Improved Outcomes in Colon and Rectal Surgery part 37 doc
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (477.42 KB, 10 trang )
improved outcomes in colon and rectal surgery
other anastomotic configurations after resection in Crohn’s
disease. Dis Col Rectum 2007; 50(10): 1674–87.
132. Landsend E, Johnson E, Johannessen H, Carlsen E. Long-
term outcome after intestinal resection for Crohn’s disease.
Scand J Gastroenterol 2006; 41(10): 1204–8.
133. Steele SR. Operative management of Crohn’s disease of
the colon including anorectal disease. Surg Clin North Am
2007; 87(3): 611–3.
134. Penner RM, Madsen KL, Fedorak RN. Postoperative Crohn’s
disease. Inflamm Bowel Dis 2005; 11(8): 765–77.
135. Yamamoto T. Factors affecting recurrence after surgery
for Crohn’s Disease. World J Gastroenterol 2006; 11(26):
3971–9.
136. Thaler K, Dinnewitzer A, Oberwalder M et al. Assessment
of long-term quality of life after laparoscopic and
open surgery for Crohn’s disease. Colorectal Dis 2005; 7:
375–81.
137. Casellas F, Vivancos JL, Badia X, Vilaseca J, Malagelada JR.
Impact of surgery for Crohn’s disease on health-related
quality of life. Am J Gastroenterol 2000; 95(1): 177–82.
Ostomies
Vance Y Sohn and Scott R Steele
CHALLENGING CASE
A 55-year-old morbidly obese male undergoes a low ante-
rior resection with concomitant defunctioning loop ileostomy
for a T2 rectal cancer. Six weeks postoperatively, he presents to
the clinic with an obvious parastomal hernia that is easily reduc-
ible. He complains of worsening pain, difficulty with application of
his ostomy appliances, and symptoms of intermittent obstruction.
CASE MANAGEMENT
In this patient, the optimal management includes reversal of the
ostomy after ensuring that the distal anastomosis has healed.
This is usually confirmed by a contrast study, often a gastrograf-
fin enema or CT scan with rectal contrast. An ostomy reversal
ameliorates and addresses all of the symptoms including the her-
nia, obstruction, and pain. After reversal, the skin of the ostomy
can be primarily closed, however extreme vigilance of the wound
is necessary secondary to an increased rate of local wound infec-
tion. Depending on the size of the fascial defect and correspond-
ing hernia, additional mesh may be needed for hernia repair.
Due to increased risk of infection of most prosthetics, biologic
materials should be considered as a first option. For patients who
are not candidates for ostomy reversal, various options are avail-
able and include both open and laparoscopic approaches. These
options include primary fascial repair, repair with biologic or
prosthetic mesh, and stoma relocation. The approach and the
method of repair is dependant on the surgeon’s preference and
experience. Certainly, observation for minimally symptomatic
parastomal hernias is the preferred option until stomal take-
down is possible.
INTRODUCTION
Intestinal stomas, either temporary or permanent, are the surgi-
cal exteriorization of either small or large bowel to the anterior
abdominal wall. An ostomy may be placed temporarily, often
when its primary purpose is to divert the fecal stream away from
an area of concern such as a high-risk anastomosis in a field of
prior radiation treatment, following a coloanal repair, or concern
for leak after a stapled end-to-end anastomosis. Once the distal
anastomosis has adequately healed, gastrointestinal continuity
can be reestablished when the ostomy is reversed. A permanent
stoma is created following an oncologic resection for rectal cancer
that includes removal of the anorectum and associated sphinc-
ter complex. In this instance, a descending colostomy would be
required to avoid perineal soiling with a coloanal anastomosis
in the absence of the sphincters. While there are various types
of ostomies described for a broad spectrum of disease processes
such as the neo-bladder construction with an ileal conduit, this
chapter will focus solely on outcomes for ostomies created with
the small or large bowel for colon and rectal diseases.
PSYCHOLOGICAL IMPACT OF LIVING WITH A STOMA
Regardless of the type of ostomy, living with a stoma exacts
a tremendous psychological burden on patients and requires
adjustments to activities of daily living. In addition to the physical
adjustment of caring for an ostomy, the possibility of participating
in simple activities, such as dining out, often becomes disrupted
in the patient’s mind. Unfortunately, this is one of the major fears
of patients whether or not it is founded in reality. Yet, it is also
one that is often not discussed in detail before the operation, nor
able to be appropriately counseled and educated when stomas are
required in the emergent setting. In 1952, Sutherland et al., (1)
published the first report on the important psychological needs
of patients living with stomas. Since then, multiple studies have
reported the negative impact ostomies have on overall quality of
life.(2–6) It is not surprising that the presence of a stoma is asso-
ciated with decreased quality of life measurements in the imme-
diate and early postoperative setting.(7) Unfortunately, it often
appears that while overall quality of life, return to prior activity
levels, pain and fatigue all improve with time following surgery,
self-impression views such as body image and sexual function do
not seem to change with time.(8) Thus, despite evidence to the
contrary that a “return to normalcy” is achievable, many patients
can never get past the idea of having to live with a stoma. More
recently, Krouse and colleagues (9) evaluated the quality of life of
239 male patients from multiple Veterans Affairs (VA) hospitals
living with stomas. Their report, which was a case-control sur-
vey study, used various previously-validated quality of life indices
to compare patients with ostomies versus 272 patients who had
undergone similar operations, but not requiring stoma forma-
tion. Their study highlighted multiple important psychosocial
facts about patients living with ostomies. There was increased
self-reported postoperative depression and suicidal ideations
among respondents living with ostomies. Such feelings may have
been compounded by issues of coping and social acceptance, as
their fears related mostly to both others’ perceptions of patients
with stomas and their own personal fears of having stoma-related
accidents. As these fears became more frequent, they clinically
translated into decreased social interactions and eventual isola-
tion. The authors’ recommendation of encouraging social net-
working among ostomates to clarify issues and limit the trial and
error approach that many patients with ostomies undergo, is a
valid conclusion which should be supported by all physicians.
This is not to say that all patients do poorly or are mentally
burdened by living with a permanent stoma. In a large meta-
analysis of 1,443 rectal cancer patients from 11 studies, there was
no difference in general quality of life scores at 2 years following
surgery between those patients undergoing an abdominoperineal
resection from those undergoing a low anterior resection with in-
continuity reconstruction.(10) These contradictory findings may
improved outcomes in colon and rectal surgery
in part depend on the questionnaire given, the disease process
for which the stoma was created, and the preoperative functional
level of the patient. For example, factors such as patient age, (11)
decreased preoperative continence, (12) and severe active peria-
nal Crohn’s fistulizing disease (13) have all been shown to have
an improved quality of life following stoma formation. Thus,
while it would be inaccurate to state that placement of stoma
will end up with a lowered quality of life and significant psycho-
logical problems, it also is naïve to think that stoma creation will
not have a significant impact on a patient’s subsequent immedi-
ate and long-term recovery. It is well-established, that in addi-
tion to networking, a close relationship with a readily available
and experienced enterostomal therapist is an invaluable aspect
of the multidisciplinary approach. These expert therapists can
significantly alleviate initial fears and anxieties that often plague
patients living with a stoma. Furthermore, in our experience,
preoperative counseling about expectations, education regard-
ing the indication for the ostomy, and even “practicing” the
wearing of an appliance before surgery all aid in lessening the
psychological impact on the patient and promotes adaptation to
their ostomy.
STOMA SITE MARKING
In 2007, the American Society of Colon and Rectal Surgeons
(ASCRS), in collaboration with the Wound, Ostomy, and
Continence Nurses (WOCN) Society developed a position state-
ment on the value of preoperative stoma marking for patients
undergoing ostomy surgery.(14) Their ultimate goal was to
decrease stomal complications and improve quality of life for
patients. In addition to precise step-by-step instructions on
the proper siting of stomas, the statement recommended that all
patients scheduled for ostomy surgery undergo preoperative stoma
marking by an experienced, trained clinician. This evaluation
includes examining the patient in the lying, sitting, and stand-
ing positions, and accounting for patient factors such as previous
incisions, waist and belt lines, abdominal habitus, and hernias, to
determine the optimal stoma position that is crucial to decreasing
the incidence of stomal complications. One of the more impor-
tant aspects of this preoperative marking evaluation is the iden-
tification of the rectus abdominus muscle, as placement of the
stoma through the rectus muscle may prevent peristomal her-
niation or prolapse (Figure 33.1).(15) Furthermore, preoperative
siting allows for patient participation and education regarding
stoma care and the use of ostomy appliances. While this posi-
tion statement has yet to be clinically validated, previous reports
have demonstrated the importance of preoperative stomal siting.
In a retrospective analysis, Bass and associates (16) reviewed a
single institution’s stoma complication rate in 593 patients over
an 18-year period. The study compared 292 patients who under-
went preoperative marking by an enterostomal therapist to the
remaining 301 remaining patients who did not undergo pre-
operative marking. The endpoints of their study, early and late
complications, were favorable for the patients who underwent
preoperative marking with a 23% versus 43% early complication
rate (p < 0.03) and 9% versus 31% late complication rate (p =
NS). This study, and the joint statement by ASCRS and WOCN,
highlights the importance of proper preoperative stoma marking
for decreasing complication rates.
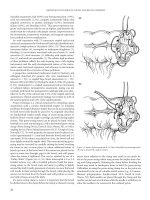
STOMAL TYPES
End Ostomies
End ostomies, either permanent or temporary, are most often
placed in the left lower quadrant of the abdominal wall using
the left colon or in the right lower quadrant when utilizing the
ileum. The indication for stoma creation is important, as this
Figure 33.1 Stomal placement. The site is selected to bring the stoma through the rectus abdominis muscle.
ostomies
often dictates whether gastrointestinal continuity can be reestab-
lished. For instance, in patients undergoing an abdominoperineal
resection (APR) for rectal cancer, a permanent end colostomy is
the only option as the anorectum and surrounding musculature
are removed. Similarly, patients who undergo a proctocolec-
tomy, usually for inflammatory bowel disease (IBD) or Familial
Adenomatous Polyposis (FAP), are candidates for an end ileos-
tomy. For patients wanting more fecal control, a continent ileos-
tomy may be offered.
Ostomies remain permanent when the altered anatomy pro-
hibits reestablishment of gastrointestinal continuity, the risks of
undergoing another surgery are prohibitive due to comorbidi-
ties, or the functional results of a reanastomosis would adversely
impact quality of life. This latter point is common with reanasto-
mosis of the ileum with the mid- or distal rectum or anus (since
the large absorptive capacity of the colon or the storage ability
of the compliant rectum is lost), or when the patient has poor
sphincter function. Barring the aforementioned contraindica-
tions, most ostomies can be reversed and thus, are temporary.
A common temporary end ostomy performed routinely by sur-
geons is the Hartmann’s procedure. Initially described by Henri
Hartmann in 1921 for rectal cancer, this versatile procedure is
indicated for a variety of benign and malignant scenarios where
primary resection of colon and reanastomosis is unsafe or not
possible. As discussed later, reversal is associated with complica-
tions and the benefits of stoma reversal must be balanced with
the potential risks to the surgery. Ideal candidates for reversal are
young, healthy patients with preserved sphincter mechanisms.
The optimal time for this colostomy reversal has been controver-
sial. Some have found that reversals after 4 months were associ-
ated with a higher complication rate; after this time, the rectal
stump was less readily accessible and therefore, led to increased
complications.(17) Others have found no outcome differences
between early or late reversals and considered the timing an insig-
nificant factor.(18)
The benefits of an end ileostomy with immediate maturation,
initially described by Brooke in 1952, have decreased the incidence
of stenosis, dysfunction, retraction, and serositis associated with
an ileostomy.(19, 20) Since that time, this has become the standard
technique for ileostomy and most colostomy formations. Despite
increasing experience with restorative continuity procedures
such as the ileal pouch-anal anastomosis (IPAA), an end ileos-
tomy remains an important part of the surgical armamentarium.
For instance, in patients with toxic megacolon undergoing total
abdominal colectomy, when the principles of “damage control”
surgery are paramount, an end ileostomy following abdominal
colectomy remains the procedure of choice. Additionally, an end
ileostomy would be preferred over an IPAA or an ileal-rectal
anastomosis (IRA) for patients with poor anal sphincter mecha-
nisms where continence is questionable. Alternatively, in young
healthy patients with inflammatory bowel disease or FAP requir-
ing proctocolectomy, IPAA should be considered, or IRA when
the rectum is spared. Purported benefits of an IPAA compared
to the end ileostomy revolve around the maintenance of conti-
nuity and thus, a more psychologically favorable outcome for
the patient. Pemberton et al. (21) evaluated this relationship by
comparing quality of life for 298 patients with IPAAs and 406
patients with end-ileostomies. Greater than 93% of patients in
both groups were satisfied with their surgeries, although 39% in
the end ileostomy group would have preferred an IPAA. After anal-
ysis, the authors concluded that patients who underwent an IPAA
experienced significant advantages in performing daily activities
with resultant improved quality of life. While the benefits of IPAA
are beyond the scope of this chapter, this procedure should be
considered as a viable alternative for patients considered for end
ileostomy. It should be noted, however, that a significant portion
of IPAA patients require a temporary stoma, with an additional
~10% developing pouch failure that requires either pouch exci-
sion with permanent stoma or permanent pouch diversion.(22)
Thus even in this select cohort, education regarding stoma care
and outcome is of utmost importance.
Continent ileostomy
Continent ileostomy, first reported by Nils Kock in 1969, is a less
frequently performed procedure due to the technical expertise
required, the significant complication rate associated with its
nipple-valve mechanism, and the preference for creation of ileoa-
nal pouches.(20) Occasionally, the continent ileostomy remains a
useful option for patients undergoing proctocolectomy for FAP or
IBD, or in those patients who develop IPAA failure. In 2006, Nessar
et al. (23) reported the long-term outcomes of patients undergo-
ing continent ileostomy at the Cleveland Clinic Foundation. Their
study population included 181 patients with continent ileostomies,
69 of whom previously had an end ileostomy, and 35 patients who
had an end ileostomy after excision of a continent ileostomy. With
a median follow-up of 11 years, 17% of patients had their conti-
nent ileostomy excised; there was only a 7 month complication-
free interval, and a 14 month revision-free interval. Long-term
complications were common, with 30% experiencing valve slip-
page, 26% developing pouchitis, 25% with fistula formation, and
15% with parastomal herniation. Other complications included
valve prolapse, difficult intubation, stoma stricture, and pouch
bleeding. Importantly, even in centers with significant experience,
the complication profile remains considerable. Similarly, in a study
by Kohler et al. (24) comparing outcomes in patients between end
ileostomy, continent ileostomy, and ileal pouch-anal anastomosis
(IPAA), those patients with IPAA had fewer restrictions in sport
and sexual activities when compared to patients with continent
ileostomy. Patients with end ileostomy fared the best with regards
to the travel capabilities when compared to the other two. In our
practice, continent ileostomies are seldom performed. Due to the
aforementioned complication profile, patients are counseled for
either an IPAA, IRA, or an end ileostomy. Yet, despite our reluc-
tance to perform this procedure, select institutions well-versed in
this procedure report excellent outcomes and overall high patient
satisfaction.(25–27)
Loop End Stomas
A loop end stoma is a variation in which a section of the bowel
several inches proximal to the divided end of the bowel is
brought through the abdominal opening (Figure 33.2). The
loop can be supported with a rod and the bowel is opened
and matured in a fashion similar to a loop stoma. This type of
stoma is helpful in challenging situations such as thick shortened
improved outcomes in colon and rectal surgery
mesentery, tenuous blood supply, or friable bowel. Its advantage
is that no blood vessels are divided and with a rod, the tension
is on the back wall of the bowel rather than the mucocutane-
ous anastomosis. This type of stoma is slightly more difficult to
pouch as it is slightly oval and may not have the protrusion of a
well-formed end stoma.
Diverting or Loop Ostomies
The ultimate purpose when creating a diverting stoma is to pre-
vent the fecal stream from reaching a distal segment of distal
small bowel or large intestine for the purpose of either treat-
ing or preventing a leak. To that end, either a loop colostomy
or ileostomy will suffice. However, an ileostomy is often pre-
ferred due to its perceived ease of closure. Proponents of a loop
colostomy cite the lower risk of high stomal output leading to
fluid and electrolyte abnormalities occasionally seen with a loop
ileostomy. The common indications for concomitant proximal
fecal diversion include protection of distal at-risk anastomo-
sis, especially low-lying colo-anal anastomosis and ileal pouch
anal-anastomosis (IPAA), complicated diverticulitis, treatment
of anastomotic leaks and pelvic sepsis, large bowel obstruction,
trauma, extensive perianal Crohn’s disease, and less commonly,
fecal incontinence.
The indication for a concomitant proximal fecal diversion for
low lying anastomosis, most commonly performed for rectal cancer,
has been intensely studied. Wong and Eu (28) reported the results
from a prospective, comparative study of 1,078 patients undergo-
ing elective low or ultra-low (defined as colonic anastomosis to the
anal canal) anterior resections from 1994 to 2004. In the diverted
group, 28% developed a clinically significant leak while of the non-
diverted group, 13% had a clinically significant leak (p = 0.86). 95%
of these leaks required a salvage operation, and analysis revealed
no statistical difference between anastomotic leak complications
between patients undergoing and not undergoing fecal diversion.
These authors concluded that a defunctioning ileostomy did not
influence the complication rate of a rectal anastomosis, rather it
minimized the clinical sequela of leaks in high risk patients. They
recommended that proximal diversion should be used on a selected
basis. In another prospective study from Sweden, the Rectal Cancer
Trial On Defunctioning Stoma (RECTODES) randomized 234
patients undergoing low rectal (<15 cm from the anal verge) anas-
tomosis to fecal diversion versus no diversion.(29) Their primary
endpoint was to assess whether there was a difference in the rate
of symptomatic anastomotic leakage in patients between the two
arms of the study. While there was a disproportionate number
of patients (72%) not undergoing randomization due to various
factors including intraoperative concerns requiring diversion, the
total number of patients with and without diversion were simi-
lar (116 pts vs 118 pts). Patient characteristics were similar with
increased operative times for those undergoing stoma placement as
the only statistically significant difference between the two patient
cohorts. In their analysis, patients without a defunctioning stoma
had significantly more symptomatic leakages (28%) when com-
pared to those without proximal diversion (10%). The group not
undergoing diversion consequently constituted 75% of all urgent
reoperations. Of the 28 out of 33 patients without initial fecal
diversion who developed a leak, urgent reoperation was accom-
panied with either a loop ileostomy or permanent end colostomy.
Consequently, these investigators recommended routine defunc-
tioning loop stoma in low anterior resections for rectal cancer.
Based on these and other studies, it is now generally acknowledged
that a proximal defunctioning stoma does not abolish the risk of
leakage, but certainly mitigates the consequences. In our practice,
defunctioning stomas are almost always placed for any anastomo-
sis within 5 cm of the anal verge, although exceptions such as the
one stage IPAA occurs occasionally. Furthermore, patient factors
such as previous irradiation, intraoperative hemodynamic insta-
bility, poor nutrition, and chronic steroid use lead us to liberally
“protect” the distal anastomosis.
When deciding to perform a proximal fecal diversion or a
defunctioning stoma, the two traditional options include a trans-
verse loop colostomy or a loop ileostomy. These two options were
compared in a prospective randomized study by Williams et al.
for elective protection of distal anastomoses.(30) In their analysis,
nearly all complications were twice as common with transverse
colostomies than ileostomies and included infection at the time
of creation and at takedown, odor, leakage, and skin problems.
Additionally, multiple visits to the stoma therapist were needed
Figure 33.2 Z-Plasty repair for stenosis. A, incisions in skin and bowel. B, completed repair.
ostomies
in 58% of colostomy patients versus 18% of ileostomy patients.
In another prospective randomized study by Edwards et al., there
was no difference in operating time required to construct either
stoma, and in fact, reversing the colostomy was easier due to the
larger fascial opening.(31) This larger defect however, resulted
in worse complications manifested as parastomal hernias, pro-
lapse, fecal fistula, and in the follow-up period, incisional hernias.
These increased rates of complications with loop colostomy and
increased rate of hernia formation at the ostomy closure site, and
has led to an almost universal preference of loop ileostomy for
diverting stoma.(31, 32) Should one choose to perform a loop
transverse colostomy, choosing a point in the colon adjacent to
the flexures may decrease the risk of prolapse to a small extent.
COMPLICATIONS WITH OSTOMIES
The incidence of stoma complications varies in surgical literature
from 10–70%, and can range from minor skin irritation to parasto-
mal herniation requiring operation.(33–35) The wide variance in
complication rates is due to the definition of complication and the
length of follow-up in the studies. Furthermore, there are a multi-
tude of factors that influence complication rates, including the type
and location of the ostomy, patient factors such as gender, BMI,
diagnosis, and urgency in which the procedure is performed. In a
study from Cook County Hospital, the incidence of stoma com-
plications was 34% in a review of 1,616 patients, with 28% hav-
ing an early complication (<30 days from time of surgery) and 7%
late complication (>30 days).(36) In a national audit, Cottam and
associates identified 1,329 (34%) patients out of a cohort of 3,970
stomal patients that developed early complications (<3 weeks from
times of surgery) defined as stoma retraction, necrosis, ischemia,
muco-cutaneous separation, and dehiscence.(37) Statistically sig-
nificant factors increasing postoperative complications were stoma
height (<20 mm for ileostomy and <7 mm for colostomy), female
gender, loop ileostomy, advanced BMI, younger age, malignant
diagnosis, and emergent procedures. Similarly, in a prospective
study of 97 patients, Arumugam et al. found elevated BMI, diabetes,
and emergency surgery as significant risk factors for the develop-
ment of stoma complications.(38) In yet another study evaluating
risk factors for stoma complications, Saghir and colleagues identi-
fied advanced age, advanced American Society of Anesthesiologists
(ASA) grade, and noncolorectal specialty-trained surgeons per-
forming the ostomy as risk factors for stoma complications.(39)
As evident in these studies, various patient and surgeon factors can
increase the risk of developing complications. Thus, it is impera-
tive for the surgeon caring for these patients to be well aware of
not only the things they can do to prevent these complications, but
also how do deal with any complications should they arise. In the
following section, the presentation and management of common
complications will be addressed.
Skin Complications
Skin conditions are common among patients living with stomas and
are more prevalent in patients with ileostomies than colostomies.
(5) Common causes include fungal or bacterial infections, irrita-
tion from the ostomy effluent, folliculitis, contact dermatitis from
the appliance, a manifestation of IBD such as pyoderma gangreno-
sum, or simple skin excoriation from frequent appliance changing.
To a certain degree, minor skin irritation is unavoidable. However,
preoperative stoma marking, precise ostomy creation, involvement
of an enterostomal therapist, and diligent postoperative care may
prevent some of the more severe complications. Proper location of
an ostomy diminishes leaking from the appliance and entails avoid-
ing previous incisions, scars, natural skin folds, and belt lines that
prevent circumferential adhesion of the appliance. Leaking around
the appliance and can lead to social embarrassment and dramatic
skin irritation. These problems can occasionally be mitigated by
careful appliance fitting which entails minimizing unprotected
skin and sealing leaks from the caustic effluent. Various commer-
cially produced barriers, powders, ointments, and creams are avail-
able especially for this purpose and should be applied with the help
of an enterostomal therapist. If skin excoriation, maceration, and
irritation persist despite these conservative measures, consider-
ation should be given for ostomy reversal, revision or repositioning
the ostomy, or if possible, converting a high output ileostomy to a
lower output colostomy.
Retraction
Stoma retraction occurs in up to 15% of patients and is most
often the result of a technical error from improper construction
and/or tension.(40–42) Postoperatively, complete retraction of
the stoma into the abdomen mandates immediate re-exploration
and re-creation of the ostomy. Fortunately, this potentially cata-
strophic complication is extremely rare. Partial stoma retraction
occurs more frequently and is more problematic for an ileostomy
than a colostomy. In ileostomies, retraction leads to difficulties
with appliance placement and subsequent skin irritation. In the
thicker viscous colostomy effluent, skin irritation is less of an issue
and can often be conservatively managed. In severe cases, opera-
tive stoma revision may be required. The principles of revision
include tension free ostomy and adequate eversion emphasizing
the Brooke method.
Ischemia and Stenosis
Ostomy necrosis, due to either arterial insufficiency or venous
engorgement, presents in the early postoperative period and is
first recognized by mucosal ischemia. Arterial insufficiency is a
complication of overaggressive mesenteric mobilization with
resultant lack of small vessel collateralization to the mucosa.
It can also be seen in patients with foreshortened or thickened
mesenteries, in obese patients with thick abdominal walls, or
after an inadequate fascial opening. Likewise, stoma necrosis
from venous engorgement as the etiology ultimately leads to the
same end result. Clinically, differentiating the etiology of necro-
sis is not important as management is the same regardless of the
cause. When both considering and managing stoma necrosis, it is
imperative to identify the proximal extent of ischemia. This can be
done by a simple bedside “test-tube test” in which a clear test tube
is inserted into the stoma and then trans-illuminated or direct
visualization is obtained via a pediatric anoscope or proctoscope.
Necrosis seen below the fascia mandates re-exploration and revi-
sion while necrosis isolated above the fascia can be conservatively
managed. Surgically, principles of revision include excision back
to healthy, viable bowel, and recreation of the stoma. This may
entail a more thorough intraabdominal mobilization to reduce
improved outcomes in colon and rectal surgery
tension through the abdominal wall, revision of the fascial open-
ing, or ensuring no kinking of the blood supply. In very difficult
cases, consideration for a loop-end ostomy is advised since less
mesenteric mobilization is required.
Conservative management of stoma necrosis is possible when
the necrosis is isolated above the level of the fascia. Simple mea-
sures, such as maintaining an adequate blood pressure for stoma
perfusion and awaiting edema resolution after bowel manipula-
tion can avoid the morbidity of a re-exploration. Even with frank
necrosis, conservative measures with local wound care should be
attempted. However, conservative management of stoma isch-
emia is a risk factor for ostomy stenosis which occurs in 2–9%
of patients.(34, 40, 41) Stoma stenosis is described as narrowing
of the lumen of the ostomy at the skin or fascia level and is due
to luminal contraction from scar tissue formation. In addition
to ischemia, stenosis can occur due to insufficient skin excision
at the stoma site, peristomal abscess, or mucocutaneous separa-
tion. Stenosis, easily diagnosed by visual inspection and digital
exam of the stoma, is rarely clinically significant and can be man-
aged with a low residue diet and stool softeners. In refractory and
symptomatic cases, dilation, excision of scar tissue, or stoma revi-
sion can be performed. A local type of revision involves a Z-plasty
repair (Figure 33.3).(43, 44)
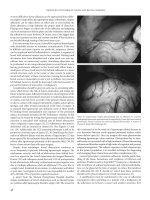
Parastomal Hernias
By definition, a stoma is a hernia in the anterior abdominal wall,
thus leading Goligher to state that the true rate of parastomal
hernias is 100%. As such, parastomal hernias are a well-known
complication of stomal surgery, and can be a major source of
morbidity (Figure 33.4).(45) The incidence of hernias ranges
from 5–10% of stomal patients with colostomies more prone
to herniate than ileostomies.(34, 46) Fortunately, most are well
tolerated and manageable nonoperatively. However, approxi-
mately 30% of hernias require operative repair for symptoms
that include bleeding, obstruction, abdominal masses, poor
fitting appliances, and leakage.(47, 48) Surgical therapy has cen-
tered on stomal relocation, primary fascial repair, and prosthetic
mesh—alone, or in combination. Each of these has been widely
touted; however, significant morbidity and complication rates up
to 88% have left surgeons searching for a better answer to this
difficult problem.(49–52) Equally frustrating is the high rate of
recurrence following initial repair. Rubin et al. found an initial
recurrence rate of 60%, with approximately 70% having subse-
quent failures following additional surgery for both primary fas-
cial repair alone and stomal relocation.(51) Although prosthetic
mesh has shown improved results over stomal relocation and
primary fascial repair, these reports are hindered by low patient
numbers and lack of long-term follow-up to draw meaningful
conclusions regarding complications and recurrences.(49–51,
53–56) A variety of surgical mesh repair techniques exist, includ-
ing a circumferential onlay mesh, two separate intraperitoneal
pieces placed lateral to the stoma, one large piece placed via a
midline approach, and an incomplete mesh ring.(49, 53, 57, 58)
Additionally, both open and laparoscopic approaches have been
used.(59, 60) Yet, fear of mesh infection and erosion has led to
concerns regarding mesh use, and the perceived need to avoid any
contact between the bowel and mesh.(57)
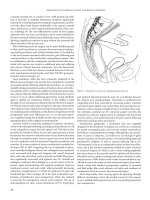
At our institution, one operative approach to symptomatic
parastomal hernias commonly used is primary fascial repair with
nonabsorbable suture and placement of mesh via a “stove-pipe”
hat repair (Figure 33.5). In this technique, one piece of mesh is
placed overlying the fascial repair, the stoma is then pulled through
Figure 33.3 Loop end colostomy. A, loop of
bowel brought through abdominal wall opening.
B, stoma rod is placed through the mesenteric
opening to support the loop on the skin and the
bowel is opened. C, Completed loop colostomy.
(A) (B)
(C)
ostomies
the center of the mesh, thus creating a 360-degree repair. An addi-
tional piece of mesh is then tacked to both the bowel circumfer-
entially and to the onlay mesh. Once constructed, this creates the
“stove-pipe hat” appearance. In selected cases, an additional piece
of mesh is placed beneath the fascia to provide additional sup-
port. Drains are routinely placed at the time of surgery.
In a recent review of our experience, we analyzed 58 patients
that underwent parastomal hernia repair with polypropylene
mesh.(64) With a mean follow-up of 50.6 + 40.1 months, the
overall complication rate related to the polypropylene mesh was
36.2%, and occurred at a mean of 27.2 months. Complications
encountered included recurrence (25.8%), surgical bowel
obstruction (8.6%), prolapse (3.4%), wound infection (3.4%),
fistula (3.4%), and mesh erosion (1.7%). No patients required
extirpation of the mesh. Data analysis demonstrated that stomas
placed for underlying colorectal cancer were associated with a
decreased rate of complications while increased complications
were significantly associated with younger age (59.6 vs. 67 years,
p < 0.05). With the increased availability and use of the biologic
mesh products, fear of contact between mesh and bowel with
subsequent erosion and infection have allowed for increased use
of these products using a similar technique. In addition, while
not extensively studied, we have periodically placed mesh dur-
ing the primary creation of a stoma in select cases, such as for
those patients with diminished fascia, prolonged steroid use, and
re-siting of stoma from prior failures. Future data on this practice
awaits further recommendations.
Prolapse
Ostomy prolapse is the telescoping of the intestine through the
stoma and can be a source of discomfort and anxiety for the patient.
Causes include a large fascial opening in the abdominal muscula-
ture, redundancy of the intestine through the abdomen, failure to
place the stoma through the rectus muscle, insufficient suturing to
the abdominal wall, distended abdomen, and increased abdominal
pressure. Prolapses are most commonly seen in loop stomas with
the distal loop more prone to prolapse. The diagnosis is easily con-
firmed by inspection and the treatment depends on the severity of
the prolapse. In severe prolapse, stoma obstruction and ischemia
may result from excessive tension on the underlying mesentery.
Ischemic changes manifested as ulceration or dusky appearance of
the bowel mandates expeditious surgical intervention and resto-
ration of blood flow. In the more chronic setting, prolapse can be
managed conservatively with manual reduction and symptomatic
relief of discomfort or pain. The application of the ostomy appli-
ance is important for patients who suffer from prolapse. The skin
barrier opening should be cut to accommodate the stoma at its larg-
est size and two piece pouching systems with plastic rings should be
avoided to prevent strangulation. Surgery may ultimately be neces-
sary to resect the prolapse and revise the stoma if symptoms persist.
Again, especially in the setting of loop colostomies, using a portion
of bowel near the flexures where it tends to be more tethered, may
aid in decreasing the incidence of prolapse.
Special Consideration
Morbidly Obese Patients
Morbid obesity, defined as a body mass index >35 kg/m
2
, is a pub-
lic health epidemic in the United States with the prevalence in the
adult population ranging from 2.8–5.1%.(62, 63) The impact of
obesity on the complication profile of patients undergoing col-
orectal surgeries have been well documented and include a higher
incidence of wound infection, dehiscence, wound herniation,
anastomotic, pulmonary, cardiovascular, thromboembolic com-
plications, increased operative time and length of hospital stay,
and overall increased morbidity and mortality.(61) Additionally,
morbid obesity has been found to increase the complication rates
associated with stomas. A prospective risk factor analysis of 97
patients for stoma complications found that elevated BMI was
independently associated with an increased rate of ostomy retrac-
tion, early skin excoriation, and overflow.(36) Furthermore, in a
retrospective review of 156 patients undergoing stoma formation,
Duchesne et al. found obesity, defined as a BMI > 30 kg/m
2
, was
significantly associated with stoma complications, most com-
monly, stoma necrosis, prolapse, and skin irritation.(65) Similarly,
Leenan and Kuypers found that obese patients had a significantly
higher percentage of overall stoma complication (47 vs 36%)
Figure 33.5 “Stove-pipe” hat repair: Parastomal hernia repair with mesh
demonstrating the onlay piece of mesh in as well as circumferential component
overlying the fascial repair. An additional piece (not shown) may be placed in the
sub-fascial location as well. (Courtesy of Patrick Y. Lee, M.D.)
Figure 33.4 Computed tomography image of a patient with a parastomal hernia.
The arrowhead represents a herniated portion of small bowel adjacent to the
ileostomy (arrow). Also note the large midline incisional hernia.
improved outcomes in colon and rectal surgery
including a higher incidence of stoma necrosis.(40) Cottam’s
group, in a nationwide audit of stoma complications, found that
increasing BMI, even that not meeting criteria for “morbid obe-
sity”, was associated with more stoma problems.(37)
Various reasons for a higher complication rate in the obese
include a relatively shortened and fatty mesentery, thicker abdomi-
nal wall through which the stoma must traverse, poor small ves-
sel circulation associated with comorbidities of obesity, and the
physical difficulties of stoma appliance application in the redun-
dant pannus. Ultimately, these factors predispose obese patients
to undergo increased mesenteric mobilization so that the bowel
reaches the skin, with the end result being arterial insufficiency to
the super-fascial stoma. Additionally, an inadequate fascial opening
or physical compression of the abdominal wall on the stoma as it
traverses the abdominal wall may lead to constriction of venous
return with resultant stoma engorgement, stenosis, or necrosis.
In morbidly obese patients undergoing stoma formation in an
elective setting, preoperative weight loss should be encouraged.
Realistically however, sufficient weight loss to favorably impact the
complication profile is unlikely. There may be a unique subset of
patients who can defer abdominal surgery requiring stoma forma-
tion until after undergoing bariatric surgery. In these cases, stoma
formation should be delayed until massive weight loss has stabilized
as significant changes on the abdominal wall may require ostomy
revision if the order of surgery is reversed. In addition to timing
of surgery, the preoperative preparation of the morbidly obese
patient is critical. This high risk patient population should undergo
age appropriate and comorbidity appropriate risk stratification and
work-up as they are at increased risk for perioperative complications.
In regards to ostomy complications, preoperative stoma marking is
important in all patients undergoing stoma formation, but is argu-
ably even more important in this patient population already at
increased risk for local skin complications. Large skin creases prone
to superficial fungal infections in the obese should be avoided, as
well as low lying ostomies which may be difficult for the patient to
adequately visualize and properly maintain (Figure 33.6).
Technically, a sufficient fascial opening should be made to
easily accommodate the bowel through the abdominal wall.
Conservative mesenteric mobilization is encouraged, with mini-
mal length required for the bowel to reach the skin without ten-
sion for proper maturation the ultimate goal. In patients with
foreshortened mesenteries or those with significant abdominal
wall thickness, a loop ostomy, or end-loop stoma in which a
loop of bowel is brought through the fascia and the distal por-
tion closed allowing a few additional centimeters of bowel length
for construction, should be considered as these are less prone
to complications associated with vascular insufficiency. Finally,
removal of some local adipose tissue through which the stoma
will traverse is reasonable, although over-aggressive “de-fatting”
may lead to skin necrosis.
Additional options include a modified abdominoplasty (abdom-
inal wall countering), localized flaps with skin or fat removal, or
liposuction. Although frequently successful, these techniques
have potential for significant morbidity. Patients who may ben-
efit from these techniques include those with stomal retraction
(especially those who have bowel limitations [e.g., continent ileo-
stomies, dense intraabdoninal adhesions or short gut], prolapse,
large peristomal hernias, abdominal wall laxity (usually resulting
from major weight loss), and peristomal skin problems such as
pyodermia. In many of these patients stomal relocation may not
be the best option.
A modified abdominoplasty or abdominal wall contouring
is similar to the technique employed by plastic surgeons.(66,
67) A low curvilinear transverse incision is made at the inferior
abdominal fold or 2–3 cm above the pubis and anterior superior
iliac spines and carried down to the fascia (Figure 33.7). A flap
of skin and subcutaneous tissue is created by electrocautery dis-
section in a cranial direction, just above the fascia. Perforating
vessels are identified and ligated or cauterized. As the dissection
continues the stoma will be encountered. With the flap on trac-
tion, the intestine is separated from the skin and subcutaneous
tissue. Care is taken to avoid injury to the bowel or its blood sup-
ply. The dissection should err on leaving additional subcutane-
ous fat attached to the intestine. This can be carefully resected
later. A similar maneuver may be performed at the umbilicus if
Figure 36.6 Loop ileostomy in an obese patient. It
is important to consider stoma placement in the
lying (A), seated (B), and standing (C) positions.
Note the placement of the ostomy with relation
to the pannus and mid-line incision. Improper
cutting of the stoma appliance cause peristomal
skin excoriations. This patient was preoperatively
marked by an enterostomal therapist with good
postoperative functional outcome.
(A) (B) (C)
Figure 33.7 Redundant abdominal wall folds of skin associated with ileostomy
retraction. (A) Frontal view. (B) Sagittal section demonstrating skin and subcutaneous
fat incisions.
(B)
(A)
ostomies
the surgeon and patient prefer to preserve it in its normal loca-
tion. Again care is taken to preserve the tissue’s blood supply. If
the umbilicus is not to be maintained, it can be amputated at the
fascial level. The flap dissection is continued cranially just above
the fascia until enough laxity or length is obtained in the upper
flap for the upper edge of the previous stomal opening to reach
the inferior portion of the incision without excessive tension or
to the costal margins. Any associated peristomal hernia can be
repaired at this time with suture repair of the fascia and/or mesh
(synthetic or biologic) reinforcement.
As the flap is retracted inferiorly, new sites for the ostomy and,
if desired, the umbilicus are selected and openings created in the
flap. Excess subcutaneous fat can be carefully removed to thin
the flap. Fortunately, there is usually less subcutaneous fat above
the umbilicus compared to below it. The excess, distal portion
of the flap is excised (Figure 33.8). The intestine and umbilicus
are brought through the respective flap openings and matured
with interrupted absorbable sutures (Figure 33.9). Excess bowel
or umbilical tissue can be carefully excised. Closed suction drains
are placed below the flap to avoid seromas and the inferior inci-
sion is closed in layers. As intraabdominal dissections are avoided
with this technique, patients usually recover quickly. Morbidity
is usually associated with infection, flap ischemia, or seromas.
These are managed with wound care.
A more localized procedure involves the use of flaps to modify
the abdominal wall around the stomas. Most involve peristomal
dissections and removal of skin and subcutaneous fat. This can be
performed via a medial or inferolateral approach (Figure 33.10).
An incision is made down to the fascia and advanced toward the
stoma. The ostomy is dissected free of the skin and subcutaneous
tissue as described above. After the stoma is freed, lateral or cra-
nial dissection will provide enough laxity to advance the previous
stoma site to the incision (advancement flap). As above, a new
ostomy opening, in fresh skin, is created. Excess fat may be excised
around the stoma and redundant midline skin is resected.
If the skin flap is not redundant enough to advance the origi-
nal ostomy opening to the midline, the subcutaneous fat can
be excised and the stoma returned to its original skin opening
through the thinned flap. Either method is performed in such a
manner to leave a smooth, flat, thinned flap that provides a flat
surface to site the appliance. The stoma is matured and the inci-
sion is closed. Subcutaneous closed suction drains are placed
above and below the stoma.
The circumstomal approach starts with an incision around the
stoma at the mucocutaneous junction. With careful dissection,
the bowel is separated from the subcutaneous tissue down to the
fascia. The subcutaneous tissue is then separated from the fascia
with electrocautery in a circumferential manner to a point 7–8 cm
out from the stoma. A wedge of subcutaneous tissue is circumfer-
entially created from the upper skin edge to meet the outer edge
of the extrafascial dissection. Small closed suction drains may be
placed and the ostomy is matured to the skin edges. If there was
a preoperative stenosis, the skin opening may be enlarged or the
bowel may be matured with a Z-plasty technique.(43, 68) If the
preoperative stomal opening was too large or it becomes too large
from the dissection, the diameter of the opening can be reduced
with interrupted sutures (Figure 33.11). This type of closure has
been referred to a “Mercedes technique”.(69)
Rapid and significant weight gain in ostomy patients may pro-
duce stomal retraction. If attempts at weight loss have not been
successful and stomal revision is not desirable or feasible (e.g., con-
tinent ileostomy or short gut patients), liposuction is an excellent
option. This method is preferred if there is no associated stomal
Figure 33.8 Excess skin and subcutaneous fat have been excised. (A) Frontal view,
(B).Sagittal section.
(A) (B)
Figure 33.9 Ileostomy relocated through upper flap and skin incisions closed.
Closed suction drains placed below flaps. (A) Frontal view, (B) Sagittal section.
(A) (B)
Figure 33.10 Medial approach. (A) Frontal view with skin incision marked,
(B) Cross section demonstrating midline incision and areas of subcutaneous fat
excision, (C) After removal of excess subcutaneous tissue, incision is closed, flaps
attached to fascia, and stoma matured with adequate eversion.
(A)
(B)
(C)