uterus or bladder.
(59) So et al. described their experience with closures and ulti-
mately found that recurrence rates were equal (20%) between
simple and mesh closures. Their repair consisted of simple
closure of the levator defect with nonabsorbable sutures. The
approach to the repair was also felt to be a point of consideration
in planning the operation. For the most part, a perineal approach
was adequate with the abdominal approach reserved for recur-
rent hernias, or those in whom laparotomy is necessary for other
reasons. The abdominal approach also provides good visualiza-
tion when suturing the mesh to the bony pelvis. A combined
AP approach is rarely necessary except under unusual circum-
stances. Skipworth et al. published their experience and tech-
nique of perineal hernia repair using Permacol® mesh. Using a
perineal approach, they isolated and ligated the sac in the stand-
ard fashion before proceeding to close the perineal defect with
4-O PDS (polydiaxonone) suture. The mesh was then fashioned
to the contours of the defect and sutured in place, tension free,
with interrupted 2-O Prolene sutures. A small suction drain was
then left superficial to the mesh and the thin, residual perineal
fascia closed with Vicryl sutures. They reported no recurrence
improved outcomes in colon and rectal surgery
in the 18 months following the repair. There are also a grow-
ing number of case reports and prospective studies in the use of
laparoscopy for perineal hernia repairs. Dulucq et al. describe
their experience in a prospective study done over the course of
a year with three patients that had received laparoscopic mesh
repairs of their perineal hernia defects. A composite mesh was
fixed laterally to the border of the levator muscle, anteriorly
to the posterior face of the vagina with nonabsorbable sutures
and posteriorly with tacks to the sacral periosteum. One suction
drain was placed. The reported benefits include adequate visu-
alization of pelvic anatomy, the ability to look for recurrence,
and fast recovery. Long term results have yet to be published for
laparoscopic perineal hernia repairs, but this may be an attrac-
tive option for patients and surgeons as it often avoids making
large incisions in areas that have already been irradiated and can
therefore be difficult to heal.(60) Before embarking on a repair
of any postoperative perineal hernia it is imperative to exclude
the possibility of cancer recurrence.
REFERENCES
1. Abraham NS, Davila JA, Rabeneck L, Berger DH, El-Serag
HB. Increased use of low anterior resection for veterans with
rectal cancer. Aliment Pharmacol Ther 2001; 21(1): 35–41.
2. Shoetz Jr DJ. Evolving practice patterns in colon and rectal
surgery. J Am Coll Surg 2006; 203(3): 322–7.
3. Swedish Rectal Cancer Registry. Available at: http://www.
oc.umu.se/rekti/rekti2002.pdf.
4. Ruo L, Guillem JG. Major 20th-century advancements in the
management of rectal cancer. Dis Colon Rectum 1999; 42(5):
563–78.
5. American Society of Colon and Rectal Surgeons Committee
Members; Wound Ostomy Continence Nurses Society Committee
Members. ASCRS and WOCN joint position statement on the
value of preoperative stoma marking for patients undergoing
fecal ostomy surgery. J Wound Ostomy Continence Nurs 2007;
34(6): 627–8.
6. Marret E, Remy C, Bonnet F. Postoperative Pain Forum
Group. Meta-analysis of epidural analgesia versus paren-
teral opioid analgesia after colorectal surgery. Br J Surg 2007;
94(6): 665–73.
7. Geerts WH, Bergqvist D, Pineo GF et al. American College of
Chest Physicians. Prevention of venous thromboembolism:
American College of chest physicians evidence-based clinical
practice guidelines (8th Edition). Chest 2008; 133(6 Suppl):
381S–453S.
8. Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery:
an advisory statement from the National Surgical Infection
Prevention Project. Clin Infect Dis 2004; 38: 1706–15.
9. Beraldo S, Dodds SR. Lower limb acute compartment
syndrome after colorectal surgery in prolonged lithotomy
position. Dis Colon Rectum 2006; 49(11): 1772–80.
10. Miles WE. A method of performing abdominoperineal exci-
sion for carcinoma of the rectum and of the terminal portion
of the pelvic colon. Lancet 1908; 2: 1812.
11. Corman ML. Carcinoma of the rectum. In: Colon and Rectal
Surgery. Fifth Edition. Lippincott, Williams, and Wilkins.
Philadelphia, 2005: 905–1061.
12. Heald RJ, Husband EM, Ryall DH. The mesorectum in rectal
cancer surgery – the clue to pelvic recurrence? Br J Surg 1982;
69: 613–6.
13. Kyo K, Sameshima S, Takahashi M, Furugori T, Sawada T.
Impact of autonomic nerve preservation and lateral node
dissection on male urogenital function after total mesorec-
tal excision for lower rectal cancer. World J Surg 2006; 30(6):
1014–9.
14. Moriya Y. Function preservation in rectal cancer surgery. Int
J Clin Oncol 2006; 11(5): 339–43.
15. Kim NK, Aahn TW, Park JK et al. Assessment of sexual and
voiding function after total mesorectal excision with pelvic
autonomic nerve preservation in males with rectal cancer.
Dis Colon Rectum 2002; 45: 1178–85.
16. Shirouzu K, Ogata Y, Araki Y. Oncologic and functional
results of total mesorectal excision and autonomic nerve-
preserving operation for advanced lower rectal cancer. Dis
Colon Rectum 2004; 47(9): 1442–7.
17. Murrell ZA, Dixon MR, Vargas H et al. Contemporary indica-
tions for and early outcomes of abdominoperineal resection.
Am Surg 2005; 71(10): 837–40.
18. Rosen L, Veidenheimer MC, Coller JA et al. Mortality, morbid-
ity, and patterns of recurrence after abdominoperineal resection
for cancer of the rectum. Dis Colon Rectum 1982; 25: 202–8.
19. Irvin IT, Goligher JC. A controlled clinical trial of three
different methods of perineal wound management following
excision of the rectum. Br J Surg 1975; 62: 287–91.
20. Silen W, Glotzer DJ. The prevention and treatment of the
persistent perineal sinus. Surgery 1974; 75: 535–42.
21. Meyer L, Bereuter M, Marusch F et al. Perineal wound
closure after abdomino-perineal excision of the rectum. Tech
Coloproctol 2004; (8 suppl 1): S230–4.
22. Gruessner U, Clemens M, Pahlplatz PV et al. Septocoll Study
Group. Improvement of perineal wound healing by local
administration of gentamicin-impregnated collagen fleeces
after abdominoperineal excision of rectal cancer. Am J Surg
2001; 182: 502–9.
23. Mendel V. Rektumexstirpation-Ergebnisse mit einem
Kollagen-Gentamycin-verbund bei der abdomino-perinealen
Rektumexstirpation. Forum Lokale Antibiose “Der Schwamm”,
Essex Pharma GmbH, 1995; 50–1.
24. Chessin DB, Hartley J, Cohen AM et al. Rectus flap recon-
struction decreases perineal wound complications after pelvic
chemoradiation and surgery: a cohort study. Ann of Surg Onc
2005; 12(2): 104–10.
25. Butler CE, Gundeslioglu AO, Rodriguez-Bigas MA. Outcomes
of immediate vertical rectus abdominis myocutaneous flap
reconstruction for irradiated abdominoperineal resection
defects. J Am Coll Surg 2008; 206(4): 694–702.
26. Page CP, Carlton PK, Becker DW. Closure of the pelvic and
perineal wounds after removal of the rectum and anus. Dis
Colon Rectum 1980; 23: 2–9.
27. Nilsson PJ. Omentoplasty in Abdominoperineal Resection:
A review of the literature using a systematic approach. Dis
Colon Rectum 2006; 49: 1354–61.
28. Pollard CW, Nivatvongs S, Rojanasakul A, Ilstrup DM.
Carcinoma of the rectum. Profiles of intraoperative and early
abdominoperineal resection
postoperative complications. Dis Colon Rectum 1994; 37:
866–74.
29. Broux ED, Parc Y, Rondelli F et al. Sutured perineal omento-
plasty after abdominoperineal resection for adenocarcinoma
of the lower rectum. Dis Col Rect 2005; 48: 476–82.
30. Baudot PE, Keighley MRB, Alexander-Williams J. Perineal
wound healing after proctectomy for carcinoma and inflam-
matory disease. Br J Surg 1980; 67: 275–6.
31. Michalson AE, Brown BP. Warnock NG, Simonson TM.
Presacral abscesses: percutaneous transperineal drainage with
use of bone landmarks and fluoroscopic guidance. Radiology
1994; 190: 574–5.
32. Wang O, Shi W, Zhaw Y, Zhou W, He Z. New concepts in
severe presacral hemorrhage during proctectomy. Arch Surg
1985; 120: 1013–20.
33. Arnaud JP, Tuech JJ, Pessaux P. Management of presacral
venous bleeding with the use of thumbtacks. Dig Sur 2000;
17(6): 651–2.
34. Nivatvongs S, Fang DT. The use of thumbtacks to stop mas-
sive presacral hemorrhage. Dis Colon Rectum 1986; 29:
589–90.
35. Rothenberger DA, Wong WD. Abdominoperineal resection
for adenocarcinoma of the low rectum. World J Surg 1992;
16: 478–85.
36. de Canniere L, RosiPre A, Michel LA. Synchronous abdomi-
noperineal resection without transfusion. Br J Surg 1993; 80:
1194–5.
37. Petrelli NJ, Nagel S, Rodriguez-Bigas M et al. Morbidity and
mortality following abdominoperineal resection for rectal
adenocarcinoma. Am Surg 1993; 59: 400–4.
38. Tompkins RG, Warshaw AL. Improved management of
the perineal wound after proctectomy. Ann Surg 1985; 202:
760–5.
39. Anthony JP, Mathes SJ. The recalcitrant perineal wound after
rectal extirpation. Arch Surg 1990; 125: 1371–7.
40. Fasth S, Hulten L, Ojerskog B. Omission of pelvic peritoneal
closure after abdominoperineal rectal excision. Ann Chir
Gynaecol 1977; 66: 181–3.
41. Scott H, Brown AC. Is routine drainage of pelvic anastomosis
necessary? Am Surg 1996; 62: 452–7.
42. Bacon HE, Nuguid TP. Problem of persistent perineal sinus
following removal of the rectum for ulcerative colitis. Dis
Colon Rectum 1962; 5: 370–2.
43. Christian CK, Kwaan, MR, Betensky RA et al. Risk factors for
perineal wound complications following abdominoperineal
resection. Dis Colon Rectum 2005; 48(1): 43–8.
44. Oomen JW, Spauwen PH, Bleichrodt RP, vanGoor H. Guideline
proposal to reconstructive surgery for complex perineal sinus
or rectal fistula. Int J Colorectal Dis 2007; 22(2): 225–30.
45. Permberton JH. How to treat the persistent perineal sinus
after excision. Colorectal Dis 5(5): 486–9.
46. Argenta LC, Morykwas MJ. Vacuum-assisted closure. A new
method for wound control and treatment: clinical experiences.
Ann Plastic Surg 38: 1997; 563–77.
47. Deva AK, Buckland GH, Fisher E et al. Topical negative pressure
in wound management. Med J Australia 2000; 173: 128–31.
48. Yamamoto T, Mylonakis E, Keighley MR. Omentoplasty for
persistent perineal sinus after proctectomy for Crohn’s disease.
Am J Surg; 181(3): 265–7.
49. Skipworth RJ, Smith GH, Anderson DN. Secondary perineal
hernia following open abdominoperineal excision of the
rectum: report of a case and review of the literature. Hernia
2007; 11: 541–5.
50. McMullin ND, Johnson WR, Polglase AL, Hughes ESR. Post-
proctectomy perineal hernia: case report and discussion.
Aust N Z J Surg 1985; 55: 69.
51. Rutledge RN, Smith JP, Wharton JT, O’Quinn AG. Pelvic
exenteration: an analysis of 296 patients. Am J Obstet Gynecol
1977; 129: 881.
52. Brotschi E, Noe JM, Silen W. Perineal hernias after proctec-
tomy. Am J Surg 1985; 149: 301–5.
53. Cattell RB, Cunningham RM. Postoperative perineal hernia
following resection of rectum: report of a case. Surg Clin
North Am 1944; 24: 679–83
54. Kelly AR. Surgical repair of post-operative perineal hernia.
Aust N Z J Surg 1960; 29: 243–5.
55. Frydman GM, Polglase AL. Perineal approach for polypro-
pylene mesh repair of perineal hernia. Aust N Z Surg 1989;
59: 895–7.
56. So JB, Palmer MT, Shellito PC. Postoperative perineal hernia.
Dis Colon Rectum 1997; 40: 954–7.
57. Delmore JE, Turner DA, Gershenson DM et al. Perineal hernia
repair using human dura. Obstet Gynecol 1987; 70: 507–8.
58. Bell JG, Weiser EB, Metz P, Hoskins WJ. Gracilis muscle
repair of perineal hernia following pelvic exenteration.
Obstet Gynecol 1980; 56(3): 377–80.
59. Remzi FH, Oncel M, Wu JS. Meshless repair of perineal
hernia after abdominoperineal resection: case report. Tech
Colo-proctol 2005; 9: 142–4.
60. Dulucq JL, Wintringer P, Mahajna A. Laparoscopic repair
of postoperative perineal hernia. Surgical Endoscopy 2006;
20(3): 414–8.
Indications and outcomes for treatment of recurrent rectal cancer
and colorectal liver and lung metastasis
Harry L Reynolds Jr, Christopher T Siegel, and Jason Robke
CHALLENGING CASE
A 74 year old male underwent low anterior resection of the rectum
one year previously after preoperative chemotherapy and radia-
tion. Final pathology revealed a yPT2N0M0 lesion. He received
post operative chemotherapy as well. In follow up he was noted to
have an elevated carcinoembrionic antigen of seven. Digital exam
revealed a palpable mass at the finger tip. It was one half circum-
ferential, posteriorly based, involving the left pelvic sidewall and
was fixed. Located at five cm from the verge on rigid proctoscopic
exam, it was just at the top of the anorectal ring and involved the
previous anastomosis. Computed Tomographic (CT) scan of the
chest abdomen and pelvis revealed a posteriorly based mass adja-
cent to the sacrum without boney erosion. It suggested involve-
ment of the left pelvic sidewall. There was a two cm hypodense
lesion in segment three of the liver. The chest was clear. Positron
Emission Tomography revealed intense uptake in the pelvis and
in the hypodense area of the left lobe of the liver noted on CT.
Magnetic Resonance Imaging of the pelvis confirmed extension
into the pelvic sidewall but did not reveal boney involvement of
the sacrum. Colonoscopy cleared the proximal colon. Biopsies
confirmed a moderately well differentiated adenocarcinoma.
CASE MANAGEMENT
The patient received a preoperative radiation boost to the pelvis
supplemented with capecitabine. He was re-explored and under-
went abdominal perineal resection. The left internal iliac artery
and vein were ligated and partially excised with a portion of the
left pelvic sidewall. He received an intraoperative radiation boost to
the left sidewall and sacrum where the tumor was adherent. It was
difficult to differentiate tumor from scarring over the sacrum, but
there was no gross boney involvement. Intraoperative ultrasound
of the liver revealed no other liver lesions and the left lateral seg-
ment metastasis was excised after the pelvic work was completed.
He was reconstructed with an end descending colostomy. He was
referred to oncology for further postoperative chemotherapy.
INTRODUCTION
Of the many challenges presented to the surgeon, patients
presenting with recurrent rectal cancer can be among the most
daunting. Likewise patients with metastatic disease to the liver
or lung can be a technical challenge and can be difficult to sort
out as to who is an appropriate operative candidate. These
patients are best treated with a multidisciplinary approach with a
Colorectal or General Surgeon serving as the team leader.(1) In
this chapter we explore, in three separate sections, the indica-
tions and outcomes for surgical intervention in patients with:
1. Recurrent rectal cancer, 2. Liver metastasis, and 3. Lung metas-
tasis. Contributing authors include a Colon and Rectal Surgeon,
a Hepatobiliary Surgeon, and a Thoracic Surgeon.
RECURRENT RECTAL CANCER
Recurrent rectal cancers are among the most challenging for
the Colon and Rectal Surgeon to manage. A multidisciplinary
approach is truly essential in planning a comprehensive treat-
ment plan if success is to be achieved. The surgeon that takes on
these cases assumes the responsibility of organizing and lead-
ing a team of sub specialists consisting of Medical and Radiation
Oncologists, Urologists, Radiologists, Pathologists, Enterostomal
Therapists, and in selected cases, Plastic Surgeons, Vascular Surgeons,
Gynecologic surgeons, Hepatobiliary Surgeons, Thoracic Surgeons,
and Intensivists. The complexity of these patients makes it essen-
tial that they be treated at centers with the staff and resources
necessary to undertake their care.(1, 2)
Assessing Resectability
A thorough workup of the patient first consists of a careful history
and physical exam. Fitness for surgery should be assessed carefully as
one can expect a significant physiologic insult in those who come to
operation. Many will require extended en bloc resections of adjacent
organs with the potential for blood loss and volume shifts not usually
seen at initial proctectomy. Preoperative assessment by appropriate
sub specialists with emphasis on cardiac and pulmonary optimiza-
tion may be necessary in these frequently elderly patients. In some
patients surgical risk may be deemed prohibitive and nonoperative
management with combinations of chemotherapy and radiation
and/or stenting or palliative diversion may be necessary.
Assessing the extent of local and metastatic disease is best
achieved with careful physical and proctoscopic exam supple-
mented with appropriate radiologic imaging. The importance
of digital examination cannot be overemphasized. Determining
location with respect to the sphincter complex and adjacent
structures including vagina, uterus, prostate, seminal vesicles,
bladder, pelvic sidewall, and sacrum can be preliminarily assessed
with a careful digital rectal and vaginal exam. Bulk, mobility, and
fixation to surrounding structures should be carefully considered
with the digital. Pelvic recurrences are frequently extramucosal
and may not be appreciated endoscopically but may be felt on
digital. Rigid proctoscopic and digital exams are essential in
determining relationships to the sphincter complex as even with
pelvic recurrences, sphincter sparing options may be available in
selected patients. Involvement of the sphincter complex typically
necessitates abdominal perineal resection (APR).
A full colonoscopy is performed to rule out synchronous
lesions. Radiographic workup typically consists of PET CT to rule
out extrapelvic metastatic disease. PET CT may identify patients
with unsuspected metastatic disease, thus preventing overly
aggressive surgical intervention. Watson reported a change in
planned surgical intervention in 37% of patients based on PET
CT imaging in patients with recurrent colorectal cancer.(3)
indications and outcomes for treatment of recurrent rectal cancer
High quality spiral CT scanning of the chest abdomen and
pelvis is frequently performed as well to define any question-
able metastatic lesions and to better define the extent of pelvic
disease. Pelvic MRI is felt by most authors to be the preferred
imaging modality for establishing the extent of adjacent organ
involvement for purposes of preoperative planning. MRI can
provide insight as to whether pelvic sidewall, seminal vesicles,
prostate, bladder, ureters, vascular structures, gynecologic struc-
tures, or sacrum are involved.(4–8) Although imaging can assist
in preop planning, no radiographic study can reliably differenti-
ate fibrosis and scarring from tumor, particularly in an irradiated
pelvis. Radiation induced inflammatory changes in the pelvis can
be PET positive as well, and can be confused for metastatic disease.
Although imaging can assist with planning, the ultimate determi-
nation of involvement and resectability is made at operation.
Reviewing previous operative notes and pathology reports can
provide insight into the adequacy of initial resection and can be
helpful in assessing likelihood of resectability at reoperation. Those
that have had an optimal cancer operation at the first exploration
with total mesorectal excision (TME) and high ligation of the infe-
rior mesenteric artery can be expected to have a much more diffi-
cult reexploration. With TME and high ligation initially, combined
with previous radiation therapy, recognizable planes are typically
absent. Those with more proximal tumors, non-TME initial resec-
tions, and/or ligation of the superior rectal vs. the inferior mesen-
teric artery (IMA) may well have some pelvic plane preservation,
making reexploration not so daunting a task. Those that have had
a proper TME with a coloanal anastomosis or abdominal perineal
resection can be expected to have more difficult lesions to deal with,
as recurrences can be expected to be adherent to adjacent struc-
tures, outside of the proper mesorectum. These tumors recur in
the sidewall, sacrum, anastomosis, perineal wound or in the gyne-
cologic or urologic organs. Those that recur laterally in the pelvic
sidewall or posteriorly on the sacrum are particularly challenging
in terms of obtaining an R0 resection. An R0 resection refers to a
complete resection with microscopically clear margins. R1 resec-
tion implies microscopic disease is left, and R2 implies gross dis-
ease is left behind. Those that recur at the anastomosis or anteriorly
in the adjacent vagina, uterus, prostate, seminal vesicle, or bladder
can frequently be completely excised with en bloc adjacent organ
resection.(9) Likewise, the occasional patient who presents with an
isolated nodal recurrence, high along the IMA after an initial low
ligation, may be resectable as well.
Pelvic sidewall recurrences can be very difficult to resect sec-
ondary to the extensive internal iliac arterial and venous branches
encountered. Anatomy is distorted by tumor and scar, and the des-
moplastic reaction associated with tumor and previous radiation
can make dissection hazardous as venous bleeding can be signifi-
cant. Likewise sacral recurrences below S1-2 typically can techni-
cally be resected, but morbidity and mortality can be significant.
(10) Local, limited anastomotic recurrences post low anterior resec-
tion and perineal recurrences post APR, without lateral or sacral
extension, are more likely to be amenable to R0 resection.(9)
Patients with extrapelvic metastatic disease are typically not offered
extended exenterative procedures. However, in selected otherwise fit
patients presenting with isolated resectable liver or lung metastasis,
it may be appropriate to proceed with extended resection. In those
with nonresectable metastatic extrapelvic disease, pelvic exenterative
procedures are felt to be contraindicated by most.
Role of Chemotherapy and Radiation
Our approach to a diagnosed pelvic recurrence initially involves
evaluation by medical and radiation oncology to determine if an
additional radiation boost can be delivered to the pelvis. This will
usually be administered with concomitant 5-FU based chemo-
therapy. Most patients will have received either preop or postop
chemoradiotherapy with there first resection. Most will receive an
additional preop boost of 30–40 Gy to the site, particularly if it is
a bulky recurrence, with plans for surgical exploration ~8 weeks
post radiation.(11–15) This is assuming the interval between radi-
ation and reirradiation is >6 months and the small intestine can
be excluded from the planned field.(11) This approach seems to
be well tolerated by most in our institution and has been validated
by others.(11–15) It is important to ensure exclusion of the small
intestine from the pelvis with reirradiation. Dresen reports that if
the small intestine is not excluded planned operation before irra-
diation may be undertaken for placement of a spacer for exclusion.
The spacer could be biologic such as the omentum or a nonbio-
logic such as a breast prosthesis or tissue expander. The patient is
typically diverted at the time of spacer placement.(11)
Intraoperative radiation therapy (IORT) can be offered via a
dedicated fixed intraop unit, a mobile unit or via after-loading
catheters place intraoperatively. This is a particularly valuable
treatment adjunct to patients with sidewall involvement, sacral
involvement, or major vascular involvement, where an extended
resection of involved areas cannot technically be accomplished or
will not be tolerated by the patient. This can be technically deliv-
ered to areas directly involved by tumor while shielding organs
particularly sensitive to radiation (i.e., ureters, small intestine, and
bladder).While no randomized trial has been performed to dem-
onstrate the value of IORT, such a trial is unlikely to be performed.
Positive circumferential margins in rectal cancer have been associ-
ated with excessively high local recurrence rates as demonstrated
by Quirke and others even after preop radiochemotherapy.(16,
17) It would seem illogical, and perhaps unethical, to randomize
patients to a nontreatment arm with a known or suspected posi-
tive margin when a modality such as IORT, with little morbid-
ity when applied appropriately, is available. There are multiple
studies, with historical controls, demonstrating decreases in local
recurrence and survival improvement, with little morbidity, when
IORT is applied appropriately.(5, 18–26) We feel that IORT is an
essential piece of the treatment algorithm. It is frequently very
difficult to differentiate a true positive margin from the fibrosis
and scarring associated with previous radiation therapy and pre-
vious pelvic surgery. Although frozen section analysis of surgical
margins can be helpful if positive, if we are clinically concerned
about margin status, IORT will be administered regardless of fro-
zen section results. Local recurrence rates in our institution have
been very favorable with this approach.(27) Likewise, others have
demonstrated favorable results with this approach.(5, 18–26)
With the addition of IORT to our armamentarium, it seems
overly aggressive to perform sacrectomy in all cases with sacral
adherence. Adherence to the presacral fascia is present in virtually
all patients who have undergone a TME and it is very difficult to