Improved Outcomes in Colon and Rectal Surgery part 34 ppsx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (437.71 KB, 10 trang )
Surgery for ulcerative colitis
Patricia L Roberts
CLINICAL VIGNETTE
Challenging Case
A 35-year-old male is undergoing an ileoanal pouch procedure
for ulcerative colitis. Following transection of the ileum flush
with the cecum, the surgeon notes that it will be difficult for the
pouch to reach the anus.
Challenging Case Management
Difficulties with the ileoanal pouch reaching the anus occur for two
main reasons: failure to mobilize the small bowel, or patient-related
factors such as obesity or a long narrow anal canal. Difficulty with
reach is more common if a mucosectomy is performed rather than
a double-stapled anastomosis. An S pouch may reach the anus eas-
ier than a J pouch. If the main reason for the pouch not reaching is
patient obesity and a thickened mesentery, an initial total abdomi-
nal colectomy, ileostomy, and Hartmann closure of the rectum may
be performed. Following weight reduction, an ileoanal pouch pro-
cedure can be performed. A series of technical maneuvers includ-
ing mobilization of the small bowel up to the duodenum, scoring
the peritoneum over the superior mesenteric artery, and the crea-
tion of mesenteric windows can facilitate pouch reach. If, despite
these maneuvers, the pouch does not reach, the pouch can be left
in the pelvis, a loop ileostomy created, and, after a period of sev-
eral months, the pouch can then be joined to the anus. Additional
details of these technical maneuvers are described in the text.
INTRODUCTION
Ulcerative colitis is an inflammatory condition involving the colon
and rectum. The incidence in the United States is 8.8 cases per
100,000 person years.(1) Thus, in this country, there are approxi-
mately 26,000 new cases of ulcerative colitis diagnosed annually and
730,000 people with ulcerative colitis.(1, 2) Although many patients
are treated effectively with medical therapy, approximately 23–45% of
patients require colectomy. The risk of requiring colectomy is higher
in patients with pancolitis than patients with left sided disease.(3, 4)
This chapter concentrates on the indications for surgery, the opera-
tive options, and the outcome of surgery for ulcerative colitis.
INDICATIONS FOR SURGERY
Surgery for ulcerative colitis is divided into two categories: urgent
or emergency surgery, and elective surgery.
Acute Colitis
Urgent or emergent surgery is indicated for patients with acute
unresolving colitis or life-threatening complications associated
with colitis, including fulminant or toxic colitis, hemorrhage,
colonic perforation, or obstruction. Severe acute colitis may occur
in 5 to 15% of patients with ulcerative colitis. The classification
system of Truelove and Witts is most commonly used and iden-
tifies clinical parameters by which colitis is categorized as mild,
moderate, and severe (5, 6) (Table 31.1). For patients with acute
colitis, stool studies should be done to rule out superinfection
with clostridium difficile, bacteria, or ova and other parasites.
A flexible sigmoidoscopy without bowel preparation with mini-
mal insufflation of air is helpful to biopsy the rectum to exclude
cytomegalovirus (CMV). In one series of patients with steroid
resistant acute ulcerative colitis, the incidence of associated
cytomegalovirus was 36%.(7) The majority of patients diagnosed
with CMV responded to administration of foscarnet or ganciclo-
vir. After exclusion of an infectious etiology, patients are treated
with intravenous steroids for 5–7 days. If there is no clinical
response, cyclosporine or infliximab is considered. Patients who
are reluctant to use cyclosporine or infliximab, or patients who
do not respond, should undergo colectomy. While administra-
tion of steroids is associated with an increase in postoperative
complications, immunosuppressives do not appear to increase
the incidence of postoperative complications.(8)
A small subset of patients may develop fulminant colitis. The
classification system of Truelove and Witts does not define ful-
minant colitis, but Hanauer (9) has modified the classification
system to define patients with fulminant colitis. In the classifica-
tion system of Truelove and Witts, severe disease is defined as >6
stools per day, a temperature >37.5 degree Celsius, a pulse of >90
beats per minute, hemoglobin <75% of normal, an erythrocyte
sedimentation rate of >30 mm/hr, the presence of air, edema-
tous wall, or thumbprinting on x-ray and abdominal tenderness.
Fulminant colitis is defined as >10 stools per day, continuous
Table 31.1 Truelove and Witts Criteria for Evaluating the Severity
of Ulcerative Colitis.
Variable
Mild disease
Severe Disease
Fulminant
Disease
Stools (Number/day) <4 >6 >10
Blood in stool Intermittent Frequent Continuous
Temperature (ºC) Normal >37.5 >37.5
Pulse (beats/min) Normal >90 >90
Hemoglobin Normal <75% of
normal value
Transfusion
required
Erythrocyte
sedimentation
rate
<30 >30 >30
Colonic features on
x-ray
Air, edematous
wall, thumb-
printing
Dilatation
Clinical Signs Abdominal
tenderness
Abdominal
distention
and
tenderness
Source: After Truelove and Witts. BMJ 1955; 2: 1041–45.
Reprinted with permission from Clinics in Colon and Rectal Surgery. Volume 17,
Number 1, 2004, page 8.
surgery for ulcerative colitis
bloody bowel movements, a temperature of >37.5 degree Celsius,
a pulse of >90 beats per minute, transfusion requirement, an
erythrocyte sedimentation rate of >30 mm/hr, dilatation of
the colon and abdominal distention and tenderness. The term
toxic megacolon has been used when the colonic distention of
the transverse colon exceeds 6 cm, but relying on this finding to
diagnose toxic colitis is not necessary, as some patients will have
“toxicity” in the absence of colonic distention. Prompt treatment
and diagnosis of toxic colitis is needed to avoid progression to
perforation. Approximately 20–30% of patients with toxic colitis
require emergency surgery.
Perforation in the setting of toxic or fulminant colitis substan-
tially increases the mortality rate. Patients whose condition wors-
ens or who fail to make substantial improvement after a period
of 48–96 hours should be considered for surgery to avoid this
complication.(10) Massive hemorrhage in patients with ulcera-
tive colitis is uncommon, accounting for <10% of emergency
colectomies performed for ulcerative colitis, and raises the pos-
sibility of Crohn’s disease.(11)
Emergency vs. Elective Procedures
The surgical options for patients who require emergency surgery
for acute colitis are aimed at restoring the patient back to a gen-
eral state of health and preserving reconstructive options for sub-
sequent surgery. The most common operation performed is total
abdominal colectomy with ileostomy, and either Hartmann closure
of the rectum or creation of a mucous fistula. Preoperative coun-
seling and marking by an enterostomal therapist is optimal. This
procedure removes the majority of the diseased bowel, avoids an
intestinal anastomosis in an ill patient, and preserves the option for
an ileoanal pouch procedure in the future. The colon is transected
at the level of the sacral promontory avoiding the need for a pel-
vic dissection. If the severity of disease as demonstrated by severe
ulcerations and friability of the bowel precludes safe closure of the
stump, a variety of other options may be employed. The stump may
be exteriorized as a mucous fistula. This requires a longer segment
of bowel and is associated with bleeding and mucus from an addi-
tional stoma. Alternatively, it has been suggested that extrafascial
placement compared with intraperitoneal closure of the Hartmann
stump may be associated with fewer infectious complications.(12)
Transanal drainage has also been suggested to decrease the inci-
dence of infectious complications associated with the Hartmann
stump.(13) Pelvic dissection and creation of a relatively short
Hartmann pouch should be avoided as this makes dissection and
subsequent ileoanal pouch creation more difficult. A laparoscopic
or open approach may be used for performance of total abdominal
colectomy and ileostomy in patients with acute colitis.(14)
Elective Procedures
The most common indication for elective surgery is intractabil-
ity to medical management defined as failure of medical ther-
apy. Intractability includes insufficient symptom control despite
intensive medical therapy. Due to loss of time from work, school
or activities in general, the patient may not have an acceptable
quality of life. The risks of medical therapy may be substantial
including potential complications from long-term steroid ther-
apy or complications of the side effects of medical therapy. In
children, growth retardation can result from poorly controlled
ulcerative colitis and is an indication for colectomy.
Patients with longstanding ulcerative colitis are at an increased
risk for the development of colorectal cancer. The exact risk is dif-
ficult to determine since many series have lacked longitudinal fol-
low-up or have included patients seen at tertiary referral facilities.
Surveillance colonoscopy with biopsy has been recommended in
patients with left-sided or pan colitis (defined as microscopic dis-
ease proximal to the splenic flexure) after 8 years of disease symp-
toms. At least 33 biopsies are necessary to obtain a sensitivity of
90%, and four quadrant biopsies are recommended every 10 cm
along the colon and in any abnormal appearing area. A recent
meta-analysis has estimated the risk of the development of color-
ectal cancer in patients with long-standing ulcerative colitis to be
2% at 10 years, 8% at 20 years, and 18% after 30 years of disease.
(15) There is no evidence to show that surveillance prolongs sur-
vival in such patients, although patients who develop cancers in a
surveillance program tend to have earlier stage cancers.(16, 17)
Proctocolectomy is indicated for patients with carcinoma,
nonadenoma-like dysplasia associated lesion or mass (DALM),
and patients with high grade dysplasia.(10) The presence of high
grade dysplasia should ideally be confirmed by two independent
expert pathologists. For those patients who underwent immedi-
ate colectomy, cancer was detected in 42% of patients with high-
grade dysplasia and 19% with low-grade dysplasia.(18) Although
patients with low-grade dysplasia should be offered colectomy,
the natural history of low-grade dysplasia is not as well defined.
The interobserver variation between pathologists confounds the
recommendations about low-grade dysplasia. Studies are con-
flicting, with one study of a surveillance program showing that in
patients with low-grade dysplasia the 5-year predictive value for
the development of cancer or high-grade dysplasia was 54%.(19)
Another study showed that only 18% of patients with low-grade
dysplasia progressed to high-grade dysplasia or a dysplasia associ-
ated lesion/mass.(20)
Strictures may also develop in 10–25% of patients with ulcera-
tive colitis, and while the majority are benign up to 25% are
malignant. Strictures which cause obstruction, develop in long-
standing disease, and are found proximal to the splenic flexure,
are most likely to be malignant and are another indication for
colectomy.(21)
PROCTOCOLECTOMY WITH BROOKE ILEOSTOMY
Proctocolectomy with ileostomy has previously been the “gold stand-
ard” operation for ulcerative colitis against which other operations
have been compared. This operation essentially cures the disease and
restores patients back to health and to a relatively normal life. It
is a one-stage procedure which removes the diseased mucosa and
has fewer potential complications than the ileoanal pouch proce-
dure. The main drawback is the presence of a permanent ileostomy,
something which most patients wish to avoid.
Indications
This operation is indicated in those patients who require surgery
for ulcerative colitis, but are not candidates for the ileoanal pouch
procedure. These patients include those who are elderly, have fecal
incontinence or an inadequate sphincter, patients with low rectal
improved outcomes in colon and rectal surgery
cancers in association with ulcerative colitis who require proc-
tectomy and possibly pelvic radiation, and those patients who
opt for a permanent Brook ileostomy for personal preferences.
Furthermore, patients who develop pouch failure and require
pouch excision essentially have a completion proctectomy.
Operative Technique
The preoperative period includes patient education about the
procedure and the effects of an ileostomy. Preoperative consulta-
tion with an enterostomal nurse is helpful. The stoma site selected
should be a flat area, away from bony prominences and creases.
Proctocolectomy is performed through either an open or
laparoscopic approach. Following mechanical bowel preparation
the day before surgery, the patient is administered preoperative
intravenous antibiotics and positioned in lithotomy position.
After performance of a standard colectomy, pelvic dissection is
performed. The retrorectal space is entered sharply and the pelvic
dissection is undertaken with careful attention to the ureters and
identification of the hypogastric nerves. The dissection is carried
out to the pelvic floor. A pack is placed posterior to the rectum
and the perineal dissection is performed. An intersphincteric dis-
section allows for a smaller wound, a relatively bloodless dissec-
tion, and presumably better healing. The perineal dissection is
carried out to the level of the pelvic dissection. After excision of
the colon and rectum, the wound is closed in layers and a Brooke
ileostomy constructed. A foley catheter is left for several days in
addition to a closed suction drain.
Outcome
Proctocolectomy with ileostomy is associated with fewer poten-
tial complications than ileoanal pouch procedure. In one series,
the long-term complication rate in patients undergoing procto-
colectomy with ileostomy compared to ileoanal pouch procedure
was 26% vs. 52%.(22) The most common long-term complica-
tions include stoma related complications. From a physiologic
standpoint, patients with an ileostomy are more prone to dehy-
dration, electrolyte abnormalities, and kidney stone formation.
Patients should be counseled to be aware of signs and symptoms
of dehydration. Although problems have decreased substantially
with modern pouching systems, preoperative stoma marking,
and the expertise of enterostomal nurses, patients may experience
peristomal skin irritation, parastomal hernia formation, stomal
retraction, fistula, and stomal stenosis. In the long-term, up to
one third of patients require operative revision.(23)
Slow or delayed perineal wound healing occurs in up to 25%
of patients after proctocolectomy with ileostomy. An intersphinc-
teric dissection may decrease the size of the perineal wound and
improve wound-related complications.(24) If infection or delayed
wound healing occurs, local wound care with examination under
anesthesia, debridement, and curettage is performed. The vacuum
assisted closure device has been helpful to treat persistent perineal
wounds.(25) In some cases, muscle transposition, such as gracilis
muscle transposition is necessary to heal persistent wounds.
As with any operation involving a pelvic dissection, sexual and uri-
nary dysfunction may occur from injury to the sympathetic and para-
sympathetic nerves. The incidence of sexual dysfunction is felt to be
less than that occurring in those patients who undergo proctectomy
for malignant disease. However, this may reflect the younger age of
patients undergoing proctocolectomy for ulcerative colitis. Impotence
occurs in 1–2% of patients and retrograde ejaculation may occur in
up to 5% of patients.(26) Dysparuenia and increase in vaginal dis-
charge occur in up to 30% of women from scarring and change in the
in-axis of the vagina.(27) Women must also be counseled about the
potential for infertility because of scarring pelvic adhesions.
Despite the fact that patients have undergone a major surgical
procedure, the quality of life remains high after proctocolectomy
with ileostomy. Overall 90–93% of patients are satisfied with their
quality of life.(28, 29) Despite the satisfaction, a number of difficul-
ties exist, including restriction of social and recreational activities in
up to 25%, and dietary restrictions in almost 30%.
PROCTOCOLECTOMY WITH CONTINENT ILEOSTOMY
Another option for patients who require surgery for ulcerative
colitis is a continent ileostomy, introduced by Nils Kock in 1969.
(30) Despite initial enthusiasm, this operation is infrequently
performed today because of the appreciable number of complica-
tions associated with the procedure, in addition to the fact that it
has been largely supplanted by the ileal pouch anal anastomosis.
Indications for a continent ileostomy include those patients who
have undergone prior proctocolectomy with ileostomy and desire
a continent stoma, selected patients who have a failed ileoanal
pouch procedure, patients with ulcerative colitis and rectal cancer
who could not undergo an ileal pouch anal anastomosis (IPAA)
and patients with poor sphincter tone in whom the functional
results would be quite poor.
Advanced age and obesity are relative contraindications to per-
formance of the procedure. As with the ileoanal pouch procedure,
Crohn’s disease is a general contraindication to the procedure
because of the risk of recurrent disease which could necessitate
resection of the continent ileostomy.
Operative Technique
The operative technique involves initial performance of a proctocolec-
tomy. The continent ileostomy is then constructed using the terminal
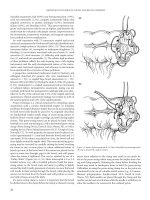
40–60 cm of the ileum. A three limb pouch with an intussuscepted
nipple valve is used (Figure 31.1). The valve is created by intussuscept-
ing the efferent loop. After being tested for integrity and continence,
the exit conduit is brought through the abdominal wall. The site of
continent ileostomy is generally determined preoperatively with an
enterostomal therapist and is lower in the abdomen than a standard
ileostomy. Catheter drainage is maintained for approximately 4 weeks
to allow complete healing of the pouch.(31, 32) Guidelines for catheter
management have been outlined by Beck.(33)
A number of technical modifications have been made over the
years to prevent nipple valve complications. Mesh was initially
used to stabilize the valve, but the technique was abandoned
because of a high incidence (42.5%) of fistula formation.(31) A
recently described modification to avoid slippage of the nipple
valve is the “T-pouch” in which a portion of the ileum is folded
into the side of the pouch.(33, 34)
Outcome
In a large series of patients undergoing continent ileostomy with
a median follow-up of 11 years, 16.6% of patients required Kock
surgery for ulcerative colitis
Figure 31.1 Continent ileostomy (A) Three limbs of small bowel are measured and the bowel wall is sutured together. (B) After opening the bowel along the dotted
lines in (A), the edges are sewn together to form a two-layered closure. (C) A valve is created intussuscepting the efferent limb into the pouch and fixing it in place with
a linear noncutting stapler. (Inset: staples in place on valve.) (D) The valve is attached to the pouch side-wall with the linear noncutting stapler. A cross-section of the
finished pouch is shown. (E) After closure of the last suture line, the pouch is attached to the abdominal wall and a catheter is inserted to keep the pouch decompressed
during healing (Reprinted with permission).
(A) (B) (C)
(D)
(E)
improved outcomes in colon and rectal surgery
pouch excision.(31) The number of complications associated
with the procedure was high with an average of 3.7 (range 1–28)
complications per patient.
Some of the most significant complications are associated with
nipple valve slippage which occurs because of the tendency of the
intussuscepted segment to slide and evert on the mesenteric aspect.
Manifestations of nipple valve slippage include difficult catheteriza-
tion, incontinence, and obstructive symptoms from obstruction of
the outflow tract. The incidence of nipple valve slippage is approxi-
mately 30%. A variety of technical modifications have been devised
to reduce the incidence of this complication. Use of prosthetic mate-
rials to wrap the valve reduces the incidence of nipple valve slippage
but is associated with abscess and fistula formation.(35) The T-pouch
modification (34) has been advocated to avoid this complication, but
there is currently no controlled data available.
Pouchitis is a well recognized complication of the Kock pouch
occurring in up to 25% of patients. It is manifested by increased
bowel frequency, often associated with blood and mucus and at
times, incontinence. The etiology of pouchitis is unknown, but
the majority of patients are treated effectively with antibiotics
and continuous pouch drainage.
Other complications associated with the procedure include
the development of fistula, parastomal hernia, and small bowel
obstruction.
Long-term results of patients with continent ilesotomies reveal
a cumulative success rate of 71% at 29 years in 96 patients followed
from 1972 to 2000.(36) The success rate with continent ileostomy
is appreciably less than with the ileoanal pouch procedure.
TOTAL ABDOMINAL COLECTOMY WITH
ILEORECTAL ANASTOMOSIS
Although the majority of patients with ulcerative colitis have rectal
involvement, a small number of patients with rectal sparing may
be treated with total abdominal colectomy and ileorectal anasto-
mosis. Such patients may subsequently require rectal excision for
diarrhea and poor functional results, ongoing proctitis, and malig-
nant transformation. Surveillance for the development of dysplasia
is recommended. Recent series have shown an average number of
bowel movements of 3–6/day after the procedure with a failure rate
of 11–57% (37, 38, 39). The incidence of developing cancer with
long-term follow-up ranges from 0–6% (40, 41, 42).
RESTORATIVE PROCTOCOLECTOMY
WITH ILEOANAL POUCH
Since its introduction in 1978, the ileoanal pouch procedure has
become the procedure of choice for patients who require surgery
for ulcerative colitis and familial adenomatous polyposis. Over
the years, the operation has undergone a series of technical modi-
fications and it can be performed with essentially no mortality
and good long-term outcomes. The procedure avoids the need
for a permanent stoma and removes the diseased bowel.
Indications
The most common indication for the ileoanal pouch procedure is
failure of medical therapy for ulcerative colitis or development of
complications from medical therapy which outweigh the benefit.
Additional indications include the development of dysplasia and
certain extraintestinal manifestations. Colon cancer is not a con-
traindication to the procedure, but performance of an ileoanal
pouch must not compromise the oncologic resection. IPAA is
usually not advisable in a low- or mid-rectal cancer because of the
need for chemoradiation therapy and the potential effects on the
pouch and the anal sphincter. Although the majority of patients
who undergo pouch surgery are young, age is not a contraindica-
tion to the performance of the procedure. We advise patients on a
case by case basis over the age of 65. Nocturnal leakage and incon-
tinence is more common in older patients who undergo pouch
surgery and preoperative assessment should include assessment
of anal sphincter function and extensive discussion about the
potential functional outcome.
Operative Technique
Preoperatively, the risks and benefits of the procedure are dis-
cussed with the patient, and consultation with an enterostomal
therapist is beneficial. An appropriate site for the intended stoma
is marked in the right lower quadrant. The procedure is perform-
ance after mechanical and antibiotic bowel preparation. Although
the procedure may be performed with an open or laparoscopic
approach, pouch surgery is increasingly being performed by a
laparoscopic approach. Retrospective case-matched comparative
studies have shown a longer operative time (median 330 min vs.
230 min), but a quicker return of bowel function (2 days vs. 4
days) and a shorter hospital stay (7 days vs. 8 days) with laparo-
scopic pouch procedures (43). A recent meta- analysis of 10 stud-
ies with 329 patients confirmed that despite a longer operative
time, patients had a lower blood loss, shorter hospital stay, and
smoother recovery compared to open surgery.(44) In a review
of 100 laparoscopic and 189 open ileoanal pouch procedures
for ulcerative colitis, patients reported excellent body image and
quality of life scores regardless of open or laparoscopic approach.
(45) In the past 5 years, the majority of the ileoanal pouch proce-
dures have been performed at our institution with a laparoscopic
hand-assisted approach.
The technical details of the procedure are outlined in videos
(CineMed-American College of Surgeons).
One of the critical maneuvers during the performance of ile-
oanal pouch surgery is the creation of a tension-free anastomosis
between the pouch and the anus. Undue tension on the anasto-
mosis leads to stricture formation, anastomotic leakage, potential
pelvic sepsis, and poor function. To perform a tension-free anas-
tomosis, the apex of the pouch should reach the inferior border
of the symphysis pubis. Assessment of potential pouch reach to
the anus is performed before pouch creation. In obese patients,
it may be necessary to perform an initial total abdominal colec-
tomy, ileostomy and Hartman closure of the rectum in anticipa-
tion of significant weight reduction and then pouch creation.(46)
An S-pouch may afford an additional 2 cm of length compared
to a J-pouch but it is more difficult to construct and has potential
efferent limb problems.(47) A tension-free anastomosis is more
difficult to achieve in male patients with a narrow pelvis, patients
with a long anal canal, obese patients, and patients who undergo
mucosectomy with handsewn anastomosis. To achieve adequate
length on the mesentery, a series of technical maneuvers is
performed, including mobilization of the posterior attachment
surgery for ulcerative colitis
of the small bowel mesentery, exposing the inferior portion of
the head of the pancreas, and scoring the peritoneum of the small
bowel mesentery serially on the anterior and posterior surfaces.
(48) Each of these relaxing incisions confers an additional 1 cm
of distal reach. At least two relaxing incisions are made along the
course of the superior mesenteric artery. If additional length is
required, the mesentery of the small bowel is transilluminated to
delineate the loop formed by the ileocolic artery and the terminal
ileal branch of the superior mesenteric artery. Traction is placed
on the small bowel by grasping the intended apex of the pouch,
and vessels between the primary and secondary arcades that are
under tension are identified and ligated. This maneuver adds 2–5
cm of additional length. The terminal branches of the superior
mesenteric artery of the ileocolic artery can be divided for addi-
tional length. These vessels are clamped for 10–15 minutes before
ligation to confirm adequate vascularity of the ileum before divi-
sion. In selected cases, interposition vein grafts have been used
to obtain adequate mesenteric length.(49) If there is inadequate
length despite these maneuvers, the pouch may be left in the pel-
vis, and not anastomosed to the anal canal with plans to return at
a subsequent date for anastomosis. The weight of the pouch and
the dependent portion of it with the aid of gravity may facilitate
reach to the anus at a later date.
Outcome
The mortality after ileoanal pouch surgery is <1%. The major-
ity of the patients undergoing the procedure are young and oth-
erwise in good health, with the exception of ulcerative colitis or
familial adenomatous polyposis. Despite refinements in surgi-
cal technique, the operation is associated with an appreciable
number of complications.
A recent meta-analysis with a review of 5,215 patients who
underwent ileoanal pouch surgery between 1988 through 2000
revealed a preoperative diagnosis of ulcerative colitis in 87.5%,
indeterminate colitis in 2%, Crohn’s disease in 0.8%, familial ade-
nomatous polyposis in 8.9%, and other diagnoses in 0.7%.(50)
A diverting ileostomy was performed in 81.6%.
FUNCTIONAL RESULTS (BOWEL, URINARY,
GYNECOLOGIC AND SEXUAL FUNCTION)
At a median follow-up of 37.2 mos after ileoanal pouch surgery
and ileostomy reversal, the mean defecation frequency was 5.2
during the day with a mean night-time frequency of 1.0.(50) Mild
fecal incontinence during the day occurred in 17%, while 3.7%
had severe fecal incontinence during the day and 7.3% had urge
incontinence. Bowel function deteriorates with advancing age.
(51) Prospective evaluation of long-term function reveals that
especially 12 years or more after surgery, major and minor incon-
tinence are worse. Twelve years following surgery, 27% of patients
vs. 9% (<12 years) had major daytime incontinence and 33% vs.
10% reported more major night time incontinence. Furthermore,
minor incontinence was seen in 48% of patients after 12 years vs.
16% of patients followed for under 12 years.(51)
The reported incidence of sexual dysfunction in a meta-analysis
of 21 studies including 5,112 patients was 3.6%.(50) The authors
point out the risk of underestimating complications due to a posi-
tive publication bias, and thus studies with negative results may be
less likely to be submitted and published. Indeed, a more recent
review has further quantified the impact of the ileoanal pouch
procedure on sexual and gynecologic function in women. A sys-
tematic review of 22 in 1,852 women who underwent restorative
proctocolectomy from 1980 to 2005 revealed a much more sig-
nificant impact on function.(52) The incidence of infertility was
12% before restorative proctocolectomy and 26% after (n = 945
women, 7 studies). Sexual dysfunction occurred in 8% preopera-
tively and 25% postoperatively (n = 419 women, 7 studies). More
Cesarean sections were performed after restorative proctocolec-
tomy, although no significant differences in pouch function and
no significant perineal trauma was seen after vaginal delivery, thus
suggesting that the mode of delivery should be based on obstet-
ric considerations. An increase in bowel actions was noted during
the third trimester but bowel activity returned to normal within 6
months of delivery. Peritoneal inclusion cysts which are associated
with pelvic sepsis and adhesions are an additional underreported
consequence of the ileoanal pouch procedure.(53, 54)
COMPLICATIONS
Despite refinements in surgical technique, restorative proctocolec-
tomy is associated with an appreciable number of complications
including pelvic sepsis, fistulas, strictures, fecal incontinence, pouch
failure, and sexual dysfunction. A recent meta-analysis on pooled
data of observational studies has been performed on 43 studies com-
prising 9,317 patients detailing the results and complications.(50)
Small Bowel Obstruction
Small bowel obstruction is a common complication after restora-
tive proctocolectomy ranging from 15–44% of patients, with
approximately half of patients requiring operation for treatment of
obstruction.(55) Small bowel obstruction occurs more commonly
after restorative proctocolectomy than after Brook ileostomy, pre-
sumably because of the cumulative increase in obstruction after
multiple procedures. Patients who develop early postoperative
small bowel obstruction are more likely to resolve with conserva-
tive measures than those patients diagnosed in later follow-up.(56)
In a series of 1,178 patients who underwent IPAA, the cumulative
risk of small bowel obstruction was 9% at 30 days, 18% at 1 year,
27% at 5 years and 31% at 10 years.(57) The most common site of
adhesions were pelvic adhesions (32%) and adhesions at the ileos-
tomy closure site (21%). Recent strategies to decrease the risk of
adhesions have focused on the use of a bioresorbable membrane
which has reduced the incidence, extent, and severity of adhesions
(58), as well as the use of laparoscopic surgery (which results in less
adhesions).
Postoperative Hemorrhage
Intraabdominal hemorrhage may occur from failure to secure the
vascular pedicles and from pelvic bleeding, in addition to bleed-
ing of the pouch suture or staple line. Pouch ischemia may also be
associated with bleeding. Pouch bleeding noted intraoperatively
is best treated by eversion of the pouch to expose the mucosa and
cauterization or suture ligation as needed. Postoperative bleeding
may require examination under anesthesia and/or pouch endos-
copy with suture or endoscopic clipping of the bleeding point.
Bleeding, especially 5–7 days after operation, may be associated
improved outcomes in colon and rectal surgery
with anastomotic dehiscence. In a series of 1,005 patients, pouch
bleeding occurred in 38 patients (3.8%) and was treated with local
irrigation with saline and adrenaline in 30 patients and transanal
suture ligation in 8.(59)
Pelvic sepsis
Pelvic sepsis is defined as pelvic abscess, anastomotic leakage or
dehiscence, or any pelvic or perineal infection. Some series distin-
guish between pelvic sepsis and anastomotic leak; pelvic sepsis gen-
erally results from a defect in the ileoanal anastomosis, anastomotic
leak, or defect of the other staple or suture lines. A meta-analysis
noted the incidence of pelvic sepsis to be 9.8%.(50) Manifestations
of pelvic sepsis include fever, leukocytosis, perineal pain, purulent
drain output, and prolonged ileus. As pelvic sepsis is a significant
cause of pouch failure and since those patients with sepsis are
more likely to have compromise of pouch function, any patient
suspected of having pelvic sepsis, should be evaluated and treated
expeditiously. CT scan confirms the diagnosis of pelvic sepsis, and
contrast in the pouch (either by instilling rectal contrast or contrast
through the efferent limb of the ileostomy) is useful in assessing
the integrity of the anastomosis. Alternatively, a pouchogram and
examination under anesthesia may be necessary. Intraabdominal
or pelvic abscess requires percutaneous or operative drainage in
addition to broad spectrum antibiotics (Figure 31.2). For patients
with leakage from the anastomotic suture or staple line the abscess
can be drained into the pouch. This potentially avoids the develop-
ment of a complex fistula. Untreated pelvic sepsis results in fibrosis,
a stiff, non-compliant reservoir, and a higher incidence of ultimate
pouch failure.(60)
Anastomotic leak or dehiscence
Anastomotic leak after the ileoanal pouch procedure occurs
between 5–18% of patients. In a recent meta-analysis, the
incidence of anastomotic leakage from either the pouch-anal
anastomosis or the pouch itself was 7.1%.(61) The incidence
of anastomotic leakage was more common in patients who did
not have a stoma at the time of pouch surgery. The presence of
a stoma may help to ameliorate the clinical manifestations of a
leak. A leak may occur at the pouch anal anastomosis or along any
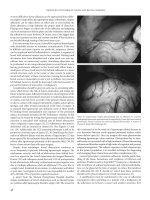
(A) (B)
Figure 31.2 Retrograde pouch study shows a presacral collection (A) confirmed on CT scan (B). Collections arising from the anastomosis are preferably drained into
the pouch to avoid a complex fistula.
Figure 31.3 Asymptomatic anastomotic sinus in patient before ileostomy closure
often requires no further treatment. Delay in ileostomy closure and repeat pouch
study generally shows healing.
surgery for ulcerative colitis
of the staple or suture lines including the top of the J-pouch, the
ileoanal anastomosis, or the pouch itself. Manifestations of a leak
include the development of an abscess, fistula, or symptoms of
pelvic pain, diarrhea, and fever. Risk factors associated with leak
include tension on the anastomosis and ischemia resulting from
tension on the anastomosis. One study suggested a lower inci-
dence of pelvic sepsis associated with a double-stapled anastomo-
sis compared with a mucosectomy and hand sewn anastomosis.
(62) Management of anastomotic leak is individualized; patients
who have an asymptomatic sinus before ileostomy closure with-
out associated sepsis can be treated by delay in ileostomy closure
and in most cases, ultimate healing of the tract.(Figure 31.3)
Patients with peritonitis who have undergone restorative proc-
tocolectomy without diverting ileostomy require diversion and
drainage. Leaks from the tip of the J pouch are challenging both
to diagnose and treat and developed in 14 out of 1,309 patients;
all required surgical repair and none healed with conservative
treatment (63) (Figure 31.4). With expertise and individual-
ized management, pouch salvage can be was achieved in 88% of
patients who developed anastomotic leak.(64)
Stricture at the ileal pouch anal anastomosis
Strictures at the ileal pouch anal anastomosis occur in approxi-
mately 10% of patients, and are more common after mucosec-
tomy and handsewn anastomosis than after double-stapled
anastomosis.(65, 66) Tension on the anastomosis and ischemia
are associated with stricture formation. A lumen which admits
the DIP joint of the index finger is generally satisfactory for good
bowel function. Soft strictures are treated with gentle finger dila-
tion or with balloon dilators. Long fibrotic strictures are more
challenging to treat and pouch advancement and neoileoanal
anastomosis may be necessary to treat such patients.(67)
Pouch vaginal fistula
The incidence of pouch-vaginal fistulas ranges from 3–16%.(68)
Pouch-vaginal fistulas are a major potential cause of pouch fail-
ure. Fistulas which occur in the early postoperative period are most
commonly a manifestation of sepsis, and can occur from anasto-
motic leak and necessitation through the vaginal wall, or may result
from technical factors including entrapment of the perivaginal tis-
sue in the staple line (Figure 31.5). An important part of the ileoanal
pouch procedure is to ensure that the vagina is not incorporated
within the stapler. Late pouch-vaginal fistulas are more commonly
associated with unsuspected Crohn’s disease.(69)
Pouch-vaginal fistulas may manifest as pelvic pain, fever, a
“Bartholin’s abscess” which when drained has fecalent mate-
rial, or passage of gas. Fistulas which occur before ileostomy
takedown are treated by management of infection, delayed ile-
ostomy closure, and local repair. A number of procedures have
been described for treatment of pouch vaginal fistulas. Ultimate
success may be achieved in over 50% (70) but often requires mul-
tiple procedures. For patients with Crohn’s disease, the use of
infliximab and other biologics may be helpful.
Pouch anal fistulas
Early fistulas are generally a manifestation of sepsis and leakage
at the ileoanal anastomosis. Late fistulas may be crytoglandular
in origin and may also be a manifestation of Crohn’s disease. Our
preference is for liberal use of draining setons and avoidance of
fistulotomy.
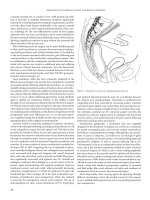
Figure 31.4 A leak from the efferent limb of the pouch may be difficult to diagnose.
Such patients rarely heal with antibiotics and drainage alone and often require
exploration and repair.
Figure 31.5 A pouch vaginal fistula is seen on retrograde study. Early fistulas
are due to infection and leak at the anastomosis while late fistulas often herald
unsuspected Crohn’s disease.
improved outcomes in colon and rectal surgery
Pouchitis
The most frequent long-term complication of the ileoanal pouch
procedure is the development of pouchitis, a nonspecific inflam-
mation of the ileal pouch mucosa. The precise etiology of pouchi-
tis has not been elucidated but it is believed to potentially result
from an overgrowth of anaerobic bacteria. It is disease specific and
more commonly seen in patients with ulcerative colitis; it is rarely
encountered in patients with familial adenomatous polyposis.
Patients with ulcerative colitis associated with extraintestinal man-
ifestations and patients with sclerosing cholangitis have a higher
incidence of pouchitis.(71, 72) Presenting signs and symptoms of
pouchitis include abdominal cramps, abdominal tenderness, fever,
and increase in stool frequency, often associated with blood or
mucus. The diagnosis may be made clinically, on the basis of endo-
scopic examination in addition to clinical findings, or on the basis
of histologic examination of the pouch mucosa; the lack of uni-
form criteria to make such a diagnosis accounts for the variation in
the incidence of pouchitis in many series. A pouchitis disease activ-
ity index has been devised which includes clinical, endoscopic, and
histologic features.(73) Pouchitis is generally treated with antibiotic
therapy and the most commonly used agents include metronida-
zole or ciprofloxacin. Some patients with pouchitis develop ongo-
ing symptoms, and for patients with refractory pouchitis or rapidly
relapsing symptoms, the use of probiotics appears to be helpful.
Probiotics may suppress the resident pathogenic bacteria, stimu-
late mucin glyocoprotein, prevent adhesion of pathogenic strains
to epithelial cells, and reduce host immune responses. Probiotics
may also be helpful in preventing recurrent pouchitis. A diagnosis
of Crohn’s disease should be considered in patients with chronic
pouchitis. In some cases, pouchitis is a cause of pouch failure.
Pouchitis has been termed by some as “the Achilles heel” of the
ileoanal pouch procedure. It is a cause of significant long-term
morbidity; elucidation of the cause of pouchitis would likely ben-
efit a large number of patients.
Dysplasia and Malignancy
Following construction, the ileoanal pouch undergoes a number
of histologic changes, and with time, the metaplastic changes result
in the ileal mucosa resembling colonic mucosa. These changes may
also occur because of inflammation in the pouch and raise concerns
of malignant transformation and the development of dysplasia.
Neoplastic changes appear to be extremely rare. The majority of ile-
oanal pouch patients who develop cancer had a prior cancer at the
time of pouch construction. The recent ASCRS guidelines do not
endorse routine surveillance of ileal pouches for dysplasia.(10)
Pouch Failure
Pouch failure defined as pouch excision or a nonfunctioning pouch
at 12 months after the ileoanal pouch procedure occurs in 5 to 15%.
While the majority of pouch failures occur within 2 years of pouch
construction, late pouch failures also occur. The common cause of
pouch failure include unsuspected Crohn’s disease, chronic pouchitis,
poor function with incontinence, persistent fistula, and other pouch
related complications such as stenosis with outlet obstruction.
Reoperative pouch surgery with an attempt to salvage the
pouch is challenging; pouch salvage is higher in patients with
ulcerative colitis than Crohn’s disease.(74)
CONTROVERSIES
Reservoir Design
While the original report by Parks used an S-pouch configu-
ration, a number of other pouch configurations have been
described, including J-pouch, lateral isoperistaltic H-pouch,
and quadruple-loop W pouch. S pouches were initially asso-
ciated with an increased need for catheterization because of a
long distal ileal conduit. Shortening of the ileal conduit helps
to initially avoid this complication, however, with time, the
exit conduit of the S-pouch seems to elongate and obstructive
defecation can occur. An S pouch may confer additional length
compared to a J-pouch and may be the preferred configuration
if achieving adequate length to performed a tension-free anas-
tomosis in selected cases. The long outlet tract associated with
an H pouch has been associated with stasis, pouch distention,
and pouchitis.
There have been no significant differences in pouch func-
tion based on the configuration of the pouch. Due to the ease of
construction and the lack of compelling data favoring a specific
pouch design, J pouches are most frequently performed. Use of
an S or W pouch adds about 45 minutes to the time of the opera-
tive procedure.
A recent meta-analysis examined the short and long-term out-
come of J-, S- and W- reservoirs in patients undergoing restora-
tive proctocolectomy.(75) A total of 18 studies of 1,519 patients
(689 J, 306 W, and 524 S pouches) were reviewed. There was no
difference in the incidence of early complications among the
3 types of pouch design. The frequency of defecation favored an
S- or W- pouch design over a J pouch, although in practical terms
the difference of 1–1.5 stools in a 24 hour period is unlikely to
be of clinical significance to the patient. Night evacuation was
significantly lower for a W than a J pouch. S pouches were associ-
ated with a greater need for pouch intubation due to a long distal
conduit; W pouches also required intubation more often than
J pouches.
Mucosectomy vs. Double-Stapled Technique
The ileoanal anastomosis may be performed with a handsewn
technique after mucosal stripping (mucosectomy) or with a dou-
ble-stapled technique.
The initial technique reported by Parks was mucosal strip-
ping commencing at the dentate line and removing all diseased
mucosa, thus eliminating the risk of recurrent proctitis or neo-
plastic transformation. A potential advantage of the double-
stapled technique is greater technical ease, and potentially less
tension on the anastomosis. Preservation of the anal transitional
zone may minimize sphincter damage and improve functional
results. Three prospective randomized trials have not shown
an advantage for the double-stapled technique vs. the muco-
sectomy technique.(76, 77, 78) These trials have all been small
and are underpowered to demonstrate a difference. A meta-
analysis of 4,183 patients (2,699 hand-sewn vs. 1,488 stapled
IPAA) found similar early postoperative outcomes; however,
stapled IPAA patients had improved nocturnal continence and
had higher resting and squeeze pressures on anorectal physi-
ologic testing.(79)
surgery for ulcerative colitis
Preservation of the anal transitional zone and performance
of a double-stapled technique leaves a residual 1–2 cm of dis-
eased rectal mucosa, which may be at risk for the development of
dysplasia and subsequent malignant transformation. It has been
suggested that patients who have had a double-stapled tech-
nique be followed in a surveillance program, with biopsies of the
retained columnar mucosa at least every 2 years beginning 8 to 10
years after the onset of symptoms of disease.(80) The recommen-
dations for biopsy are controversial and is an area where further
study is needed to define the natural history of the retained 1–2
cm of columnar mucosa. Other authors have not found the devel-
opment of dysplasia with long-term follow-up.(81)
Omission of ileostomy
Restorative proctocolectomy is most commonly performed in
two stages with an initial proctocolectomy, pouch construction,
and diverting ileostomy, followed by ileostomy takedown after
demonstration of satisfactory pouch healing. However, construc-
tion of a loop ileostomy may be associated with excessive stoma
output, dehydration, hernia, bowel obstruction, and subsequent
anastomotic complications associated with ileostomy takedown;
these have been cited as a reason to potentially avoid diverting
ileostomy in selected patients after ileoanal pouch construction.
Conversely, many feel that loop ileostomy construction will mini-
mize the potential consequences of pelvic sepsis (and potentially
reduce the chance of pouch failure).
This issue has been characterized by a great deal of passion and
no randomized controlled studies. A one stage procedure without
loop ileostomy is associated with a more difficult initial recovery
and most likely a slight increased rate of anastomotic disruption
and pelvic sepsis. An alternate view of this is that with fecal diver-
sion, some patients with minor leaks and sepsis may not be clini-
cally detected.
Loop ileostomy avoids some of the consequences of pelvic sep-
sis, which is a major cause of pouch failure. Despite aggressive
treatment the risk of pouch failure after pelvic sepsis is 20%, 31%
and 35% at 3, 5 and 10 years respectively.(61) A single stage IPAA
without loop ileostomy decreases the risk of ileostomy related
complications, and complications including small bowel obstruc-
tion associated with an additional operative procedure.
A recent review compared 17 studies with 1,486 patients (765
without ileostomy and 721 with ileostomy).(61) While there was
no significant difference in the functional outcome of the two
groups, those patients without an ileostomy had a higher inci-
dence of pouch related leak and stricture formation.
Selective omission of an ileostomy may be considered when an
anastomosis is intact and under no tension, the procedure is not
complicated by excessive bleeding or other technical difficulties
and the patient is not on high dose steroids before the procedure.
(10) The patient should be adequately counseled preoperatively
concerning the pros and cons of ileostomy omission.
Crohn’s Disease and Indeterminate Colitis
Crohn’s disease has been considered to be a contraindication to
the performance of an ileoanal pouch procedure because of the
risks of recurrent disease and the potential need for pouch exci-
sion with subsequent loss of substantial amounts of bowel.
However, there are some patients who undergo the procedure
for ulcerative colitis and an ultimate diagnosis of Crohn’s disease
is made. In general these patients are found to have a higher risk of
pouch failure from 28–52% (82, 83, 84, 85) compared to patients
with ulcerative colitis or familial adenomatous polyposis. In a cohort
of 32 patients out of 790 patients with an ultimate diagnosis of
Crohn’s disease, 93% had complications including perineal abscess/
fistula (63%), pouchitis (50%), and anal stricture (38%) (85). It is
not known whether administration of agents such as infliximab to
such patients will ultimately impact the incidence of pouch failure,
or whether it will delay the diagnosis or pouch failure. All efforts
should be made to confirm a diagnosis of ulcerative colitis and
exclude a diagnosis of Crohn’s disease preoperatively. In addition to
a thorough history and examination, a recent study suggested that
a family history of Crohn’s disease and serology positive for anti-
Saccharomyces cerevisiae immunoglobulin-A were more likely to be
diagnosed with Crohn’s s after IPAA (67%) than patients with either
risk factor (18%) or neither risk factor (4%) (86).
While techniques of restorative surgery for ulcerative colitis
have shown substantial advances over the past several decades,
further study focusing on improvements in complications and
functional outcomes will ultimately further improve a patient’s
quality of life.
REFERENCES
1. Loftus CG, Loftus EV, Sandborn WJ et al. Update on inci-
dence and prevalence of Crohn’s disease (CD) and ulcer-
ative colitis (UC) in Olmsted County, Minnesota [abstract].
Gastroenterology 2003; 124: A36.
2. Loftus EV Jr. Clinical epidemiology of inflammatory bowel
disease: incidence, prevalence, and environmental influences.
Gastroenterology 2004; 126: 1504–7.
3. Leijonmarck CE. Surgical treatment of ulcerative colitis
in Stockholm county. Acta Chir Scand Suppl 1990; 554:
1–56.
4. Wexner SD, Rosen L, Lowry A et al. Practice parameters for
the treatment of mucosal ulcerative colitis. Dis Colon Rectum
1997; 40: 1277–85.
5. Truelove SC, Witts L. Cortisone in ulcerative colitis: final
report on a therapeutic trial. BMJ 1955; 2: 1041–8.
6. Mahadevan U. Medical treatment of ulcerative colitis. Clin
Colon Rectal Surg 2004; 17: 7–19.
7. Cottone M, Pietrosi G, Martorana G et al. Prevalence of
cytomegalovirus infection in severe refractory ulcerative and
Crohn’s colitis. Am J Gastroenterol 2001; 96: 773–5.
8. Mahadevan U, Loftus EV Jr, Tremaine WJ et al. Azathioprine
or 6-mercaptopurine before colectomy for ulcerative colitis
is not associated with increased postoperative complications.
Inflamm Bowel Dis 2002; 8: 311–6.
9. Hanauer SB. Drug therapy: inflammatory bowel disease.
N Engl J Med 1996; 334: 841–8.
10. Cohen JL, Strong SA, Hyman NH et al. Practice parameters
for the surgical treatment of ulcerative colitis. Dis Colon
Rectum 2005; 48: 1979–2009.
11. Robert JH, Sachar DB, Aufses A et al. Management of severe
hemorrhage in ulcerative colitis. Am J Surg 1990; 159:
550–5.