Improved Outcomes in Colon and Rectal Surgery part 42 pot
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (560.91 KB, 10 trang )
improved outcomes in colon and rectal surgery
can be used to aid in diagnosis of ureteral injury by retrograde
injection of methylene blue through the ureteral catheter. They
can also be used to place a retrograde wire under fluoroscopic
guidance for placement of an indwelling ureteral double-J stent
after a ligation/crush injury.
Types of Injury
Laceration
A laceration or transection of the ureter can usually be repaired
with primary anastamosis (ureteroureterostomy with spatulated
ends), ureteral stent, and placement of a closed suction drain in
the area of the repair (Figure 36.3).
Ligation
If a ligation injury is apparent intraoperativly, the clamp or tie can
be removed followed by ureteral stent placement for up to one
month. The patient should undergo repeat imaging either with a
renal ultrasound or intravenous pyelogram (IVP) at 3 months to
ensure a ureteral stricture has not developed. If the injury is not
identified until post operatively, a retrograde ureterogram and
stent placement or percutaneous nephrostomy tube placement
may be needed before surgical correction.
Devascularization
A devascularization injury will not be evident intraoperatively
and results from the sacrifice of the segmental ureteral blood supply.
Intraoperativley a devascularized ureter may appear discolored,
lack peristalsis, and may not bleed at a transected site. The irradi-
ated ureter is especially susceptible to this type of injury, as the
normal healthy ureter has numerous collaterals and is very resist-
ant to devascularization, even with extensive dissection. The anat-
omy of the blood supply to the ureter (as previously described)
should be known as the surgeon is carrying his dissection over
the pelvic brim.
Thermal
Thermal injuries will usually present in the early postoperative period
with either fistula or stricture formation. These injuries are repaired
in the same fashion as above depending on the location of the injury.
Many laparoscopic surgeons use alternatives to monopolar dissec-
tors because of the risk of thermal injury and delayed presentation of
injuries. Even with these newer technologies, collateral tissue dam-
age can be produced depending on the energy level and duration of
exposure. In animal models, use of the ultrasonic dissector (Ethicon
or USSC) at a level of 3 for <10 seconds per burst resulted in little to
no collateral tissue damage.(27) When using an ultrasonic dissector
at levels of 4 or 5, energy time should be reduced to <5 seconds to
prevent collateral damage due to spread of thermal.(27)
lOCatiOn dePendent rePair OF the
iatrOgeniC Ureteral injUrY
Repair of the injured ureter does not necessitate open conversion
if a urologist is available with advanced laparoscopic skills. The
basic principles of a ureteral anastamosis: a tension free anasta-
mosis; well-vascularized spatulated ends anastamosed over an
indwelling ureteral stent ; use of an absorbable suture material
4–0 or 5–0; and placement of a closed drain near the area of the
repair. Do not use nonabsorbable suture, as stone formation is
inherent with these nonabsorbable materials.
Proximal One Third
The boundaries of the proximal one-third ureter is from the ure-
teropelvic junction (level of the kidney) to the pelvic brim (sac-
roiliac joint on KUB). Repairs of injuries to the proximal ureter
depend on the length of the damaged segment. Simple spatulated
ureteroureterostomy with ureteral stent placement is the pre-
ferred method of repair if there is significant length of the unin-
jured ureter. A nephropexy can be performed to bring the kidney
caudad to allow a tension free anastamosis. In cases with long
segments of damaged ureters, a bowel interposition with tapered
ileum or an apendiceal interposition can be used (Figure 36.4).
At specialized centers, autotransplantation with reanastamosis to
the iliac vessels, and native more distal ureter can be performed.
Middle One Third
The preferred technique for mid-ureteral repair is ureteroureter-
ostomy, either laparoscopically or through the open technique.
Distal One Third
The procedure of choice for the lower one-third ureteral injury is
the ureteroneocystotomy. This may be accomplished primarily for
very distal ureteral injuries or may require a Psoas hitch or Boari
flap for patients with small capacity bladders and injuries near the
iliac vessels.(24) Care must be taken to maintain a tension free
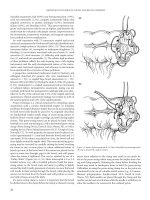
Figure 36.3 Ureteroureterostomy. (A) Spatulation of ureteral margins and placement
of running locked sutures. Preferred technique. (B) Oblique anastomosis.
(A)
(B)
urologic complications of colorectal surgery
anastamosis. This can usually be accomplished with a Psoas Hitch
(Figure 36.5). The bladder is mobilized by ligating the superior
vesical pedicle on the contralateral side of the injury. It is prudent
to locate the contralateral ureter and ensure its integrity before
this maneuver. The bladder can then be opened through an ante-
rior cystotomy and then secured to the Psoas muscle and tendon
using several 0–0 SAS sutures through the seromuscular layer of
the bladder. Care must be taken not to include the genitofemoral
nerve which is located within the belly of the Psaos muscle. Suture
should be placed in a linear fashion inline with the fascicles of the
muscle to prevent underlying nerve entrapment. The ureter can then
be tunneled by passing a clamp from the lumen through all layers of
the bladder and then withdrawn with the distal aspect of the proxi-
mal salvaged ureter. The ureter should then be widely spatulated and
interrupted mucosal stitches (4–0 SAS) should be used circumferen-
tially to create the neo-orifice. A ureteral stent can also be placed. The
anterior cystotomy is then closed as previously described. A closed
suction drain and foley catheter is then left in place.
The Boari flap is another effective yet more complex method
for replacing an extensive loss of the distal and mid-ureter. A flap
of the anterior bladder wall is raised in a rectangular fashion and
affixed to the Psoas muscle in same fashion as a Psoas hitch. The
ureter is tunneled through the most proximal portion of the flap
and a neo-orifice is created as previously described. The bladder
flap is then tabularized and closed in a two-layer fashion using
running 3–0 SAS to close the mucosa followed by closure of the
seromuscular layer using 2–0 SAS (Figure 36.6).
The final option is the transureteroureterostomy. The surgeon
tunnels the injured ureter under the posterior peritoneum over-
lying the great vessels. The allows a spatulated end to side anasta-
mosis of the injured ureter to the patient’s native uninjured ureter
(Figure 36.7).
renal injUries
Direct renal injury is a rare occurrence in colorectal surgery.
McAnich et al. have reported that 90% of renal injuries can be
managed without nephrectomy.(28) Though this work does not
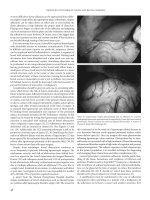
Figure 36.4 Ureteral replacement by ileum. Left colon retracted medially. Ileum
brought through a hiatus in the colonic mesentary. Ileal ureter is in retroperitoneal
position.
Figure 36.5 Psoas bladder hitch. Mobilized bladder being anchored to psoas
muscle and the ureter is reimplanted.
improved outcomes in colon and rectal surgery
address iatrogenic injuries, the principle of renal salvage should
be applied. Every attempt to evaluate the extent of the injury as
well as an assessment of the entire genitourinary tract should be
done before undertaking repair. A one shot IVP can confirm con-
tralateral renal function. This can be done by giving the patient
2 ml of contrast per kg up to a maximal of 150 ml IV. An on the
table KUB is then done 10 minutes later. Simple palpation of the
contralateral kidney does not ensure function. The literature is
full of anomalous solitary kidneys which were removed neces-
sitating dialysis or transplantation.(29, 30) Pelvic kidneys have an
anomalous blood supply generally arising from multiple arteries
along the aorta and iliac vessels. A total of 10% are solitary and
may easily be taken for a pelvic mass as they are not reniform
and have a discoid shape.(23) If caliceal or renal pelvis injury is
suspected, intravenous methylene blue or indigo carmine can be
administered.
Once the injury is well defined, repair can be decided. Minor
renal lacerations or penetrating injuries may be repaired primarily
with absorbable sutures and retroperitonealized with perinephric
fat, omentum, or hemostatic materials. Hilar control is paramount
if an attempt at repair is to be performed. If the injury is to the
collecting system or renal parenchyma and the ensuing blood loss
is able to be managed by pressure and hemostatic agents alone, a
ureteral stent and foley catheter can be placed from below and the
area drained with a closed suction to prevent urinoma formation.
Conservative management is optimal as renorraphy and explora-
tion can lead to unnecessary nephrectomy. If a major vascular
injury occurs and the patient’s intraoperative condition permits,
every attempt should be made to reestablish vascular integrity.
Bladder dYsFUnCtiOn
The reported incidence of difficulty in reestablishing micturation
ranges from 15 to 25% after low anterior resection and up to 50%
after abdominoperineal resection.(31) A thorough understand-
ing of the neuroanatomy of the pelvis and the technique of total
mesorectal excision (TME) and autonomic nerve preservation
(ANP) can enable both local tumor control and preservation of
autonomic nerve structures thus reducing the risk of urogenital
dysfunction.(34, 35) Favorable oncologic outcomes have been
Figure 36.6 Boari or bladder
flap procedure. (A) Creation
of tapered bladder flap, based
posteriorly. (B) Submucosal
ureteral reimplantation. (C)
Closure of bladder flap.
(A) (B) (C)
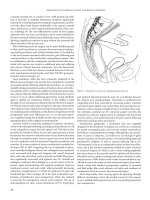
Figure 36.7 Transureteroureterostomy. Right-to-left, showing retroperitoneal
tunnel anterior to the great vessels.
reported for these nerve sparing techniques.(35–39) APR, when
performed in accordance with the principles of TME and ANP,
ensures the greatest likelihood of resecting all regional disease
urologic complications of colorectal surgery
while preserving both urinary and sexual function.(39) Locally
advanced tumors and preoperative chemotherapy and radia-
tion can make identification of the autonomic nerves and plexus
more difficult and sometime impossible.(34) The most common
sequela from autonomic nerve damage during surgery of the
colon and rectum is detrusor denervation and areflexia. This nor-
mally requires clean intermittent catheterization, foley catheter
placement, or suprapubic tube placement depending on the over-
all dexterity and functional status of the patient. Damage to the
pudendal nerve or its branches from Alcocks canal can result in
weakening of the striated urinary sphincter with resultant stress
urinary incontinence and intrinsic sphincter deficiency.
Detrusor function (bladder contractility) is predominantly
mediated by the parasympathetic nervous system, namely the
pelvic nerve.(33) These parasympathetic fibers originate from
the spinal cord at the S2–S4 level. Pelvic nerve branches are
redundant within the pelvis. The main trunks to the bladder and
proximal urethra course in the visceral pelvic fascia, also called
the posterior endopelvic fascia.(33) These preganglionic auto-
nomic fibers course alongside the superior vesical vasculature to
synapse with postganglionic autonomic fibers within the bladder
wall. Multiple pelvic preganglionic nerves pass laterally from the
pelvic floor over the rectal fascia investments en route medially to
the bladder (Figure. 36.8).(33)
Sympathetic innervation to the bladder arises at the level of
L2–L4 with a presynaptic fiber to the sympathetic ganglion adja-
cent to the spinal cord. Synapse occurs in the ganglion and a long
post ganglionic fibers travels through the pelvis to innervate the
bladder. Through different end receptors located within the blad-
der, the sympathetic component of the autonomic nervous sys-
tem helps to cause relaxation of the bladder body (compliance
for storage) and contraction of the trigone and bladder neck at
resting/storage states.
Somatic motor innervation to the striated pelvic floor muscu-
lature and sphincter arises from the S2–S4 level and travels via
the pudendal nerve through Alcocks canal. The perineal branches
of the pudendal nerve follow the perineal artery into the super-
ficial pouch to supply the ischiocavernosus, bulbospongiosus,
and transverse perinei muscles. Some branches continue anteri-
orly to supply sensation to the posterior scrotum and perineum.
Additional perineal branches pass deep to the perineal membrane
to supply the levator ani and striated urethral spincter.(40)
In the study by Junginger on total mesorectal excision (TME),
identification of the pelvic autonomic nerves was complete in
72%, partial identification in 10.7%, and not at all in 17.3% of
patients.(34) Univariate analysis showed that the case number
(experience), gender (males > females), and T stage (T1-2 vs.
T3-4) exerted an independent influence on the achievement of
complete pelvic nerve identification. In this series of 150 patients
with adenocarcinoma of the rectum, identification and preser-
vation of the autonomic nerves was achieved in a majority of
patients and led to the prevention of urinary dysfunction (4.5%
vs. 38.5%; p < 0.001).(34)
Management of the postoperative patient with bladder dys-
function after colorectal surgery includes teaching clean inter-
mittent catheterization (CIC) and having the patient return for
full urodynamic evaluation around 2–3 months postoperatively.
Urodynamics can be a combination of fluoroscopic pressure/flow
studies with EMG tracings and sometimes urethral pressure profil-
ing. It may take up to 6 months for bladder function to return to its
new baseline and CIC may be a lifelong therapy. CIC is performed
with a 12–14 french low friction catheter every 4–6 hours and the
duration can be adjusted based on the storage pressures and bladder
capacity at the time of urodynamic evaluation. There are no drugs
with acceptable pharmacokinetics and side-effect profiles that have
been shown to clinically increase contractility in the bladder.
In a meta-analysis, Branagan et al., reviewed the colorectal
surgery literature on suprapubic catheter placement followed
by voiding trial versus urethral catheter placement and standard
trial of voiding postoperatively.(31) They found favorable results
for the suprapubic catheter in terms of incidence of urinary tract
infection, and a shorter magnitude and duration of pain and dis-
comfort. The ability to simply clamp and unclamp the suprapubic
catheter makes management and voiding trials relatively simple
especially in patients unable to perform CIC or those at especially
high risk for postoperative bladder dysfunction. Suprapubic
catheters are particularly useful if autonomic nerves have to be
removed during radical pelvic surgery, because normal voiding
may be difficult to reestablish and may take several months to
recover. In the select patient with voiding dysfunction and delayed
recovery, suprapubic catheter placement results in less morbidity
and patient discomfort than urethral catheterization.(32)
seXUal dYsFUnCtiOn
In the urologic community, an emphasis on postoperative sexual
function has arisen from studies by Walsh on the anatomic ret-
ropubic prostatectomy with preservation of the neurovascular
bundles that contribute to erectile function.(41) Most recently,
post operative penile rehabilitation is being performed in mul-
tiple settings with a theoretical benefit of reducing the time of
neuropraxia to the penis and prevention of apoptosis induced
atrophy. Although no standardization exists with these rehabili-
tation programs, patients are very interested and at the authors’
institution this is discussed preoperatively. Sexual dysfunction
has long been associated with rectal surgery in both male and
female patients. In male patients, erectile dysfunction is reported
in 5 to 65% of patients and ejaculatory dysfunction is reported in
Figure 36.8 Innervation of lower urinary tract
improved outcomes in colon and rectal surgery
14 to 69%.(43) Damage to the sacral splanchnic nerve (parasym-
pathetic) or the hypogastric nerve (sympathetic) during surgery
is the propsed mechanism of injury.(43)
Sexual dysfunction is a broad term that encompasses failure of
arousal, erection, orgasm, ejaculation, and emission. Complaints
from patients after radical pelvic surgery are usually mixed.
Erection is parasympathetically mediated and is governed by
impulses traveling along the nervi ergentes (S2–S4).(41) The pel-
vic plexus is located retroperitoneally on the lateral surface of the
rectum 5–11 cm from the anal verge with its midpoint located at
the tip of the seminal vesicles. The preganglionic fibers from the
nervi ergentes coalesce on the pelvic wall with contributions from
the sympathetic fibers and from the hypogastric plexus (T10–L4).
Damage to the sympathetic plexus will result in problems with
ejaculation including retrograde ejaculation or anejaculation.
In a study by Henderson et al., eighty one women and 99 men
that had undergone curative rectal cancer surgery were given a
validated sexual function questionnaire.(42) Thirty-two percent
of women and 50% of men were sexually active compared with
61% and 91% preoperatively. Twenty-nine percent of women
and 49% of men reported that “surgery made their sexual lives
worse”. Specific sexual problems in women were libido 41%,
arousal 29%, lubrication 56%, orgasm 35%, and dyspareunia
46%. In men complaints were impotence/erectile dysfunction
84%, libido 47%, orgasm difficulty 41%, and ejaculation diffi-
culties 43%. Patients seldom remembered discussing sexual risks
preoperatively and were seldom referred or treated for symptoms
postoperatively. Sexual dysfunction should be discussed with rec-
tal cancer patients, and when appropriate, efforts to prevent and
treat sexual dysfunction should be instituted.(42)
In a study of patients by Nam et al., on patients undergoing TME
and ANP for rectal carcinoma, factors that most affected postop-
erative sexual dysfunction were age older than 60 (sexual desire, p =
0.019), time period within 6 months of surgery (erectile function,
p = 0.04), and lower rectal cancer (erectile function p = 0.02).(43)
In the urologic literature, penile rehabilitation is started at approxi-
mately 1 month postoperatively with evidence suggesting that lack
of natural erections during this period of time produces cavern-
osal hypoxia.(44) Prolonged periods of cavernosal hypoxia induce
fibrosis, which later increases the incidence of venous leak and thus
potentiates long-term or permanent erectile dysfunction
In consultation with a urologist, sexual dysfunction in the man
can be treated with many different modalities. For erectile dys-
function, oral phosphodiesterase inhibitors, intraurethral vasoac-
tive suppositories, intracavernosal injections, vacuum errection
devices, and implantable devices are all options. For ejaculatory
dysfunction in a patient desiring pregnancy, semen may be col-
lected from the bladder in the case of retrograde ejaculation.
Sympathomimetic agents may also be used. For refractory cases,
electro-vibratory ejaculation can be performed at specialized cent-
ers. It is important to discuss sexual function with the patient both
pre and postoperatively as there are many therapeutic options that
have been shown to be very satisfactory for both partners.
artiFiCial deViCes
Thousands of artificial urinary sphincters (AUS) and inflatable
penile prosthesis (IPP) have been implanted worldwide for the
treatment of stress urinary incontinence and erectile dysfunction,
respectively (Figure 36.9). The IPP has one to three components,
while the AUS has three components. The three component sys-
tems have a reservoir, pump, and cuff or prosthesis that is inter-
connected with reinforced tubing. These devices are silicone but
develop a capsule around them after implantation. The reservoir
is typically placed suprapubically in the space of Retzius. One
should make every attempt to refrain from entering this capsule
and to prevent contamination of these silicone devices. If con-
tamination occurs, either device removal or salvage therapy with
copious antibiotic irrigation is recommended, preferably the lat-
ter. The risk of device contamination, post operative infection,
and damage to the tubing necessitating device removal or reop-
eration should be discussed with the patient preoperatively. It is
the authors practice to be very conservative in patients with AUS,
and we recommend all patients have their device de-activated by
a urologist familiar with the AUS before placement of a urethral
catheter. There are numerous reports of patients “turning off” their
own AUS when in reality they only cycle them, followed by urethral
catheterization at the time of surgery and the result is a device ero-
sion through the urethra. This is a medico-legal issue that usually
can be averted with a preoperative consultation with a urologist.
The FDA approved sacral neuromodualtor is the Interstim
device manufactured by Medtronic Corp.(45) It is approved for
use in patients with refractory urgency and frequency or nonob-
structive nonneurogenic urinary retention. A tined lead is placed
through the S3 foramen and an implanted generator is placed in
a pocket created in the gluteal area/upper hip. The manufacturer
recommends against using electrocautery near the generator and
to not perform a MRI on any patients with the Interstim device.
Figure 36.9 Artificial urinary sphincter (AVS-800); American Medical Systems
Inc, Minnetonka, MN. (A) reservoir. (B) cuff. (C) pump.
(A)
(B)
(C)
urologic complications of colorectal surgery
It is the authors practice to turn off the device with a Medtronic
supplied magnet before any radical pelvic operation. In small
patients, appropriate padding must be applied to the area of the
implanted generator. MRI is contraindicated although there has
been at least one study to show deactivation of the device before
MRI to be safe.(46, 47)
reFerenCes
1. Rivera R, Barboglio PG, Hellinger M, Gousse AE. Staging
rectourinary fistulas to guide surgical treatment. J Urol 2007;
177: 586–8.
2. Fengler SA, Abcarian H. The York Mason approach to repair
of iatrogenic rectourinary fistulae. Am J Surg 1997; 173:
213–7.
3. Dreznik Z, Alper D, Vishne TH, Ramadan E. Rectal flap
advancement-a simple and effective approach for the treat-
ment of rectourethral fistula. Colorectal Dis 2003; 5: 53–5.
4. Culkin DJ, Ramsey CE. Urethrorectal fistula: transanal, trans-
sphincteric approach with locally based pedicle interposition
flaps. J Urol 2003; 169: 2181–3.
5. Mason AY. Surgical access to the rectum-a transsphincteric
exposure. Proc R Soc Med 1970; 63 suppl: 91–4.
6. Crippa A, Dall’oglio MF, Nesrallah HJ et al. The York-
Mason technique for recto-urethral fistulas. Clinics 2007; 62:
699–704.
7. Yousseff AH, Fath-Alla M, El-Kassaby AW. Perineal subcuta-
neous dartos pedicled flap as a new technique for repairing
urethrorectal fistula. J Urol 1999; 161: 1498–500.
8. Visser BC, McAninch JW, Welton ML. Rectourethral fistulae:
the perineal approach. JACS 2006; 195: 138–43.
9. Zmora O, tulchinsky H, Gur E et al. Gracilis muscle transpo-
sition for fistulas between the rectum and urethra or vagina.
Dis Colon Rectum 2006; 49(9): 1316–21.
10. Bruce RG, El-Galley RE, Galloway NT et al. Use of rec-
tus abdominis muscle flap for the treatment of complex
and refractory urethrovaginal fistulas. J Urol 2000; 163:
1212–5.
11. Elliott SP, McAninch JW, Chi T et al. Management of severe
urethral complications of prostate cancer therapy. J Urol
2006; 176; 2508–13.
12. Moore EE, Cogbill TH, Jurkovich GJ et al. Organ injury scal-
ing III: Chest wall, abdominal vascular, ureter, bladder, and
urethra. J Trauma 1992; 33: 337–9.
13. Armenakas NA, Pareek G, Fracchia JA. Iatrogenic bladder
perforations: longterm followup of 65 patients. JACS 2004;
198: 78–82.
14. Van Goor H. Consequences and complications of peritoneal
adhesions. Colorectal Dis 2007; 9(Suppl 2): 25–34.
15. Deck AJ, Shaves S, Talner L, Porter JR. Computerized tomog-
raphy cystography for the diagnosis of traumatic bladder
rupture. J Urol 2000; 164: 43–6.
16. Jarrett TW, Vaughan ED Jr. Accuracy of computerized
tomography in the diagnosis of colovesical fistula secondary
to diverticular disease. J Urol 1995; 153: 44–6.
17. Kwon EO, Armenakas NA, Scharf SC et al. The poppy seed
test for colovesical fistula: big bang, little bucks! J Urol 2008;
179: 1425–7.
18. Nam YS, Wexner SD. Clinical value of prophylactic ureteral
stent indwelling during laparoscopic colorectal surgery.
J Korean Med Sci 2002; 17: 633–5.
19. Larach SW, Gallagher JT. Complications of laparoscopic sur-
gery for rectal cancer: Avoidance and management. Semin
Surg Oncol 2000; 18: 265–8.
20. Chahin F, Dwivedi AJ, Paramesh A et al. The implications
of lighted ureteral stenting in laparoscopic colectomy. JSLS
2002; 6: 49–52.
21. Scala A, Huang A, Dowson HM, Rockall TA. Laparoscopic
colorectal surgery - results from 200 patients. Colorectal Dis
2007; 9: 701–5.
22. Fry et al. Iatrogenic Ureteral Injury. Arch Surg 1983; 118:
454–7
23. Perlmutter AD, Retik AB, Gauer SB. Anomalies of the upper
urinary tract. In Harrison JH, Gittees RF, Perlmutter AD,
et al., eds. Campbell’s Urology, 4th ed. Philadelphia: WB
Saunders, 1979: 1309–98.
24. Anderson, Kabalin, Cadeddu et al. Surgical anatomy of the
Retroperitoneum, Adrenals, Kidneys, and Ureters. 34–37.
Campbell-Walsh Urology, Elsevier Inc. 9th Edition; 2007
25. Kyzer S, Gordon PH. The prophylactic use of ureteral
catheters during colorectal operations. Am Surg 1994; 60:
212–6.
26. Larach SW, Patankar SK, Ferrara A et al. Complications of lap-
aroscopic colorectal surgery. Analysis and comparison of early
vs. latter experience. Dis Colon Rectum 1997; 40: 592–6.
27. Emam TA, Cuschieri A. How safe is high-power ultrasonic
dissection. Ann Surg 2003; 237: 186–91.
28. McAninch JW, Carroll PR, Klosterman PW et al. Renal
reconstruction after injury. J Urol 1991; 145: 932–7.
29. Granat M, Gordon T, Issaq E, Shabtai M. Accidental punc-
ture of a pelvic kidney: a rare complication of culdocentesis.
Am J Obstet Gynecol 1980; 138: 233–5.
30. Zusmer NR Maturo V, Stern M. Pelvic kidney masquerading
as adnexal mass. Rev Interam Radiol 1980; 5: 95–6.
31. Branagan GW, Moran BJ. Published evidence favors the
use of suprapubic catheters in pelvic colorectal surgery. Dis
Colon Rectum 2002; 45: 1104–8.
32. Chaudhri S, Maruthachalam K, Kaiser A et al. Successful
voiding after trial without catheter is not synonymous with
recovery of bladder function after colorectal surgery. Dis
Colon Rectum 2006; 49: 1066–70.
33. Hollabaugh RS Jr, Steiner MS, Sellers KD et al. Neuroanatomy
of the pelvis: implications for colonic and rectal resection.
Dis Colon Rectum 2000; 43: 1390–7.
34. Junginger T, Kneist W, Heintz A. Influence of identification
and preservation of pelvic autonomic nerves in rectal cancer
surgery on bladder dysfunction after total mesorectal exci-
sion. Dis Colon Rectum 2003; 46: 621–8.
35. Shirouzu K, Ogata Y, Araki Y. Oncologic and functional
results of total mesorectal excision and autonomic nerve-
preserving operation for advanced lower rectal cancer. Dis
Colon Rectum 2004; 47:1442–7.
36. Saito N, Koda K, Nobuhiro K et al. Nerve-sparing surgery for
advanced rectal cancer patients: special reference to Dukes C
patients. World J Surg 1999; 23: 1062–8.
improved outcomes in colon and rectal surgery
37. Yamakoshi H, Ike H, Oki S et al. Metastasis of rectal cancer
to lymph nodes and tissues around the autonomic nerves
spared for urinary and sexual function. Dis Colon Rectum
1997; 40: 1079–84.
38. Moriya Y, Sugihara K , Akasu T, Fujita S. Importance of
extended lymphadenectomy with lateral node dissection
for advanced lower rectal cancer. World J Surg 1997; 21:
728–32.
39. Enker WE, Havenga K, Polyak T et al. Abdominoperineal resec-
tion via total mesorectal excision and autonomic nerve preser-
vation for low rectal cancer. World J Surg 1997; 21: 715–20.
40. Brooks. Anatomy for the Lower Urinary Tract and Male
Genitalia. 2;p69. Campbell-Walsh Urology, 9th Edition,
Elsevier Inc; 2007.
41. Walsh PC, Schlegel PN. Radical pelvic surgery with preserva-
tion of sexual function. Ann Surg 1988; 208: 391–400.
42. Hendren SK, O’Connor BI, Liu M et al. Prevalence of male
and female sexual dysfunction is high following surgery for
rectal cancer. Ann Surg 2005; 242: 212–23.
43. Kim NK, Aahn TW, Park JK et al. Assessment of sexual and
voiding function after total mesorectal excision with pelvic
autonomic nerve preservation in males with rectal cancer.
Dis Colon Rectum 2002; 45: 1178–85.
44. Raina R, Pahlajani G, Ararwal A, Zippe CD. Early penile
rehabilitation following radical prostatectomy: Cleveland
clinic experience. Int J Impot Res 2008; 20: 121–6.
45. Interstim Device Trademarked by Medtronic Corp.
46. Holley et al. MRI Following Interstim Therapy. Presentation
at SESAUA Annual Meeting; 2007.
47. Elkelini MS, Hassouna MM. Safety of MRI at 1.5 Tesla in
Patients with Implanted Sacral Nerve Neurostimulator. Eur
Urol 2006; 50: 311–6.
Index
5-Aminosalicylates (5-ASA) 336–7
5-FU 300
Bevacizumab 301
Cetuximab 301
indications for 301
Irinotecan-Containing Regimens 301
with leucovorin 300
with levamisole 300
Oxaliplatin-Containing Regimens 300–1
see also chemotherapy
6-methyl-mercaptopurine (6-MMP) 337–8
6-methylprednisolone 337
6-Thioguanine Nucleotide (6-TGN) 337–8
AAST grades 378
abdominal colectomy
ileorectal anastomosis with 322
abdominal CT 377
abdominal discomfort 367
abdominal radiography 97
bowel obstruction and dilatation 97–8
cecal volvulus 99
pneumoperitoneum 97
sigmoid volvulus 98–9
toxic megacolon 98
abdominal surgery 263
functional outcomes 267
oncologic outcomes 263
preoperative evaluation 263–4
surgical technique 264
patient-centered outcomes 267–8
surgical outcomes
anastomotic bleeding 265–6
anastomotic complications 264
anastomotic leak 264–5
anastomotic stricture 265
autonomic nerves injury 267
pelvic hemorrhage 266
splenic injury 266–7
ureteral injuries 267
abdominal trauma
colostomy closure
outcomes of 384
antibiotic therapy 385–6
blunt colon injury 383
diagnosis 376
epidemiology 375
military perspective 384–5
physical exam
computed tomography 377
diagnostic peritoneal lavage (DPL) 376
injury scale 377–8
laparoscopy 377
peritoneal sign 376
seat belt sign 376
ultrasound 376–7
preoperative assessment 375–6
retained fragments 386–7
World War I 375
World War II 375
abdominal wall contouring 356–7
caring 356–7
flap dissection 356, 357
abdominoperineal resection (APR) 278
closure, methods of 280–1
complications 281
abscess 281
intraoperativ hemorrhage 281
non-healing wound and
perineal sinus 282
perineal wound complications 282
postoperative hemorrhage 281–2
epidemiology 278
evisceration 283
operative technique 278–80
perineal hernia 283
positioning 278
leg positioning 279
preparation 278
risk factors 282–3
carcinoma 282
fecal contamination 282–3
radiation therapy 282
sexual and urinary function 280
treatment 283
abscess 340
absorbable regenerated cellulose 266
Acticon
TM
Neosphincter device 231
acute anal fissure 200
calcium channel antagonists versus nitrates
acute pilonidal disease 216
Adalimumab 338
adhesions 47–9
grading system for bowel 47, 47
adjuvant chemotherapy
for T3 302–3
stage II and IV colon cancer 301–2
stage III colon cancer 302
Advanced Trauma Life Support principles 375
adynamic ileus 110
Agency for Healthcare Research and Quality (AHRQ) 164
aging 8
Altmeier procedure 241–2
alvimopan 367
American College of Surgeons (ACS) 3, 164
American Heart Association (AHA) 134
American Joint Committee on Cancer (AJCC) 301
American Society of Anesthesiologists (ASA) 2, 19
classification 2
American Society of Clinical Oncology (ASCO) 164, 165
American Society of Colon and Rectal Surgeons (ASCRS) 14, 15, 27,
164, 200, 251, 253
aminosalicylates 336
anal dilation 207
index
anal encirclement 242, 243–4
anal fissure
acute 200–1
chronic 201
classification 199
conservative therapy 200
diagnosis 200
pathophysiology 199
posterior and anterior 199
surgery therapy 206–8
see also acute anal fissure; chronic anal fissure
anal fistula 174
Crohn’s disease 192
diagnosis 184–5
etiology 183–4
HIV-positive patient 193
incontinence 190–1
non-surgical management 192–3
recurrence 189–90
surgical therapy
advancement flap 186–7
anal fistula plug 188–9
extrasphincteric fistulas 186
fibrin glue 187–8
fistulotomy 186
incision and drainage 185–6
seton placement 186
anal fistula plug 188–9
Anal Fistula Plug™ (AFP) 188
anal skin tags 175
anal sphincter 226–7
anal sphincter injury 389
life-threatening injuries 389
anal sphincteroplasty 229
anal stenosis 174, 208–210
anal ultrasonography 91–2
anastomosis 380
anastomotic complications, postoperative 56
bowel preparation , mechanical 58
case management 56
clinical presentation 59–60
considerations 56–7
dehiscence 56
diagnosis 60
diagnosis 64–5
management 60–2
asymptomatic 60–1
colocutaneous fistula 62
leak with associated abscess 61–2
leak without abscess 61
peritonitis 62
omental pedicle 58
operative intervention 62–3
colostomy creation 62
leaking anastomosis
@4:exteriorization of 63
@4:leaving, in place 63
@4:repeat anastomosis after resection 63
@4:resection of 62
@4:short and long-term implications of 63
pelvic drains 59
proximal diversion 57–8
radiation 58–9
stricture 63–4
techniques 58
treatment 64–5
anastomotic leak 264, 324
anemia 1
anesthesia 1
awareness 23
local anesthesia 19
Monitored Anesthetic Care (MAC) 20–1
regional anesthesia 21–3
anoderm 172
anorectal foreign bodies
bedside extraction 389
initial assessment 388–9
operative removal 389
sexual implements 388
anorectal manometry 117, 364
anorectal physiology tests (ARP) 228–9
limitations of 87
case management 87
anal ultrasonography 91–2
constipation 92
balloon expulsion test 94
biofeedback 94
colonic transit studies 93
defecography 93
MRI 94
small bowel transit 94
electromyography
concentric needle 89–90
single fiber 90
surface 90
fecal continence 87
fecal incontinence, investigations for
biofeedback 92
electomyography 91
manometry 87–8, 88
ultrasound 92
pudendal nerve terminal motor latency 90, 90
rectal capacity and sensation 89
Recto-Anal inhibitory reflex 89
sphincter pressure measurement 88–89
anorectal sexually transmitted disease 156–7
anorectal varices 175
antegrade colonic enema 233–4
anthraquinones 367
antibiotic prophylaxis 14
antibiotic therapy
abdominal trauma 385–6
anti-Saccharomyces cerevisiae mannan antibodies (ASCA) 332
anti-Saccharomyces cerevisiae 327
apocrine sweat glands 221
appendicitis 117
areflexia 401
argon plasma coagulation 306
arteriovenous sinusoids 178
artificial bowel sphincter 231
artificial urinary sphincters (AUS)
components 402
traumatic foley catheter placement 395
ASA Closed Claims Project 21
ASCRS see American Society of Colon and Rectal Surgery
Aspirin 135
index
ATLS principles see Advanced Trauma Life Support principles
AUS see artificial urinary sphincters
Australian Safety and Efficacy Register of New Interventional
Procedures-Surgical 232
autonomic nerves injury 267
AVASTIN® 301
azathioprine (AZA) 337
Babcock clamp 140
bacteremia 22
bacterium Clostridium botulinum see botulinum toxin
Bacteroides 386
balloon expulsion test 94, 364
balloon proctography 117
balsalazide 337
barium enema 112–15, 367
Crohn’s disease 114–15
diverticulitis 115
diverticulosis 115
double contrast 112–13
limitations of 113–14
lymphoma 115
single contrast 112–13
ulcerative colitis 114
Bascom II procedure 220
Bascom operation 219
bedside extraction 389
benzodiazepines 133
Bevacizumab (AVASTIN®) 301
biofeedback therapy
failure of 368
for constipation 94
for fecal incontinence 92
limitations 92
pelvic floor dyssynergia
dyssynergic-type constipation 368
treatment of 367–8
on slow-transit constipation 368
Bioplastique® 233
bispectral index (BIS) 23
bladder dysfunction
autonomic nerve structures 400
innervations 401
oncologic outcomes 400–1
postoperative patient
clean intermittent catheterization (CIC) 401
resection 400
total mesorectal excision 401
bladder flap see Boari flap
bladder injury
delayed bladder injury 396
iatrogenic injury
stages 396
risk factor 396
two layer technique 396
bleeding complications 69
bleeding 178, 265–6
blunt colon injury (BCI) 383
Boari flap 399
body temperature and oxygenation 28
botulinum toxin (BT) 205
vs nitrates 206
vs placebo 205–6
randomized controlled trials 205
sphincterotomy 206
bowel function
adhesions, grading system for 47, 47
preparation 33–4
mechanical 58
status 34
bowel obstruction and dilatation 97–8
Bridgewater 135
brooke ileostomy
proctocolectomy with 319–20
budesonide 337
bupivicaine 19
calcium channel antagonists 203–5
vs nitrates 204
vs placebo 203–4
vs sphincterotomy 204
calcium polycarbophil 179
Cancer Care Outcomes Research and Surveillance Consortium”
(CanCORS) 165
cardiovascular disease 3–4
functional status, assessment of 4
preoperative cardiac evaluation
Goldman risk model 3
Lee index 3
care paths
benefits of 80
case management 79
challenges and concerns 83–4
in colon and rectal surgery 79
development of 80–3
fast track surgery 84–5
guidelines 79
implementation of 80–3
institutional experience 83
objectives of 79
outcome measures, defining and improving 80
realistic expectations 84
cathartic colon” 367
caudal anesthetic 21
cecal volvulus 99
Centers of Medicare and Medicaid Services (CMS) 161, 162
central nervous system (CNS) 20, 227
central neuraxial blockade 21–2
caudal 21
contraindications 22
epidural 21–2
heparin 22
spinal 21
cerebrospinal fluid (CSF) 21
cerebrovascular accidents (CVA) 8
Cetuximab (ERBITUX®) 301
Chance fractures” 376
chemotherapy 287, 300, 314
with colorectal cancer 300–1
future directions 303–4
indications and timing 301–3
side effects 303
chlamydia trachomatis infections 157
chronic anal fissure 201
botulinum toxin (BT) 205
vs nitrates 206