Improved Outcomes in Colon and Rectal Surgery part 41 ppsx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (291.43 KB, 10 trang )
improved outcomes in colon and rectal surgery
(96, 108) Given the relative ease of the procedure and subse-
quent reversal, a loop colostomy is recommended (100, 102, 103)
if solely for the purpose of diversion in rectal injury, while an
end colostomy is performed if there are other indications, such as
associated colonic injury. Extraperitoneal injuries can be treated
without repair, unless they are easily accessible or uncovered in
the course of treating other injuries.(96, 102, 103, 108) There is
some evidence to support this principle. Gonzalez et al. (111),
implemented a protocol for the management of extraperitoneal
rectal injury without fecal diversion, presacral drainage, or dis-
tal irrigation in patients with nondestructive penetrating injury.
Although they had no mortality or infectious complications, the
series included only 14 patients, making these results difficult to
generalize. Interestingly, in all 14 patients a barium enema was
performed and demonstrated complete healing by postinjury
day 10, demonstrating the rapid healing capacity of the rectum,
likely due to its rich blood supply. Abdominoperineal resec-
tion has been described in the setting of traumatic rectal injury
(102), but should be regarded as an extraordinary measure under
extremely rare circumstances. The steps in the classic, conserva-
tive management of rectal injury have been described by Stewart
and Rosenthal (20) and are summarized in Table 35.8. A modi-
fication to these steps as suggested by modern series is presented
in Table 35.9.
summary
Rectal injury is uncommon and often accompanied by significant
associated injury, most commonly genitourinary. Data is scarce,
and is mostly limited to retrospective reviews. Diagnosis is chal-
lenging, and is most often made by clinical suspicion, digital exam,
and proctoscopy or sigmoidoscopy. Intraperitoneal injury can be
treated with primary repair in a manner analogous to colon injury.
Extensive destructive injury can be diverted with lower expected
complication rates than colon injury. Extraperitoneal injury can be
treated with diversion alone, although selected cases of partial or
nondestructive injury can be treated with nonoperative manage-
ment. Presacral drainage is sometimes recommended in these cases
in order to prevent pelvic sepsis. Presacral drainage and distal rectal
washout are more appropriate in high-velocity injuries similar to
combat injuries but have less efficacy in civilian settings.
Foreign bodies
Anorectal foreign bodies are almost always inserted during sexual
conduct.(113–117) The most common objects found are sexual
implements such as vibrators and dildos (115, 116) (Figure 35.2).
Other, less common causes are ingested material, most often
bones, or iatrogenic causes such as thermometers and enema tips.
(113) A case of a live eel inserted into the anus as a folk remedy for
constipation has been reported, in which the eel migrated proxi-
mally and was found biting the perforated splenic flexure.(118)
The patient presented with peritonitis, which led the clinicians to
note “the shadow of an eel on abdominal radiograph”, confirming
the diagnosis. There is a predominance of males, ranging from
93–100% in the largest series.(114–116, 119)
Goals of initial assessment are to create an atmosphere that
allows the patient to give a detailed history, to recognize the
potential of rape or assault, and to recognize signs of perfora-
tion that require more urgent therapy. Multiple-view plain radio-
graphs should be obtained. Plain films will help localize the
object, although rubber will not be apparent on radiography. Free
air or obvious perforation can be ruled out. Patients with signs
and symptoms of obstruction or perforation should have basic
labs drawn, intravenous fluids initiated, antibiotics started and
proceed to urgent laparotomy with no further attempt at removal
of the object.(117) A perforation should be treated as any trau-
matic rectal injury, with removal of the foreign body, which will
be discussed subsequently.
Table 35.8 Traditional steps in the management of rectal injury.(20)
Perineolithotomy position
Management of concomitant injuries
Debridement
Proximal diversion
Remove foreign bodies
Presacral drainage
Distal rectal washout
Repair injury if possible
Repair sphincters if possible
External wound drainage
Broad spectrum antibiotics
Skin left open
Figure 35.2 Foreign body requiring operative extraction. This patient sustained a
full-thickness rectal injury from the foreign body placement.
Table 35.9 Modified steps in management of rectal injury.
Perineolithotomy position
Management of concomitant injuries
Debridement
Intraperitoneal injury Extraperitoneal injury
Primary repair Diversion
Diversion if destructive injury (Loop colostomy
preferred)
Selective presacral drainage
Repair if easily accessible
Repair sphincters if possible
Selective external wound drainage
Broad spectrum antibiotics
Skin left open
colorectal trauma
The majority of rectal foreign bodies can be removed at the
bedside, which is successful in 60–75% of cases.(114, 115, 119)
An attempt at bedside extraction is reasonable in patients without
signs of peritonitis.(113–115, 119) Sedation and local anesthesia
can assist with relaxation and extraction, and an awake patient
can be asked to perform a valsalva maneuver. If these maneuvers
are unsuccessful, a stable patient can be admitted and observed
for 12 hours; during this time the object will often descend into
the rectum.(114) Foreign bodies removed nonoperatively require
a postprocedure sigmoidoscopy to assess the viability of the rec-
tum and rule out perforation.(113–117, 119)
Operative removal can be accomplished with local, regional,
or general anesthesia. Either the lithotomy or prone position
can be used, but one advantage of lithotomy is that pressure
can be applied to the abdomen to move the object distally.(117)
Retractors can be placed and the anus dilated. Obstetric forceps
and balloon-tipped catheters are commonly employed.(113–117,
119) Balloon-tipped catheters are useful in the case of jars or
containers that are positioned with the mouth facing proximally,
where the suction generated can prevent removal. The passage of
a Foley catheter past the object can serve to break the suction and
can be used to aid in extraction.(113)
Rarely, laparotomy is required (0–6% of cases).(114, 115, 119)
Attempts should be made at distally displacing the object with-
out entering the bowel. If this is unsuccessful, an enterotomy
can be made through which the object can be removed.(117,
119) Even more uncommonly, a lateral sphincterotomy may be
required. Lake and colleagues (119) performed a recent review of
93 retained colorectal foreign bodies in 87 patients to determine
predictors of operative intervention. Two patients (2%) presented
with signs of peritonitis and were taken to the operating room.
Seventy five percent of attempts at bedside extraction were suc-
cessful. Of 23 cases requiring operative management, 6 required
laparotomy and 5 (6%) required creation of a colotomy. Size of
object (greater than 10 cm) and time to presentation (greater
than 48 hours) were not associated with an increase in opera-
tive intervention. Only location in the sigmoid was predictive of
failure of nonoperative management (55% versus 24%, p = 0.04),
with an associated OR of 2.25.
Anal sphincter and perineal injury
Anal sphincter injury
Anal sphincter function is extremely complex, and a full dis-
cussion is outside the scope of this discussion. Anal sphincter
trauma is highly unusual due to its protected anatomic location
and abundant blood supply (113). The most common cause of
anal sphincter injury is obstetric trauma, followed by sequelae of
anorectal operations, and uncommonly by etiologies similar to
those causing rectal injuries.(113, 120) Stapling procedures such
as for hemorrhoidectomy have been shown to cause anal sphinc-
ter injuries as well.(113)
Life-threatening injuries should be addressed first in trauma
patients, particularly massive, complex perineal injury (discussed
subsequently). In a comprehensive review, Hellinger (113) out-
lines the initial management of anal sphincter injury. As men-
tioned, documentation of the extent and nature of the sphincter
injury is imperative. Superficial injuries can be debrided and
repaired without proximal diversion and minimal injury iso-
lated to the internal sphincter can be left unrepaired. Destructive
injury requires diversion following the same principles as rectal
injury. In most cases primary repair should be undertaken, com-
monly in an end-to-end or overlapping fashion. Overlapping
repair is accomplished by dissecting out the sphincter muscles
and wrapping them anteriorly around the anus. Although over-
lapping repair increases the surface area of muscular apposition,
this repair is difficult to achieve without tension in the acute
setting, and thus end-to-end repair may be the principal option
in the setting of acute trauma.(113, 120)
Other techniques for repair include muscle transpositions (e.g.
gracilis or gluteal) and artificial sphincters.(113,120) However,
these procedures are best undertaken in the delayed setting and
should be performed by surgeons with extensive experience. For
example, graciloplasty for fecal incontinence has been shown in
several large series to have success rates of 60–66% by various
measures (121–123), but infectious complications in 34–39%
of patients and donor-site morbidity (pain, paresthesias) have
been reported in 22–72%.(121, 123) Artificial anal sphincters
have been associated with success rates of 75–98% by vari-
ous measures, but infection rates range from 13–34%, erosions
from 8–21%, explants in 19–37%, and revisionary procedures in
26–45%.(124, 125)
In the long-term, sphincter repairs tend to degenerate (120),
with rates in the elective population ranging between 2.8–10%.
(126, 127) It is important to arrange appropriate follow-up for
the assessment of anal function in these patients. Physical exam,
myography, manometry, and contrast studies are all routinely
employed to assess sphincter function.(113) In addition, endo-
scopic ultrasound has been shown to be a useful adjunct to visu-
alizing the anal sphincters and predicting defects. A sensitivity of
100% and specificity ranging from 83–100% has been reported
when compared to intraoperative findings in elective operations
for fecal incontinence.(128, 129) Delayed repair has been shown
to have good results in approximately 70% of patients with fecal
incontinence due to nonobstetric trauma.(130)
Complex perineal injury
Kudsk and Hanna (131) have published a complete review of
complex perineal injuries, describing a 15-year experience in the
comprehensive care of these patients. Figures 35.3a–3c illustrate
complex perineal injury. The authors demonstrate the synthe-
sis of the principles of ATLS, damage control, and distal rectal
injury required to manage these potentially devastating injuries.
Their review included only those patients with evidence of severe
degloving (25 total) and the reported mortality was 24% during
the first 2 hours of admission. Two additional patients died for
reasons unrelated to their perineal injury, for an overall mortal-
ity of 32%. Their review of the literature revealed similar mor-
tality rates. Roughly half of the patients were pedestrians hit by
cars, one-third were involved in motor vehicle crashes, and the
remainder sustained industrial accidents.
The second most common cause of mortality after exsan-
guinating hemorrhage is pelvic sepsis. The authors’ review of
the literature revealed a 21–25% death rate from this cause. For
improved outcomes in colon and rectal surgery
authors emphasize that in complex pelvic injuries, lower extremity
central access is contraindicated in that it may contribute to fur-
ther hemorrhage by delivering fluids and blood products directly
into the abdominal cavity through lacerated vessels. Access above
the diaphragm is recommended. Laparotomy should be performed
and intraabdominal injuries addressed. Early pelvic fixation and
hemorrhage control should proceed by packing, direct ligation
or clamping, and angiography as necessary. The lithotomy posi-
tion is essential to appropriate exposure. Associated genitourinary
injuries should be addressed. Debridement should continue only
in the absence of refractory hemorrhage and the patient should
be returned to the ICU for further resuscitation before prolonged
operative interventions.
Following stabilization, fecal diversion should be undertaken
early. Aggressive debridement and irrigation should be under-
taken with frequent return trips to the operating room. Kudsk
and Hanna report an average of 8 trips to the operating room
using pulse-lavage before closure or coverage was attempted.
These principles are similar to the management of Fournier’s
gangrene. Enteral feeding should be initiated as early as possible.
In the delayed setting, coverage can be achieved with skin grafts
and muscle flaps as indicated. Using these techniques, the authors
were able to discharge 17 of 19 patients (89%) to home. Feeding
jejunostomies were placed in 6 patients and enteral nutrition was
initiated in all 6 within 48 hours.
summary
Anal sphincter and complex perineal injuries are uncommon in
civilian settings. Life-threatening hemorrhage and pelvic fracture
are the first concerns. Documentation of the extent of sphincter
injury is imperative. Genitourinary and rectal injuries should be
suspected until ruled out by careful investigation. Primary repair
of sphincter injury should be undertaken if feasible. Referral
is recommended for cases where complex repair is required.
Follow-up is important for assessment of long-term function, as
Figure 35.3a Complex perineal injury. The patient was run over by heavy road-
repair equipment. The patient also sustained urethral injury, sigmoid colon injury,
and severe pelvic fracture.
Figure 35.3b CT scan of complex perineal injury. Note the large soft-tissue
defect.
Figure 35.3c Pelvic fracture associated with complex perineal injury.
example, Maull et al. (132), reported a 25% mortality due to pel-
vic sepsis in their series. Kudsk and Hanna report a 21% pelvic
sepsis rate but no mortality, which they attribute to their aggres-
sive, multisystem approach as described.
Immediate assessment of the ABCs, intravenous access, and lim-
ited radiographic imaging are the initial steps. In cases of severe
injury resuscitation should occur in the operating room. The most
serious and most common associated injury was severe complex
pelvic fracture, which occurred 74% of the time (Figure 35.3c). The
colorectal trauma
functional deterioration is common after repair. Fecal diversion
may be necessary in cases of massive injury. The primary concern
initially is hemorrhage control, while infection and nutrition are
more important in the next several days. In the longer-term, tis-
sue coverage and functional issues become more important. With
aggressive, comprehensive management, the majority of patients
have good outcomes.
referenCes
1. Yaw PB, Smith RN, Glover JL. Eight years experience with
civilian injuries of the colon. Surg Gynecol Obstet 1977;
145: 203–5.
2. Perry WB, Brooks JP, Muskat PC. The history of military
colorectal trauma management. Semin Colon Rectal Surg
2002; 15: 70–9.
3. LoCicero J, Tajima T, Drapanas T. A half-century of experi-
ence in the management of colon injuries: Changing con-
cepts. J Trauma 1975; 16: 575–9.
4. Ogilvie WH. Abdominal wounds in the Western desert. Surg
Gynecol Obstet 1944; 78: 225–38.
5. Singer MA, Nelson RL. Primary repair of penetrating colon
injuries: A systematic review. Dis Colon Rectum 2002; 45:
1579–87.
6. Burch JM, Brock JC, Gevirtzman L et al. The injured colon.
Ann Surg 1986; 203: 701–11.
7. Steichen FM, Ravitch MM. Contemporary stapling instru-
ments and basic mechanical suture techniques. Surg Clin N
Am 1984; 64: 425–40.
8. Curran TJ, Borzotta AP. Complication of primary repair
of colon injury: Literature review of 2964 cases. Am J Surg
1999; 177: 42–7.
9. Stone HH, Fabian TC. Management of perforating colon
trauma: Randomization between primary closure and exte-
riorization. Ann Surg 1979; 190: 430–5.
10. Eshraghi N, Mullins RJ, Mayberry JC et al. Surveyed opin-
ion of American trauma surgeons in management of colon
injuries. J Trauma 1998; 44: 93–7.
11. Pezim ME, Vestrup JA. Canadian attitudes toward use of
primary repair in management of colon trauma. Dis Colon
Rectum 1996; 39: 40–4.
12. Demetriades D, Velmahos G, Cornwell E et al. Selective
nonoperative management of gunshot wounds to the ante-
rior abdomen. Arch Surg 1997; 132: 178–83.
13. Burch JM, Martin RR, Richardson RJ et al. Evolution of the
treatment of the injured colon in the 1980s. Arch Surg 1991;
126: 979–84.
14. Chappuis CW, Frey DJ, Dietzen CD et al. Management of
penetrating colon injuries: A prospective randomized trial.
Ann Surg 1991; 213: 492–7.
15. Adkins RB, Zirkle PK, Waterhouse G. Penetrating colon
trauma. J Trauma 1984; 24: 491–9.
16. Flint LM, Vitale GC, Richardson JD, Polk HC. The injured
colon. Ann Surg 1981; 193: 619–23.
17. Watts DD, Fahkry SM. Incidence of hollow viscus injury in
blunt trauma: An analysis from 2,75,557 trauma admissions
form the EAST multi-institutional trial. J Trauma 2003; 54:
289–94.
18. Ricciardi R, Paterson CA, Islam S et al. Independent predic-
tors of morbidity and mortality in blunt colon trauma. Am
Surg 2004; 70: 75–9.
19. Ross SE, Cobean RA, Hoyt DB et al. Blunt colonic injury—a
multicenter review. J Trauma 1992; 33: 379–84.
20. Stewart RM, Rosenthal D. Colorectal Trauma. In: Corman,
M, ed. Colon and Rectal Surgery. Philadelphia, PA: Lippincott
Williams & Wilkins; 2005: 427–49.
21. Nelson R, Singer M. Primary repair for penetrating colon
injuries. Cochrane Database of Syst Rev 2003; 3: CD002247
22. Singer MA, Nelson RL. Primary repair of penetrating colon
injuries. Dis Colon Rectum 2002; 45: 1579–87.
23. Sasaki LS, Allaben RD, Golwala R, Mittal VK. Primary
repair of colon injuries: A prospective, randomized study.
J Trauma 1995; 39: 895–901.
24. Gonzalez RP, Merlotti GJ, Holevar MR. Colostomy in pen-
etrating colon injury: Is it necessary? J Trauma 1996; 41:
271–5.
25. Gonzalez RP, Falimirski ME, Holevar MR. Further evalu-
ation of colostomy in penetrating colon injury. Am Surg
2000; 66: 342–7.
26. Kamwendo NY, Modiba MCM, Matlala NS, Becker PJ.
Randomized Clinical trial to determine if delay from time
of penetrating colon injury precludes primary repair. Br J
Surg 2002; 89: 993–8.
27. Demetriades D, Murray JA, Chan L et al. Penetrating colon
injuries requiring resection: Diversion or primary anasto-
mosis? An AAST prospective multicenter study. J Trauma
2001; 50: 765–75.
28. Schreiber MA. Damage control surgery. Crit Care Clin 2004;
20: 101–18.
29. Lee JC, Peitzman AB. Damage control laparotomy Curr
Opin Crit Care 2006; 12: 346–50.
30. Demetriades D, Salim A. Colon and rectal trauma and rec-
tal foreign bodies. In: Wolff BG, Fleshman JW, Beck DE, et
al eds. The ASCRS Textbook of Colon and Rectal Surgery.
New York, NY:Springer, 2008: 322–34.
31. Sokolove PE, Kuppermann N, Holmes JF et al. Association
between the “Seat Belt Sign” and intraabdominal injury in chil-
dren with blunt torso trauma. Acad Emer Med 2005; 12: 808–13.
32. LeGay DA, Petrie DP, Alexander DI et al. Flexion-distraction
injuries of the lumbar spine and associated abdominal
trauma.J Trauma 1990; 30(4): 436–44.
33. Hoff WS, Holevar M, Nagy KK et al. Practice Management
Guidelines for the evaluation of blunt abdominal trauma,
EAST Practice Management Guidelines Work Group.
J Trauma 2002; 53: 602–15.
34. Otomo Y, Henmi H, Mashiko K et al. New diagnostic peri-
toneal lavage criteria for diagnosis of intestinal injury.
J Trauma 1998; 44: 991–9.
35. Liu M, Lee CH, P’eng FK. Prospective comparison of diag-
nostic peritoneal lavage, computed tomographic scanning,
and ultrasonography for the diagnosis of blunt abdominal
trauma. J Trauma 1993; 35: 267–70.
36. Boulanger BR, Brenneman FD, McLellan BA et al. A pro-
spective study of emergent abdominal sonography after
blunt trauma. J Trauma 1995; 39: 325–30.
improved outcomes in colon and rectal surgery
37. Smith SR, Kern SJ, Fry WR et al. Institutional learning curve
of surgeon-performed trauma ultrasound. Arch Surg 1998;
133: 530–6.
38. McKenney MG, Martin L, Lentz K et al. 1000 consecutive
ultrasounds for blunt abdominal trauma. J Trauma 1996;
40: 607–12.
39. Kern SJ, Smith RS, Fry WR et al. Sonographic examination
of abdominal trauma by senior surgical residents. Am Surg
1997; 63: 669–74.
40. Rozycki GS, Ochsner MG, Schmidt JA et al. A prospective
study of surgeon-performed ultrasound as the primary
adjuvant modality for injured patient assessment. J Trauma
1995; 39: 492–500.
41. Malhotra AK, Fabian TC, Katsis SB et al. Blunt bowel and
mesenteric injuries: the role of screening computer tomog-
raphy. J Trauma 2000; 48: 991–1000.
42. Gutela ST, Federle MP, Huang LF et al. Performance of CT in
detection of bowel injury. Am J Radiol 2001; 176: 129–35.
43. Rizzo MJ, Federle MP, Griffiths BG et al. Bowel and mesen-
teric injury following blunt abdominal trauma: evaluation
with CT. Radiology 1989; 173: 143–8.
44. Ahmed N, Whelan J, Brownlee J et al. The Contribution
of Laparoscopy in Evaluation of Penetrating Abdominal
Wounds. J Am Coll Surg 2005; 201: 213–6.
45. Mitsuhide K, Junichi S, Atsushi N et al. Computed
Tomographic Scanning and Selective Laparoscopy in the
Diagnosis of Blunt Bowel Injury: A Prospective Study.
J Trauma 2005; 58: 696–703.
46. Moore EE, Dunn EL, Moore JB, Thompson JS. Penetrating
abdominal trauma index. J Trauma 1981; 21: 439–45.
47. Nallathambi MN, Ivatury RR, Shah PM et al. Aggressive
definitive management of penetrating colon injuries—136
cases with 3.7 percent mortality. J Trauma 1984; 24: 500–5.
48. Nelken N, Lewis F. The influence of injury severity on com-
plication rates after primary closure or colostomy for pen-
etrating colon trauma. Ann Surg 1989; 209: 439–47.
49. Moore, EE, Cogbill TH, Malangoni MA et al. Organ injury
scaling, II: Pancreas, duodenum, small bowel, colon, and
rectum. J Trauma 1990; 30: 1427–9.
50. Pontius RG, Creech O, DeBakey ME. Management of large
bowel injuries in civilian practice. Ann Surg 1957; 146:
291–5.
51. Falcone RE, Wanamaker SR, Santanello SA, Carey LC.
Colorectal trauma: Primary repair or anastomosis with
intracolonic bypass vs. ostomy. Dis Colon Rectum 1992; 35:
957–63.
52. Stewart RM, Fabian TC, Croce MA et al. Is resection with
primary anastomosis following destructive colon wounds
always safe? Am J Surg 1994; 168: 316–9.
53. George SM, Fabian TC, Voeller GR et al. Primary repair of
colon wounds. Ann Surg 1989; 209: 728–34.
54. Miller PR, Chang MC, Hoth JJ, Homes JH, Meredith JW.
Colonic resection in the setting of damage control laparotomy:
Is delayed anastomosis safe? Am Surg 2007; 76: 606–10.
55. Chavarria-Aguilar M, Cockerham WT, Barker DE et al.
Management of destructive bowel injury in the open abdo-
men. J Trauma 2004; 56: 560–4.
56. Johnson JW, Gracias VH, Schwab CW et al. Evolution in
damage control surgery for exsanguinating penetrating
abdominal injury. J Trauma 2001; 51: 261–71.
57. Miller PR, Fabian TC, Croce MA et al. Improving outcomes
following penetrating colon wounds: Application of a clini-
cal pathway. Ann Surg 2002; 235: 775–81.
58. Fahkry SM, Watts DD, Luchette FA et al. Current diagnos-
tic approaches lack sensitivity in the diagnosis of perforated
blunt small bowel injury: analysis from 275,557 trauma
admissions from the EAST multi-institutional HVI trial.
J Trauma 2003; 54: 295–306.
59. Fahkry SM, Brownstein M, Oller D et al. Relatively short
diagnostic delays (<8 hrs) produce morbidity and mortality
in blunt small bowel injury: an analysis of time to operative
intervention in 198 patients from a multicenter experience.
J Trauma 2000; 48: 408–15.
60. Williams MD, Watts D, Fakhry S et al. Colon Injury after
Blunt Abdominal Trauma: Results of the EAST Multi-
institutional Hollow Viscus Injury Study.J trauma 2003; 55:
906–12.
61. Dauterive AH, Flancbaum L, Cox EF. Blunt intestinal trauma:
A modern-day review. Ann Surg 1985; 201: 198–203.
62. Ross SE, Cobean RA, Hoyt DB et al. Blunt Colonic Injury –
A Multicenter Review. J Trauma 1992; 33: 379–84.
63. Nance ML, Peden GW, Schwab CW et al. Solid viscus injury
predicts major hollow viscus injury in blunt abdominal
trauma. J Trauma 1997; 43: 618–23.
64. Yegiyants S, Abou-Lahoud G, Taylor E. The management of
blunt abdominal trauma patients with computed tomogra-
phy scan findings of free peritoneal fluid and no evidence of
solid organ injury. Am Surg 2006; 72: 943–6.
65. Hughes TMD, Elton C. The pathophysiology and manage-
ment of bowel and mesenteric injuries due to blunt trauma.
Injury 2002; 33: 295–302.
66. Parks SE, Hastings PR. Complications of colostomy closure.
Am J Surg 1985; 149: 672–5.
67. Bulger EM, McMahon K, Jurkovich GJ. The morbidity of
penetrating colon injury. Injury 2003; 34: 41–6.
68. Berne JD, Velmahos GC, Chan LS, Asensio JA, Demetriades
D. The high morbidity of colostomy closure after trauma:
Further support for the primary repair of colon injuries.
Surgery 1998; 123: 157–64.
69. PokornyH, Herkner H, Jakesz R, Herbst F. Mortality and com-
plications after stoma closure. Arch Surg 2005; 140: 956–60.
70. Dente CJ, Tyburski J, Wilson RF et al. Ostomy as a risk factor
for posttraumatic infection in penetrating colonic injuries:
Univariate and multivariate analyses. J Trauma 2000; 49:
628–37.
71. Fabian TC. Infection in penetrating abdominal trauma:
risk factors and preventive antibiotics. Am Surg 2002; 68:
29–35.
72. Hudolin T, Hudolin I. The role of primary repair for colonic
injuries in wartime. Br J Surg 2005; 92: 643–7.
73. Steele SR, Wolcott KE, Mullenix PS et al. Colon and rec-
tal injuries during Operation Iraqi Freedom: Are there any
changing trends in management or outcome? Dis Colon
Rectum 2006; 50: 870–7.
colorectal trauma
74. Welling DR, Hutton JE, Minken SL, Place RJ, Burris DG.
Diversion defended—military colon trauma. J Trauma
2008; 64: 1119–22.
75. Duncan JE, Corwin CH, Sweeney WB et al. Management
of colorectal injuries during Operation Iraqi Freedom:
Patterns of stoma usage. J Trauma 2008; 64: 1043–7.
76. American College of Surgeons Committee on Trauma, eds.
Advanced Trauma Life Support Course, 7th ed. Chicago, Ill:
American College of Surgeons; 2004.
77. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia
to reduce the incidence of surgical-wound infection and
shorten hospitalization. Study of Wound Infection and
Temperature Group. N Engl J Med 1996; 9: 1209–15.
78. Van den Berghe G, Wouters P, Weekers F et al. Intensive
insulin therapy in the critically ill patients. N Engl J Med.
2001; 345: 1359–67.
79. Dunne JR, Riddle MS, Danko J, Hayden R, Petersen K.
Blood transfusion is associated with infection and increased
resource utilization in combat casualties. Am Surg. 2006; 72:
619–25.
80. Taylor RW, Manganaro L, O’Brien J et al. Impact of allogenic
packed red blood cell transfusion on nosocomial infection rates
in the critically ill patient. Crit Care Med 2002; 30: 2249–54.
81. Taylor RW, O’Brien J, Trottier SJ et al. Red blood cell trans-
fusions and nosocomial infections in critically ill patients.
Crit Care Med 2006; 34: 2302–8.
82. Kudsk KA, Croce MA, Fabian TC et al. Enteral versus par-
enteral feeding. Effects on septic morbidity after blunt
and penetrating abdominal trauma. Ann Surg 1992; 215:
503–13.
83. Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson
VM. TEN versus TPN following major abdominal trauma-
-reduced septic morbidity. J Trauma 1989; 29: 916–23.
84. Moore FA, Moore EE, Kudsk KA et al. Clinical benefits of an
immune-enhancing diet for early postinjury enteral feed-
ing. J Trauma 1994; 37: 607–15.
85. Polk HC, Lopez-Mayor JF. Postoperative wound infection:
A prospective study of determinant factors and prevention.
Surgery 1969; 66: 97–103.
86. Stone HH, Hooper CA, Kolb LD, Geheber CE, Dawkins EJ.
Antibiotic prophylaxis in gastric, biliary and colonic sur-
gery. Ann Surg 1976; 184: 443–52.
87. Mazuski JE, Sawyer RG, Nathens AB et al. The Surgical
infection society guidelines on antimicrobial therapy for
intra-abdominal infections: An executive summary. Surg
Infect 2002; 3: 161–73.
88. Bozorgzadeh A, Pizzi WF, Barie PS et al. The duration of
antibiotic administration in penetrating abdominal trauma.
Am J Surg 1999; 177: 125–31.
89. Cornwell EE, Dougherty WR, Berne TV et al. Duration of
antibiotic prophylaxis in high-risk patients with penetrat-
ing abdominal trauma: a prospective randomized trial.
J Gastrointest Surg 1999; 3: 648–53.
90. Kirton OC, O’Neill PA, Kestner M, Tortella BJ. Perioperative
antibiotic use in high-risk penetrating hollow viscus injury:
a prospective randomized, double-blind, placebo-control
trial of 24 hours versus 5 days. J Trauma 2000; 49: 822–32.
91. Poret HA, Timothy TC, Croce MA, Bynoe RP, Kudsk KA.
Analysis of septic morbidity following gunshot wounds to
the colon: The missile is an adjuvant for abscess. J Trauma
1991; 31: 1088–95.
92. Demetriades D, Charalambides D. Gunshot wounds of the
colon: Role of retained bullets in sepsis. Br J Surg 1993; 80:
772–3.
93. Sarmiento JM, Yugueros P, Garcia AF, Wolff BG. Bullets and
their role in sepsis after colon wounds. World J Surg 1997;
21: 648–52.
94. Edwards DP, Brown D, Watkins PE. Should colon-penetrat-
ing small missiles be removed? An experimental study of
retrocolic wound tracks. J Invest Surg 1999; 12: 25–9.
95. Flint LM, Voyles CR, Richardson JD, Fry DE. Missile tract
infections after transcolonic gunshot wounds. Arch Surg
1978; 113: 727–8.
96. Merlino JI, Reynolds HL. Management of rectal injuries.
Semin Colon Rectal Surg 2004; 15: 95–104.
97. Lüning TH, Keemers-Gels ME, Barendregt WB, Tan AC,
Rosman C. Colonoscopic perforations: a review of 30,366
patients. Surg Endosc 2007; 21: 1975–7.
98. Gedebou TM, Wong RA, Rappaport WD et al. Clinical
presentation and management of iatrogenic colon perfora-
tions. Am J Surg 1996; 172: 454–8.
99. Morken JJ, Kraatz JJ, Balcos EG et al. Civilian rectal trauma:
A changing perspective. Surgery 1999; 126: 693–700.
100. evy RD, Strauss P, Aladgem D et al. Extraperitoneal rectal
gunshot injuries. J Trauma 1995; 38: 273–7.
101. McGrath V, Fabian TC, Croce MA, Minard G, Pritchard FE.
Rectal trauma: Management based on anatomic distinc-
tions. Am Surg 1998; 64: 1136–41.
102. Burch JM, Feliciano DV, Mattox KL. Colostomy and drain-
age for civilian rectal injuries: Is that all? Ann Surg 1989;
209: 600–11.
103. Navsaria PH, Edu S, Nicol AJ. Civilian extraperitoneal rec-
tal gunshot wounds: Surgical management made simpler.
World J Surg 2007; 31: 1345–51.
104. Velmahos GC, Gomez H, Falabella A, Demetriades D.
Operative management of civilian rectal gunshot wounds:
Simpler is better. World J Surg 2000; 24: 114–8.
105. Rubesin SE, Levine MS. Radiologic diagnosis of gastrointes-
tinal perforation. Rad Clin N Am 2003; 41: 1095–115.
106. Navsaria PH, Shaw JM, Zellweger R et al. Diagnostic lap-
aroscopy and diverting sigmoid loop colostomy in the man-
agement of civilian extraperitoneal rectal gunshot injuries.
Br J Surg 2004; 91: 460–4.
107. Navsaria PH, Graham R, Nicol A. A new approach to extra-
peritoneal rectal injuries: Laparoscopy and diverting sig-
moid colostomy. J Trauma 2001; 51: 532–5.
108. Weinberg JA, Fabian TC, Magnotti LJ et al. Penetrating rec-
tal trauma: Management by anatomic distinction improves
outcome. J Trauma 2006; 60: 508–14.
109. Marti MC, Morel P, Rohner A. Traumatic lesions of the rec-
tum. Int J Colorectal Dis 1986; 1: 152–4.
110. Gonzalez RP, Falimirski ME, Holevar MR. The role of pre-
sacral drainage in the management of penetrating rectal
injuries. J Trauma 1998; 45: 656–61.
improved outcomes in colon and rectal surgery
111. Gonzalez RP, Phelan H, Hassan M, Ellis N, Rodning CB.
Is fecal diversion necessary for nondestructive penetrating
extraperitoneal rectal injuries? J Trauma 2006; 61: 815–9.
112. Steinig JP, Boyd CR. Presacral drainage in penetrating extra-
peritoneal rectal injuries: is it necessary? Am Surg 1996; 62:
765.
113. Hellinger MD. Anal trauma and foreign bodies. Surg Clin N
Am 2002; 82: 1253–60.
114. Barone JE, Sohn N, Nealon TF. Perforations and foreign
bodies of the rectum: Report of 28 cases. Ann Surg 1976;
184: 601–4.
115. Barone JE, Yee J, Nealon TF. Management of foreign bodies and
trauma of the rectum. Surg Gynecol Obstet 1983; 156: 453–7.
116. Busch DB, Starling JR. Rectal foreign bodies: case reports
and a comprehensive review of the world’s literature.
Surgery 1986; 110: 512–9.
117. Kann BR, Hicks TC. Anorectal foreign bodies: Evaluation
and treatment. Semin colon rectal surg 2004; 15: 119–24.
118. Lo SF, Wong SH, Leung LS, Law IC, Yip AWC. Traumatic
rectal perforation by an eel. Surgery 2004; 135: 110–1.
119. Lake JP, Essani R, Petrone Pet al. Management of retained
colorectal foreign bodies: Predictors of operative interven-
tion. Dis Colon Rectum 2004; 47: 1694–8.
120. Brill SA, Margolin DA. Anal sphincter trauma. Semin Colon
Rectal Surg 2004; 15: 90–4.
121. Thornton MJ, Kennedy ML, Lubowski DZ, King DW. Long-
term follow-up of dynamic graciloplasty for faecal inconti-
nence. Colorectal Dis 2004; 6: 470–6.
122. Wexner SD, Baeten C, Bailey R et al. Long-term efficacy of
dynamic graciloplasty for fecal incontinence. Dis Colon
Rectum 2002; 45: 809–18.
123. Madoff RD, Rosen HR, Baeten CG et al. Safety and efficacy
of dynamic muscle plasty for anal incontinence: lessons
from a prospective, multicenter trial. Gastroenterology.
1999; 116: 549–56.
124. Wong WD, Congliosi SM, Spencer MP et al. The safety and
efficacy of the artificial bowel sphincter for fecal inconti-
nence. Dis Colo Rectum 2002; 45: 1139–53.
125. Devesa JM, Rey A, Hervas PL et al. Artificial anal sphincter:
Complications and functional results of a large personal
series. Dis Colon Rectum 2002; 45: 1154–63.
126. Rotholtz NA, Bun M, Mauri MV et al. Long-term assess-
ment of fecal incontinence after lateral internal sphinctero-
tomy. Tech Coloproctol 2005; 9: 115–8.
127. Casillas S, Hull TL, Zutchi M et al. Incontinence after a lat-
eral internal sphincterotomy: Are we underestimating it?
Dis Colon Rectum 2005; 48: 1193–9.
128. Deen KI, Kumar D, Williams JG, Olliff J, Keighley MRB.
Anal sphincter defects: Correlation between endoanal ultra-
sound and surgery. Ann Surg 1993; 218: 201–5.
129. Meyenberger C, Bertschinger P, Zala GF, Buchmann P. Anal
sphincter defects in fecal incontinence: Correlation between
endosonography and surgery. Endoscopy 1996; 28: 217–24.
130. Engel AF, Kamm MA, Hawley PR. Civilian war injuries
of the perineum and anal sphincters. Br J Surg 1994; 81:
1069–73.
131. Kudsk KA, Hanna MK. Management of complex perineal
injuries. World J Surg 2003; 27: 895–900.
132. Maull KI, Sachatello GR, Ernst CB. The deep perineal lac-
eration—an injury frequently associated with open pel-
vic fractures: A need for aggressive surgical management.
J Trauma 1977; 17: 685–96.
6
Urologic complications of colorectal surgery
Scott Delacroix Jr and J Christian Winters
CHALLENGING CASE
A laparoscopic left hemicolectomy was performed for an asympto-
matic 2.0 cm sigmoid adenocarcinoma found on screening colon-
oscopy. Due to her previous three cesarean sections and abdominal
hysterectomy with bilateral oopherectomy, she required extensive
lysis of adhesions in order to mobilize the left colon. The procedure
was uneventful and the patient was discharged home on postoper-
ative day number three. On follow-up at 10 days, patient was doing
well except for a new complaint of left “side pain” rated as a 3 out
of 10. Management was expectant and patient was scheduled for
a follow-up visit. She presented to the emergency room one week
later with significant left flank pain and a fever of 102.1. Her WBC
count was 21,000 and serum creatinine was 1.2 (preop 0.9). A CT
scan of the abdomen and pelvis with and without intravenous con-
trast was ordered and left hydroureteronephrosis was seen down to
the level of the mid-ureter.
CASE MANAGEMENT
The patient was admitted and placed on intravenous antibiotics.
Urology was consulted and the patient was taken to the cystos-
copy suite where a cystoscopy and retrograde stent placement was
attempted but unsuccessful. No contrast was seen beyond the level of
the mid-ureter. The patient was taken to the interventional radiology
suite where a percutaneous nephrostomy tube was placed. Patient
improved clinically over the next 48 hours with IV antibiotics. An
anterograde nephrostogram was performed and a 2.0 cm stenotic
segment of ureter was visualized in the middle-third of the left ure-
ter. Iatrogenic injury was presumed and treatment options were dis-
cussed with the patient. After a full treatment course of antibiotics
for her pyelonephritis, the patient underwent a robotic ureteral reim-
plant with Psoas hitch and ureteral stent placement. Her percutane-
ous nephrostomy tube was removed 1 week later (as outpatient). The
ureteral stent was removed at 4 weeks postoperatively and patient
remained asymptomatic thereafter. At 4 months postreimplantation,
her serum creatinine was 0.9 and renal ultrasound showed a normal
left kidney without evidence of obstruction.
URETHRAL INJURIES
The most common urologic injury in surgery is the traumatic
foley catheter placement. It is essential to adequately lubricate the
entire foley and insert the catheter past the point at which urine
is returned into catheter tubing. Inserting the catheter in a male
to the inflation port can help prevent urethral injury. The usual
preoperative catheter is either a 16 french or 18 french catheter. It
is not necessary to inflate the balloon before placement as this will
increase the size and decrease the rigidity of the distal aspect of the
catheter. If catheter placement is unsuccessful, a trial of passage with
an 18 French coude’ tipped foley catheter is appropriate. Patient
dehydration secondary to bowel preparation can make it difficult
to determine proper placement if one only looks for urine return.
Placement of the entire foley catheter (to port)before inflation will
aid in proper placement. If still unsure, usage of a 60 cc catheter
tipped syringe to irrigate the bladder can confirm placement before
inflation of the balloon. Intraoperative urologic consultation for
cystoscopy and foley catheter placement should be performed if
the above measures fail. If cystoscopy cannot accurately deline-
ate urethral anatomy, a suprapubic catheter can be placed either
through a percutaneous or an open technique.
Artificial urinary sphincters (AUS) must be deactivated before
insertion of a foley catheter. Deactivation is a different mecha-
nism than the normal operating “on” and “off ” cycling. It is
the author’s experience that most patients do not know how to
deactivation their AUS beyond the normal cycling mode. This de-
activation must be performed before placement of a foley cath-
eter. Either a device representative or urologist can deactivate the
sphincter preoperatively as an outpatient or on the day of surgery.
A 12 French foley catheter can then be placed with lubrication
and care to ensure placement in the bladder before inflation of
balloon. Failure to deactivate the AUS can result in erosion of the
urinary sphincter by means of pressure necrosis between the foley
catheter and sphincter device. Removal of the catheter should be
done in standard fashion postoperatively. If unable to obtain uro-
logic consultation before or intraoperatively, a suprapubic cath-
eter can be placed either by open or percutaneous methods. Care
must be taken to avoid intraabdominal prosthetic components,
which are normally placed below the rectus muscle suprapubi-
cally. An inflatable penile prosthesis (IPP) should not pose any
additional difficulty in placing a urethral catheter if lubrication
and the aforementioned guidelines are adhered.
Urethral injuries are associated with extensive rectal neoplasm
or any inflammatory processes that alter surgical planes includ-
ing pelvic radiation. Urethral injuries are usually identified at
the time of surgery secondary to identification of the indwell-
ing foley catheter. Repair of a small urethral laceration can be
performed with absorbable 3–0 or 4–0 synthetic absorbable suture
(SAS) on a tapered needle. If the patient has had prior radiation or
there is poor tissue composition, placement of either an omental flap
or local tissue flap to support coverage of the repair is recommended.
Injuries not identified at surgery can present postoperatively as urine
drainage per rectum, pneumaturia, or fecaluria if fistula is present.
It can also present as a delayed urethral stricture with difficult void-
ing and bladder outlet obstruction. A retrograde urethrogram will
confirm the presence of a urethral stricture but must be done in the
bilateral oblique as well as anterior-posterior views. A retrograde
urethrogram (RUG) can be performed by affixing a 14-gauge angi-
ocatheter to a 60 cc syringe filled with standard water-soluble con-
trast. A RUG should be performed around an indwelling catheter
if already in place. If a radiographic enema is performed, water-
soluble contrast is preferred as it does not form concretions in the
bladder. Spontaneous closure of a urinary fistula is rare but a trial
6
improved outcomes in colon and rectal surgery
of conservative urinary diversion (foley catheter) for low-grade fis-
tulas is recommended for 4–6 weeks.
Urinary fistulas are staged according to location, size, and
patient’s history.(1)
Stage 1—low (<4 cm from the anal verge and non-irradiated) •
Stage 2—high (>4 cm for the anal verge and non-irradiated) •
Stage 3—small (<2 cm irradiated fistula) •
Stage 4—large (>2 cm irradiated fistula) •
Stage 5—large (ischial decubitus fistula) •
Enteric diversion by means of a diverting colostomy or ileos-
tomy is recommended for Stages 3–5. The choices for repair are
diverse and depend on local tissue integrity and staging. It is rec-
ommended to place a suprapubic catheter at the time of repair in
addition to a foley catheter for maximal drainage.(2) Transanal
rectal flap advancement can be used for Stage 1 fistulas or in com-
bination with other techniques for higher stage fistulas.(3) Other
techniques described include:
transanal-transphincteric approach (dorsal lithotomy anterior •
sphincterotomy).(4)
York Mason/transphincteric with rectal advancement flap (2, 5, •
6) (jack-knife posterior sphincterotomy)
Perineal approach (Jack knife or dorsal lithotomy).(7, 8)
•
Gracillus and Rectus Abdominus Flaps.(9, 10) •
Surgical selection is based on fistula stage and the experience of
the reconstructive surgeon. Higher stage fistulas and recurrences
normally require regional flaps and possibly even urinary diver-
sion.(11) Outcomes for surgically corrected rectourethral fistulas
are overall favorable with recurrences mostly dependent on stage
and appropriate choice in initial surgical treatment. Success rates
vary from >90% for low-grade fistulas to 70% for higher-grade
fistulas.(1–11) A retrograde urethrogram around foley catheter at
4–6 weeks postoperatively should be performed before urethral
catheter removal.
BLADDER INJURIES
The location of the bladder within the pelvis and its, close prox-
imity to the sigmoid colon and rectum predisposes the bladder to
injury during surgery of the colon and rectum. Iatrogenic injuries
to the bladder can be staged as:(12, 13)
Grade 1 : contusion, intramural hematoma, or partial thickness
•
laceration
Grade 2: extraperitoneal bladder wall laceration <2 cm •
Grade 3: extraperitoneal >2 cm or intraperitoneal <2 cm blad- •
der laceration
Grade 4: intraperitoneal bladder wall laceration >2 cm •
Grade 5: intra or extra peritoneal bladder wall laceration extend- •
ing into the bladder neck or trigone (near ureteral orifice)
Risk factors for bladder injury include any process that distorts
tissue planes and reduces surgical exposure.(14) This includes adhe-
sions or scarring from prior surgery, radiation, malignant infiltra-
tion, chronic inflammation, or infection. Injuries can be apparent
intraoperatively or present in a delayed fashion. Intra operative iden-
tification of the injury allows for immediate cystorraphy usually in
a two layer fashion. In open surgery, the mucosa is closed in a run-
ning fashion using a 3–0 SAS suture followed by a seromuscular run-
ning suture of 2–0 SAS. The bladder can then be irrigated to ensure
a watertight closure. In the laparoscopic setting, a running one layer
closure is performed using a 2–0 SAS to close all three layers of the
bladder. Care must be taken to ensure closure of the mucosal layer
in the laparoscopic one layer technique. Again, the bladder should
be irrigated to ensure a watertight closure. Repair can also differ
depending on the location of the injury. Anterior and dome injuries
can be repaired primarily as above. Posterior injuries involving the
trigone or near the ureteral orifices (possible Grade 5) dictate a more
thorough inspection of the bladder and an assurance of ureteral
integrity before closure. This is done through an anterior cystotomy
in the sagittal plane extending down toward the pubic symphisis.
This will allow placement of a Balfour or Bookwalter self-retaining
retractor and placement of bilateral ureteral open-ended catheters.
Giving the patient indigo carmine with Lasix can aid in identifica-
tion of the ureteral orifices. Closure of the posterior bladder injury
can then be done from the bladder lumen—closing the muscular
layer first using 2–0 SAS followed by closure of the mucosal layer
using 3–0 SAS. The anterior cystotomy is then closed as described
above. In cases where neoadjuvant radiotherapy has been used, an
interposition of omentum or perivesical fascia is prudent to decrease
the risk of fistula formation.
A delayed bladder injury will usually manifest in the early postop-
erative period, especially after removal of foley catheter. The injury
can present as drainage from surgical incision; increased output
from surgical drain; vaginal leakage; ileus; apparent oliguria; uri-
nary ascites with increasing BUN and serum creatinine secondary
to reabsorption of urine through parietal peritoneum—in the case
of an unrecognized intraperitoneal injury; pneumaturia or fecaluria
in the cases of an enterovesical or colovesical fistula. Delayed urine
leaks can be diagnosed radiographically by fluoroscopic cystogram
or the CT cystogram.(15) It is important when ordering a CT cysto-
gram that passive filling of the bladder from the upper tracts is not
the sole method of bladder opacification. A foley catheter should be
placed and the bladder filled in a retrograde fashion with 300–400
cc’s of water-soluble contrast before the scan.
The development of a colovesical or enterovesical fistula is a delayed
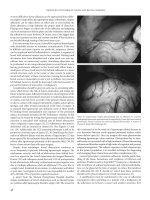
complication of cystotomy.(16–17) Abdominal-pelvic CT scan with
oral and/or rectal water-soluble contrast has a greater sensitivity than
cystoscopy in diagnosing an enterovesical fistula (Figure 36.1). The
most sensitive test to diagnose an enterovesical or colovesical fistula
is the poppy seed test.(17) A 1.25-ounce container of poppy seed is
mixed into a 12-ounce beverage o r a 6-ounce serving of yogurt and
orally ingested by each patient. Urine was visually inspected during
48 hours, during which identification of poppy seed in the urine was
a positive confirmatory test for gastrointestinal fistula to the urinary
tract. The sensitivity and specificity was 100%.(17) This test does
not provide anatomical information as in the case of the abdominal-
pelvic CT scan but it is a much more cost effective screening test in
patients with equivocal symptoms (5 dollars vs. over 600 dollars).
(17) When using Barium contrast, it is the authors recommendation
to empty the bladder after a fistulae is diagnosed as there have been
reports of Barium concretions within the bladder.
urologic complications of colorectal surgery
URETERAL INJURIES
Injury to the ureter is one of the most common intraoperative
urologic injuries in colorectal surgery. The incidence of iatro-
genic injury to the ureter is reportedly from 1 to 10%.(18–22)
Iatrogenic ureteral injuries are of 4 types: laceration, ligation,
devascularization, and thermal or energy related. Optimal treat-
ment is early recognition and repair of any ureteral injury.
Anatomy
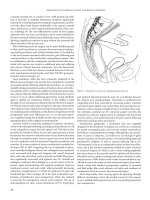
Iatrogenic ureteral injuries in colorectal surgery usually occur in
three distinct locations: at the takeoff of the inferior mesenteric
artery, where the infundibulopelvic ligament/uterine vessels crosses
the pelvic brim, and between the lateral rectal ligaments (Figure
36.2).(23) The course of the ureter begins posterior to the renal
artery and continues along the anterior edge of the psoas muscle.
The gonadal vessels cross the ureter from lateral to medial in this
region. The ureter next passes over the iliac vessels, generally
marking the bifurcation of the common iliac into internal and
external iliac arteries.(24) Of greatest importance to the surgeon
is that arterial branches to the abdominal ureter approach from
the medial direction whereas arterial branches to the pelvic ureter
approach from the lateral direction.(24) For the abdominal ure-
ter, these branches originate from the renal artery, gonadal artery,
abdominal aorta, and common iliac artery. After entering the pel-
vis, additional small arterial branches may arise from the internal
iliac artery or its branches, and also from the middle rectal and
vaginal arteries.(24)
The ureter will tend to adhere to the peritoneum during its
reflection rather than staying adherent to the Psoas muscle and
underlying tissue. The ureter can be identified by visualization
and by its peristaltic activity. Gentle pressure applied to the ureter
will frequently cause peristalsis—termed the Kelly sign. The right
ureter is adjacent to the cecum, terminal ileum, and the appendix.
The left ureter is related to the descending and sigmoid colon and
their mesenteries.
Prevention
Ureteral catheterization is used to aid in identification of the
ureters and to help identify ureteral injury, but catheters do not
prevent ureteral injury.
The clinical value of prophylactic ureteral catheter placement
before 162 laparoscopic segmental left and right colectomies was
assessed by Nam et al. There were no complications from place-
ment of ureteral catheters.(18) Postoperative urinary tract infec-
tion was not increased. Total operative time was increased by
11.3 minutes. The ureteral catheter group included more difficult
cases including patients with Crohn’s disease and diverticulitis.
There were no ureteral injuries in any of the one hundred sixty
two patients.(18) An earlier study deemed ureteral catheteriza-
tion necessary in 27.5% of patients when assessed in a stand-
ardized retrospective fashion.(22) There were 4 complications
presumably due to ureteral catheterization which included renal
colic, oliguria, and one case of anuria attributed to ureteral edema
after removal of the ureteral catheters.(20, 25) Chahin et al. stud-
ied lighted ureteral stents/catheters placed before laparoscopic
colectomy in 66 patients.(20) The most common complication
was self-limiting hematuria in 98.4% of patients with an aver-
age duration of 2.5 days for unilateral stenting and 3.3 days with
bilateral stenting.
It is the authors’ opinion that the choice for ureteral stenting is
a surgeon preference and depends on multiple variables includ-
ing complexity of case, anatomy, and experience—especially with
the laparoscopic approach in a hostile abdomen. With greater
experience, iatrogenic injury decreases. In a study by Larach et al.,
the incidence of conversions due to iatrogenic injuries showed a
decline from 7.3% in the early group to 1.4% in the latter expe-
rience group.(26) Once again ureteral catheters have not been
shown to decrease ureteral injuries but aid in identification of
the ureters and any iatrogenic ureteral injury. Ureteral catheters
Figure 36.1 Enterovesical fistula (arrow).
Figure 36.2 Anatomy of the ureter.