Chemistry part 4, Julia Burdge,2e (2009) pdf
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (15.68 MB, 26 trang )
58
CHAPTER
2 Atoms, Molecules, and Ions
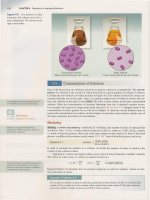
Figure 2.15 An electron
is
transferred from the sodium atom to the
chlorine atom, giving a sodium ion and
a chloride ion. The oppositely charged
ions are attracted to each other and
form a solid lattice.
Sodium atom (Na)
Chlorine atom
(CI)
Loses an
electron
Gains an
electron
Electron transfer
Sodium ion (Na
+)
lOe-
Chloride ion
(CI
- )
Sodium chloride crystal (NaCl)
charge on the anion and a subscript for the anion that is numerically equal to the charge
on
the
cation.
If
the charges are numerically equal, then no subscripts are necessary.
Let
us consider some examples.
Potassium Bromide The potassium ion (K+) and the bromide ion
(Br-)
combine to form the
ionic compound
potassium bromide.
The
sum
of
the charges is 1 +
(-
1) = 0, so no subscripts are
necessary. The formula is KEr.
Zinc
Iodide
The
zinc ion (Zn
2
+) and the iodide ion
(r-)
combine to form zinc iodid
e.
The sum
of
the charges
of
one
Zn
2+
ion and one
1-
ion is
+2
+ (- 1) = +
1.
To make the charges add up
to zero,
we
multiply the
-1
charge
of
the anion by 2 and add the subscript "2" to the symbol for
iodine. Thus, the formula for zinc iodide is ZnI
2
.
Ammonium Chloride The cation is
NHt
and the anion is cr. The
sum
of
the charges is 1 +
(-1)
=
0,
so the ions combine in a
1:
1 ratio and the resulting formula is
NH
4
Cl.
Aluminum Oxide
The
cation is
AI3+
and the anion is 0
2
The
following diagram can be used to
determine the subscripts for this compound:
AI3+ 0
2
-
The
sum
of
the charges for aluminum oxide is 2( +
3)
+ 3( - 2) =
0.
Thus, the formula is
A1
2
0
3
.
Calcium Phosphate The cation is
Ca2+
and the anion is
PO~
The
following diagram can be
used to determine the subscripts:
Ca
2
+
The
sum
of
the charges is
3(+2)
+
2(-3)
=
0.
Thus, the formula for calcium phosphate is
Ca
3
(P04h
When
we
add a subscript to a polyatomic ion,
we
must first
put
parentheses around the
ion's
formula to indicate that the subscript applies to all the atoms in the poly atomic ion.
Naming Ionic Compounds
An ionic compound is named using the name
of
the cation followed by the name
of
the anion,
eliminating the word
ion from each. Several examples were given earlier in the Formulas
of
Ionic Compounds section. Other examples are sodium cyanide (NaCN), potassium permanganate
(KMn04), and ammonium sulfate
[(NH4hS04]. Unlike the naming
of
molecular compounds, no
Greek prefixes are use
d.
For example, Li
2
C0
3
is lithium carbonate, not dilithium carbonate, even
though there are two lithium ions for every carbonate ion.
Prefixes are unnecessary because the
•
How
Are
Oxoanions
and
Oxoacids
Named?
Oxoanions are polyatomic anions that contain one
or
more oxy-
gen atoms and one atom (the
"central atom")
of
another element.
Examples include the chlorate
(CIO}
),
nitrate (
NO
}), and sulfate
(SO~
-
)
ions. Often, two or more oxoanions have the s
ame
central
atom
but
different numbers
of
0 atoms (e.g.,
NO
} and
N0
2
).
Starting with the oxoanions whose names end in -ate,
we
can
name these ions as follows:
no net charge.
For
example, the formulas
of
oxoacids
ba
sed on
the nitrate
(NO}) and sulfate (SOi- ) ions are
HN0
3
and H
2
S0
4
,
respectively. The names
of
oxoacids are derived from the names
of
the corresponding oxoanions using the following guidelines:
1.
An acid
ba
sed on an -ate ion is called . . . ic acid. Thus,
HCl0
3
is called chloric acid.
2. An acid
ba
sed
on
an -ite ion is called . . . ous acid.
Thus,
HCI0
2
is called chlorous acid.
1.
The
ion with one more 0 atom than the -ate ion is called
the
per .
ate ion. Thus, CIO} is the chlorate ion, so CIO
';-
is the perchlorate io
n.
2.
The
ion with one less 0 atom than the -ate anion is called
the
-ite ion. Thus,
CI0
2
is the chlorite ion.
3.
Prefixes in oxoanion names are retained in the names
of
the
corresponding oxoacids. Thus,
HCI0
4
and HCIO are called
perchloric acid and hypochlorous acid, respectively.
3.
The
ion with two fewer 0 atoms than the -ate ion is called
the
hypo .
ite ion.
Thu
s, CIO- is the hypochlorite ion.
At
a minimum, you must commit to memory the formulas
and charges
of
the oxoanions whose names
end
in
-at
e so that
you can apply these guidelines when necessary.
Many oxoacids, such as
H
2
S0
4
and H
3
P0
4
,
are
polyprotic-
meaning that they have more than one ionizable hydrogen atom.
In these cases, the names
of
anions in which one or more (but not
all)
of
the hydrogen ions have been removed must indicate the
number
of
H ions that remain, as shown for the anions derived
from phosphoric acid:
In addition to the simple acids discussed in Section 2.6, there
is another important class
of
acids known as oxoacids, which ion-
ize to produce hydrogen ions and the corresponding oxoanion
s.
The
formula
of
an oxoacid can
be
determined by adding enough
H+ ions to the corresponding oxoanion to yield a formula with
Sample Problem 2.7
Name the following species: (a) BrO; , (b) HCO
:;-,
and (c) H
2
C0
3
.
H
3
P0
4
H
2
PO
"
HPO
~-
PO
~
-
Phosphoric acid
Dihydrogen phosphate ion
Hydrogen phosphate ion
Phosphate ion
Strategy Each species
is
either an oxoanion or an oxoacid. Identify the "reference oxoanion" (the
one with the
-ate
ending) for each, and apply the rules to determine appropriate names.
Setup (a) Chlorine, bromine, and iodine (members
of
group 7 A) all form analogous series
of
oxoanions with one to four oxygen atom
s.
Thus, the reference oxoanion is bromate (BrO
:;-
), which
is analogous to chlorate
(ClO
:;-)
. In parts (b) and (c), HCO
:;-
and H
2
C0
3
have one and two more
hydrogens, respectively, than the carbonate ion
(Co j-
).
Solution (a) BrO; has one more 0 atom than the bromate ion (BrO
:;-),
so
Br0
4 is the perbromate
ion. (b)
coj
- is the carbonate ion. Because HCO
:;-
has one ionizable hydrogen atom, it is called the
hydrogen carbonate ion. (c) With two ionizable hydrogen atoms and no charge on the compound,
H
2
C0
3
is carbonic acid.
Practice Problem A Name the following species: (a)
HErO
, (b)
HS0
4
,
and (c) H
2
C
2
0
4
.
Practice Problem B Name the following species: (a)
HI0
3
,
(b)
HCr0
4, and (c) HC
2
0 ; .
~I ~"
Determine the formula of sulfurous acid.
Strategy
The
-ous
ending in the name
of
an acid indicates that the acid is derived from an oxoanion
ending in
-it
e.
Determine the formula and charge
of
the oxoanion, and add enough hydrogens to
make a neutral formula.
Setup The sulfite ion is SO j
Solution The formula
of
sulfurous acid is H
2
S0
3
.
Practice Problem A Determine the formula
of
perbromic acid. (Refer to the
inf
ormation in Sample
Problem 2.7.)
Practice Problem B Determine the formula
of
chromic acid.
59
60
CHAPTER 2 Atoms,
Mo
lecules, and Ions
Figure 2.16 Steps for naming
molecular and ionic compounds.
Think
About
It
Be
careful not to
confuse the subscript in a formula
with the charge on the metal ion. In
part (c), for example, the subscript
on
Fe
is 2, but this is
an
iron (III)
compound.
.
~
!z-
Molecular
.s
l
Binary compounds
of
nonmetals
~
;z.
Naming
• Use pre
fi
xes for
both elements
pre
sent.
(
Pr
efix
mOI1O-
usually omitted for
the flrst element.)
• Add - ide to the
root
of
second
element.
Compound
.s(
)z.
Ionic
S
~
Cation: metal
or
NHt
Anion: monatomic
or
polyatomic
s!.
Z
s
."z.
Cation has Cation has more
a
ni
y
one
charge .
than one charge.
• Alkali metal cations • Other metal cations
• A I
kal
i ne earth metal
ca
ti
ons
• A
o-
+
AI
3+
Cd
2+
Zn
2
+
to'
, ,
Naming
• Na
me
metal firs
t.
•
If
monatomic anion,
add
- ide to root
of
element name.
•
If
polyatomic anion,
use name
of
anion.
Naming
•
Name
metal
fi
rs
t.
• Specify charge
of
metal cation
wi
th
Roman numeral
in parentheses.
•
If
monatomic anion,
add
-ide
to
root
of
element name.
•
If
po
lyatomic anion,
use
name
of
anion.
ions have
known
charges. Lithium ion always has a charge
of
+
1,
and carbonate ion always has
a charge
of
-2.
The
only ratio
in
which they
can
combine
to
form
a neutral
compound
is two
Li
+
ions for every one
CO
~
-
ion. Therefore, the
name
lithium carbonate is sufficient to convey the
compound's
empirical formula.
In cases where a metal cation
may
have
more
than
one
possible charge, recall that
the
charge
is indicated
in
the
name
of
the ion with a
Roman
numeral
in
parentheses. Thus, the
compounds
FeCI
2
and FeCI
3
are
named
iron(
Il
) chloride
and
iron(IIl) chloride, respectively. (These are
pro
-
nounced
"iron
-two chloride"
and
"iron
-three chloride.")
Figure
2.16 summarizes the steps
for
naming
molec
u
lar
and
ionic
compounds
.
Sample
Problem
s 2.9 and 2.10 illustrate
how
to name ionic
compounds
and write formulas
for ionic
compounds
based
on
the
information given in
Figure
2.16 and Tables 2.8 and 2.9.
Sample Problem 2.9
Name
the following ionic compounds: (a)
MgO,
(b) AI(OH)3' and (c)
FeiS04h
Strategy
Begin by identifying the cation and the anion in
each
compound, and then
combine
the
names for each, eliminating the word
i OI1.
Setup
MgO
contains
Mg
2+ and 0
2
- , the magnesium ion and the oxide ion; AI(OH)3 contains Al
3+
and
OH
- , the aluminum ion and the hydroxide ion; and Fe2(S04)3 contains
Fe
3+
and
SO
~
-
,
the
iron (III)
ion
and the sulfate ion. We know that the iron in
F~(S0
4
) 3
is iron(III),
Fe
3+
, because it is
combined with the sulfate ion in a
2:3 ratio.
Solution
(a) Combining the cation and anion names, and eliminating the word ion from each
of
the individu
al
ions' names, we
get
magnesium oxide as the name
of
MgO; (b) AI(OH)3
is
aluminum
hydroxide;
and (c)
Fe
2(
S0
4)3 is iron(J/l) sulfate.
Practice Problem A
Name
the following ionic compounds: (a)
Na
2
S0
4, (b) Cu
(N0
3
)2,
(c)
Fe2
(C0
3h
Practice Problem B
Name
the following ionic compounds: (a) K2
Cr
20 7, (b)
Li
2
C
2
0
4
,
(c)
CuN0
3
.
SECTION 2.7 Ions and Ionic Compounds
61
Deduce
the
formulas
of
the following ionic compounds: (a)
mercury
(I) chloride, (b) lead(
II
)
chromate, and (c)
potassium
hydrogen
pho
sphate.
Strategy
Identify
the
ions in
each
compound,
and
determine their ratios
of
combination
using
the
charges
on
the
cation
and anion in each.
Setup
(a) Mercury(I) chloride is a
combination
of
Hg
~
+
and CI- . [Mercury(I) is
one
of
the few
cations listed in Table 2.9.]
In
order
to
produce
a neutral
compound
,
the
se two ions
must
combine
in
a 1:2 ratio. (b) Lead(II)
chromate
is a
combination
of
Pb
2+
and
CrO
~
-
.
These
ions
combine
in
a
1:
1
ratio. (c)
Potassium
hydrogen
phosphate
is a c
ombination
of
K+ and
HPO
~
-
.
The
se ions
combine
in
a
2:
1 ratio.
I Solution
The
formulas
are
(a)
Hg
2
Cl
b
(b)
PbCr0
4,
and
(c) K
2
HP0
4
·
Practice Problem A
Deduce
the
formulas
of
the following ionic
compound
s: (a) lead(II) chloride,
(b)
magnesium
carbonate,
and
(c)
ammonium
phosphate.
Practice Problem B
Deduce
the
formula
s
of
the following ionic
compounds:
(a) iron(III) sulfide,
(b) mercury(II) nitrate,
and
(c)
potassium
sulfite.
~ ~
Hydrates
Hydrates are compounds that have a specific number
of
water molecules within their solid struc-
ture. In its normal state, for example, each unit
of
copper(II) sulfate has five water molecules asso-
ciated with it. The systematic name for this compound is copper(II) sulfate pentahydrate, and its
formula
is
written as CUS04 .
SH
2
0.
The water molecules can be driven off by heating. When this
occurs, the resulting compound is
CUS04, which is sometimes called anhydrous copper(II) sulfate;
anhydrous means that the compound no longer has water molecules associated with it. Hydrates
and the corresponding anhydrous compounds often have distinctly different physical and chemical
properties (Figure 2.17).
Some other hydrates are
BaCl
2
'
2H
2
0
L
iCl·
H
2
0
.\1gS0
4
. 7H
2
0
Sr(
N0
3
h .
4H
2
0
Barium chloride dihydrate
Lithium chloride monohydrate
Magnesium sulfate heptahydrate
Strontium nitrate tetrahydrate
Familiar Inorganic Compounds
So
me compounds are better known by their common names than by their systematic chemical
names. Familiar examples are listed
in
Table 2.10.
Think
About
It
Make sure that the
charges sum to zero
in each compound
formula.
In part (a), for example,
Hg
~
+
+
2Cl-
= (
2+
) + 2
(-
1) = 0;
in part (b),
(+
2) + (- 2) =
0;
and
in
part (c),
2(+1)
+
(-2)
=
O.
Figure 2.17 CUS04 is white.
The
pentahydrate, CUS04 .
5H
2
0,
is blue.
62
CHAPTER
2 Atoms, Molecules, and Ions
Formula
H
2
0
NH3
CO
2
NaCI
N
2
0
CaC0
3
NaHC0
3
MgS0
4
·7H
2
0
Mg(OHh
Common
Name
Water
Ammonia
Dry
ice
Salt
Laughing gas
Marble, chalk, limestone
Baking soda
Epsom
salt
Milk
of
magnesia
Systematic
Name
Dihydrogen monoxide
Trihydrogen nitride
Solid carbon dioxide
Sodium
chloride
Dinitrogen monoxide
Calcium carbonate
Sodium
hydrogen carbonate
Magnesium sulfate heptahydrate
Magnesium hydroxide
Checkpoint
2.7
Ions and Ionic Compounds
2.7.1
2.7.2
2.7.3
What
is the correct name
of
the
compound
PbS0
4
?
a)
Lead
sulfate
b) Lead(I) sulfate
c)
Lead(II) sulfate
d)
Monolead sulfate
e)
Lead monosulfate
What
is the correct formula for the
compound iron
(ill)
carbonate?
a)
FeC0
3
b)
Fe
3
C0
3
c)
Fe2C0
3
d) Fez(C0
3
)3
e)
Fe
i
C0
3
)2
Which
of
the following is the correct
formula for nitrous acid?
a)
HNO
b)
HN
2
0
c)
N
2
0
d)
HN0
2
e)
HN0
3
•
2.7.4
2.7.5
2.7.6
What
is the formula
of
nickel(II) nitrate
hexahydrate?
a)
NiN0
3
·6H
2
O
b)
Ni
2
N0
3
•
6H
2
O
c)
Ni(N0
3
)2
. 6H
2
O
d)
NiN0
3
•
12H
2
O
e)
Ni(N0
3
h . 12H
2
O
What
is the correct formula for sodium
nitride?
a)
NaN
b)
NaN
3
c)
Na
3N
d)
NaN0
3
e)
NaN0
2
What is the correct n
ame
of
the
compound
Hg
2Cr04?
a)
Mercury(I) chromate
b)
Mercury(II) chromate
c)
Mercury dichromate
d)
Dimercury chromate
e)
Monomercury chromate
APPLYING
WHAT
YOU'VE LEARNED 63
Applying
What
You've Learned
Although iron is an essential element, it is also a potentially toxic substance. Hemochromato-
sis is one
of
the most common hereditary disorders, causing "iron overload" or the storage
of
excess iron in the tissues and organs. Individuals with hemochromatosis often must undergo
periodic phlebotomy (removal
of
blood) in order to remove excess stored iron, which would
otherwise cause irreversible damage to internal organs including the liver and kidneys. Those
who have a tendency to store too much iron are advised to avoid combining iron-rich foods
with substances that enhance iron absorption, such as ascorbic acid (vitamin C).
Iron
~
~
_
_
~~_
I_.n.
Ascorbic acid
Because
of
iron's toxicity, iron supplements are potentially dangerous, especially
to
children.
In
fact, iron poisoning is the
most
common
toxicological emergency in
young children due in
part
to the resemblance many iron supplements
bear
to candy.
Most
vitamins
that
contain iron are sold with childproof caps to help prevent accidental
overdose.
The
Food
and
Drug
Administration (FDA) requires supplements containing
more
than
30
mg
of
iron
per
dose
to
be
sold
in
single-dose blister packs to
make
it
more
difficult for a child to consume a dangerous amount.
Problems:
a) Iron has
four
naturally occurring isotopes:
54
Fe
(53.9396 amu),
56
Fe
(55.9349 amu),
57Fe
(56.9354 amu), and
58Fe
(57.9333 amu).
For
each
isotope,
detennine
the number
of
neutrons in the nucleus.
[
~~
Sample
Problem
2.1]
b) Calculate the average atomic mass
of
iron
given that the natural abundances
of
the
four isotopes are 5.845, 91.754, 2.119, and
0.282 percent, respectively.
[
~~
Sample
Problem
2.2]
c) Write the molecular formula for ascorbic acid (see the ball-and-stick model).
[
~~
Sample
Problem
2.3]
d) DeteIlIIine the empirical fOImula
of
ascorbic acid.
[
~~
Sample
Problem
2.6]
e) Write the fOIIllula for ferrous sulfate [iron
(II)
sulfate].
[
~
Sample
Problem
2.10]
•
64
CHAPTER
2 Atoms, Molecules, and Ions
CHAPTER SUMMARY
Section 2.1
o Dalton's atomic theory states that all matter is made up
of
tiny
indivisible, immutable particles called
atoms. Compounds form,
moreover, when atoms
of
different elements combine in fixed ratios.
According to the
law
of
definite proportions, any sample
of
a given
compound will always contain the same elements in the same mass
ratio.
o The law
of
multiple proportions states that
if
two elements can form
more than one compound with one another, the mass ratio
of
one will
be related to the mass ratio
of
the other by a small whole number.
o
The
law
of
conservation
of
mass states that matter can be neither
created nor destroyed.
Section 2.2
o On the basis
of
Dalton's atomic theory, the
atom
is the basic unit
of
an element. Studies with radiation indicated that atoms contained
subatomic particles, one
of
which was the electron.
o Experiments with radioactivity have shown that some atoms give off
different types
of
radiation, called alpha
(ex)
rays, beta
(f3)
rays, and
gamma
(y)
rays. Alpha rays are composed
of
ex
particles, which are
actually helium nuclei. Beta rays are composed
of
f3
particles, which
are actually electrons. Gamma rays are high-energy radiation.
o
Most
of
the mass
of
an atom resides in a tiny, dense region known as
the
nucleus.
The
nucleus contains positively charged particles called
protons and electrically neutral particles called neutrons. Protons
and neutrons are referred to collectively as nucleons. The charge
on
a
proton is equal in magnitude but opposite in sign to the charge on an
electron. The electrons occupy the relatively large volume around the
nucleus. A neutron has a slightly greater mass than a proton, but each
is almost
2000 times as massive as an electron.
Section 2.3
o
The
atomic
number
(Z) is the number
of
protons in the nucleus
of
an atom. The atomic number determines the identity
of
the atom.
The
mass
number
(A) is the sum
of
the protons and neutrons in the
nucleus.
o Atoms with the same atomic number but different mass numbers are
called
isotopes.
Section 2.4
o
The
periodic table arranges the elements in rows (periods) and
columns
(groups orfamilies). Elements in the same group exhibit
similar properties.
o All elements fall into one
of
three categories: nonmetal, metal, or
metalloid.
o Some
of
the groups have special names including alkali metals
(Group
lA,
except hydrogen), alkaline earth metals (Group 2A),
chalcogens (Group 6A), halogens (Group 7A), noble gases (Group
8A), and
transition elements
or
transition metals (Group IB and
Groups 3B-8B).
Section 2.5
o
Atomic
mass is the mass
of
an atom in atomic mass units. One atomic
mass
unit
(amu), is exactly one-twelfth the
ma
ss
of
a carbon-12 atom.
o
The
periodic table contains the average atomic mass (sometimes
called the
atomic weight)
of
each element.
Section 2.6
o A molecule is an electrically neutral group
of
two
or
more atoms.
Molecules consisting
of
just
two atoms are called diatomic.
Diatomic molecules may
be
homonuclear
(just one kind
of
atom)
or
heteronuclear (two kinds
of
atoms
).
In general, molecules containing
more than two atoms are called
polyatomic.
o A chemical
formula
denotes the composition
of
a substance. A
molecular
formula
specifies the exact numbers
of
atoms in a molecule
of
a compound. A structural
formula
shows the arrangement
of
atoms
in a substance.
o
An
allotrope is one
of
two or more different forms
of
an element.
o Molecular compounds are named according to a set
of
rules, including
the use
of
Greek prefixes to specify the number
of
each kind
of
atom
in the molecule.
o Binary compounds are those that consist
of
two elements.
An
acid is
a substance that generates hydrogen ions when it dissolves in water.
An
ionizable hydrogen atom is one that can
be
removed in water to
become a hydrogen ion, H+.
o Inorganic compounds are generally those that do not contain carbon.
Organic compounds contain carbon and hydrogen, sometimes
in combination with other elements.
Hydrocarbons contain only
carbon and hydrogen.
The
simplest hydrocarbons are the alkanes. A
functional
group is a group
of
atoms that determines the chemical
properties
of
an organic compound.
o
Empiricalformulas
express, in the smallest possible whole numbers,
the ratio
of
the combination
of
atoms
of
the elements in a compound.
The empirical and molecular formulas
of
a compound
mayor
may not
be identical.
Section 2.7
o
An
ion is an atom or group
of
atoms with a net charge. An atomic ion
or a monatomic ion consists
of
just one atom.
o
An
ion with a net positive charge is a cation. An ion with a net
negative charge is
an
anion. An ionic
compound
is one that consists
of
cations and anions in an electrically neutral combination. A three-
dimensional array
of
alternating cations and anions is called a lattice.
o Ionic compounds are named using rules similar
to
those for molecular
compounds.
In
general, prefixes are not used to denote the number
of
ions
in
the names
of
ionic compounds.
o Polyatomic ions are those that contain more than one atom chemically
bonded together.
Oxoanions are polyatomic ions that contain one
or
more oxygen atoms.
o Oxoacids are acids based on oxoanions. Acids with more than one
ionizable hydrogen atom are called
polyprotic.
o Hydrates are compounds whose formulas include a specific number
of
water molecules.
KEyWORDS
Acid,
51
Alkali metal, 45
Alkaline earth metal, 45
Alkane,
51
Allotrope,
48
ex
particle,
38
Alpha
(ex)
ray, 38
Anion,
55
Atom, 36
Atomic ion, 55
Atomic mass,
46
Atomic
mass
unit (amu),
46
Atomic
number
(2),
40
Atomic weight,
46
f3
particle,
39
B
eta
(13)
ray,
39
Binary,
49
Cation, 55
Chalcogens, 45
Chemical formula,
48
Diatomic
molecule, 48
Electron,
37
Empirical formula, 51
Family,
44
Functional group, 51
Gamma
('I)
rays,
39
Group,
44
Halogens, 45
Heteronuclear, 48
Homonuclear,
48
Hydrate,
61
Hydrocarbon,
51
Inorganic
compounds
, 51
Ion, 55
,
.
QUESTIONS AND PROBLEMS
QUESTIONS
AND
PROBLEMS
Ionic
compound
, 55
Ionizable hydrogen atom, 51
Isotope,
41
Lattice,
57
Law
of
conservation
of
mass,
36
Law
of
definite proportions, 35
Law
of
multiple proportions, 35
Mass
number
(A), 40
Metal,
44
Metalloid,
44
Molecular formula, 48
Molecule,
47
Monatomic
ion, 55
Neutron,
40
Noble gases, 45
Nonmetal,
44
Nucleons, 41
-
Nucleus,
40
Organic compounds, 51
Oxoacid,
59
Oxoanion,
59
Period
,
44
Periodic table,
44
Poly atomic ion, 56
Polyatomic molecule,
48
Polyprotic acid,
59
Proton,
40
Radiation,
36
Radioactivity, 38
Structural formula, 48
Transition elements,
45
Transition metals, 45
65
========================
=====-==~
Section 2.1: The Atomic Theory
Review Questions
2
.1
What
are the hypotheses on which
Dalton's
atomic theory is
based?
2.2 State the laws
of
definite proportions and multiple proportions.
lllustrate each with an example.
Section 2.
2:
The Structure
of
the
Atom
R
eview
Questions
2.3
1.6
Define the following terms: (a)
ex
particle, (b)
13
particle,
(c)
'I
ray, (d) X ray.
Name
the types
of
radiation known to
be
emitted
by
radioactive
elements.
Compare
the properties of the following:
ex
particles, cathode
rays, protons, neutrons, and electrons.
Describe the contributions
of
the following scientists to
our
knowledge
of
atomic structure: J. J. Thomson, R. A. Millikan,
Ernest
Rutherford, and James Chadwick.
.7 Describe the experimental
basi!>
for believing that the nucleus
occupies a very small fraction
of
the volume
of
the atom.
Problems
The
diameter
of
a neutral helium
atom
is about 1 X
10
2
pm.
Suppose that
we
could
line up helium atoms side by side in
contact with
one
another. Approximately how many atoms would
it
take to
make
the distance 1
cm
from end to end?
•
2.9 Roughly speaking, the radius
of
an atom
is
about 10,000 times
greater than that
of
its nucleus.
If
an
atom
were magnified so
that the radius
of
its nucleus became
2.0
cm, about the size
of
a
marble,
what
would
be
the radius
of
the atom in miles?
(1
mi = 1609 m.)
Section 2.3: Atomic Number, Mass Number, and Isotopes
Review Questions
2.10
2.11
2.12
2.13
Use
the helium-4 isotope to define atomic
number
and
mass
number.
Why
does knowledge
of
the atomic
number
enable us to
deduce the
number
of
electrons present in an atom?
Why
do
all atoms
of
an
element
have the
same
atomic number,
although they
may
have different
mass
numbers?
What
do
we
call atoms
of
the
same
elements with different mass
numbers?
Explain the meaning
of
each term
in
the
symbol1X.
Problems
2.14
What
is the mass
number
of
an iron
atom
that
ha
s 28 neutrons?
2.15
2.16
2.17
Calculate the
number
of
neutrons
of
239
Pu.
For
each
of
the following species, determine the
number
of
protons and the number
of
neutrons
in
the nucleus:
~
He,
iHe,
24 25M 48
T'
7
9B
19
5
Pt
I2Mg,
12
g,
22
1, 35
r,
78
Indicate the
number
of
protons, neutrons,
and
electrons
in
each
of
th
II'
. 15N
33
S
63C
84
S
I30B
186W 202H
e
10
owrng species: 7 ,
16
,2
9 u,
38
r, 56
a,
74
, 80 g
66
2.18
CHAPTER 2
Atoms,
Molecules,
and
Ions
Write the appropriate
symb
ol for each
of
the following isotopes:
(a)
Z =
11
, A = 23; (b) Z = 28, A =
64
, (c) Z = 50, A = 115,
(
d)Z=
20
, A = 42.
2.19 Write the appropriate symbol for each
of
the following isotopes:
(a)
Z = 74, A = 186; (b) Z = 80, A = 201, (c) Z = 34, A =
76
,
(d)
Z = 94, A = 239.
2.20 Determine the mass number
of
(a) a boron atom with 5 neutrons,
(b) a magnesium atom with 14 neutrons, (c) a bromine
atom
with
46 neutrons, and (d) a mercury atom with 116 neutrons.
2.21 Determine the mass number
of
(a) a fluorine
atom
with 10
neutrons, (b) a sulfur atom with 18 neutrons, (c) an arsenic atom
with
42
neutrons, and (d) a platinum atom with 114 neutrons.
2.
22
The
following radioactive isotopes are used in medicine for
imaging organs, studying blood circulation, treating cancer,
and so on. Give the number
of
neutrons present
in
each isotope:
1
98
Au
47
Ca
6O
Co
18F
12
5
1
131
1
42
K
43
K
24
N
32
p
85
S 99
T
, , , , , , , , a, ,
f , C.
Section 2.4: The Periodic Table
Review Questions
2.23
2.24
2.25
2.26
2.27
What
is the periodic table, and what is its significance
in
the
study
of
chemistry?
State two differences between a metal and a nonmetal.
Write the names and symbols for four elements in each
of
the
following categories: (a) nonmetal, (
b)
metal, (c) metalloid.
Give two examples
of
each
of
the following: (a) alkali metals,
(b) alkaline earth metals, (c) halogens, (d) noble gases, ( e)
chalcogens,
(f)
transition metals.
The
explosion
of
an atomic
bomb
in
the atmosphere releases
many radioactive isotopes into the environment.
One
of
the
isotopes is
9O
Sr. Via a relatively short food chain,
it
can
enter
the human body. Considering the position
of
strontium in the
periodic table, explain why it is particularly harmful to humans.
Problems
2.28 Elements whose names
end
with -ium are usually metals; sodium
is
one
example. Identify a nonmetal whose name also
end
s with
•
-tum.
2.29 Describe the changes in properties (from metals to nonmetals
or
from nonmetals to metals) as
we
move (a) down a periodic group
and (b) across the periodic table from
left to right.
2.30 Consult a handbook
of
chemical and physical data (ask your
instructor where you can locate a copy
of
the handbook) to find
(a) two metals less dense than water, (b) two metals more dense
than mercury, (c) the densest known solid metallic element, and
(d) the densest known solid nonmetallic element.
2.31 Group the following elements
in
pairs that you would expect to
show similar chemical properties: K, F,
P, Na, Cl, and N.
2.32
2.33
Group the following elements in pairs that you would expect to
show similar chemical properties: I, Ba,
0,
Br
, S, and Ca.
Write the symbol for each
of
the following biologically
important elements in the given periodic table: iron (present
in hemoglobin for transporting oxygen), iodine (present in the
thyroid gland), sodium (present
in
intracellular and extracellular
fluids), phosphorus (present
in
bones and teeth), sulfur (present in
proteins), and magnesium (present in chlorophyll molecules).
lA
D2A
3A
4A
SA
6A
7A
3B
4B
5B
6B
7BI
8B
1
lB
2B
Section 2.5: The
Atomic
Mass Scale and Average
Atomic
Mass
Review Questions
8A
2.34
What
is an atomic
ma
ss unit?
Why
is
it
necessary to introduce
such a unit?
2.35
2.36
What is the
ma
ss (
in
amu)
of
a carbon-12 atom?
Why
is the
atomic mass
of
carbon
li
sted as 12.01 amu in the table on the
inside front cover
of
this book?
Explain clearly what is meant by the statement
"The
atomic mass
of
gold is 197.0 amu."
2.37
What
information would you need to calculate the average
atomic mass
of
an element?
Problems
2.38
The
atomic masses
of
n
Cl
(75.53 percent) and n
Cl
(24.47
percent) are 34.968 and 36.956 amu, respectively. Calculate the
average atomic
ma
ss
of
chlorine.
The
percentages in parentheses
denote the relative abundances.
2.39
The
atomic masses
oe
04
Pb (1.4 percent), 2
06Pb
(24.1
percent),207Pb (22.1 percent), and 2
08
Pb
(52.4 percent) are
203.973020,
205.974440,206
.975872, and 207.976627 amu,
respectively. Calculate the average atomic mass
of
lead. The
percentages in parentheses denote the relative abundances .
2.40
The
atomic
ma
sses
of
20
3
T
l and 20s
TI
are 202.972320 and
204.974401 amu, respectively. Calculate the natural abundances
of
these two isotopes.
The
average atomic mass
of
thallium is
204.4 amu.
2.41
The
atomic masses
of
6Li and 7
Li
are 6.0151 amu and
7.0160 amu, respectively. Calculate the natural abundances
of
these two isotopes.
The
average atomic mass
of
Li is 6.941 amu.
2.42
What
is the mass in
gram
s
of
13.2 amu?
2.43 How many atomic mass units are there in 8.4
g?
Section 2.6: Molecules
and
Molecular
Compounds
Review Questions
2.44
2.45
What is the difference between an atom and a molecule?
What
are allotropes? Give an example.
How
are allotropes
different from isotopes?
2.46
2.47
Describe the two commonly used molecular models.
What
does a chemical formula represent? Determine the ratio
of
the atoms in the following molecular formula
s:
(a) NO, (b) NCI
3
,
(c) N
2
0
4
, (d) P406.
2.48 Define molecular formula and empirical formula.
What
are the
similarities and differences between the empirical formula and
molecular formula
of
a compound?
2.49 Give an example
of
a case in which two molecules have different
molecular formulas but the
sa
me
empirical formula.
2.50
What
is the difference between inorganic compounds and organic
compounds?
2.51 Give one example each for a binary compound and a ternary
compound. (A ternary compound is
one
that contains three
differen t elements.)
2.52 Explain why the formula
HCl
can represent two different
chemical systems.
Problems
2.53
For
each
of
the following diagrams, determine whether it
represents diatomic molecules, poly atomic molecules, molecules
that are not compounds, molecules that are compounds,
or
an
elemental form
of
the substance.
(a) (b) (c)
_.54
For
each
of
the following diagrams, determine whether it
represents diatomic molecule
s,
polyatomic molecules, molecules
that are not compounds, molecules that are compounds,
or
an
elemental form
of
the substance.
~
L"
1_
57
: -8
(a)
(b)
(c)
Identify the following as elements
or
compounds: NH
3
,
N
2
,
S8,
NO, CO, COlo
Hz,
SOz·
Give two examples
of
each
of
the following: (a) a diatomic
molecule containing atoms
of
the
sa
me
element, (b) a diatomic
molecule containing atoms
of
different elements, (c) a
polyatomic molecule containing atoms
of
the same element, (d) a
polyatomic molecule containing atoms
of
different elements.
Write the empirical formulas
of
the following compounds:
(a) CzN
z
, (b) C
6
H
6
, (c) C
9
H2o,
(d) P
4
0
lO
,
(e) BzH6.
Write the empirical formulas
of
the following compounds:
(a)
A12Br6,
(b) Na2SZ04, (c)NzOs, (d)K2Cr207.
2.59
2.60
2.61
2.62
2.63
2.64
F
QUESTIONS
AND
PROBLEMS
Write the molecular formula
of
alanine, an amino acid used
in protein synthesis.
The
color codes are black (carbon), blue
(nitrogen), red (oxygen), and white (hydrogen).
Write the molecular formula
of
ethanol.
The
color codes are:
black (carbon), red (oxygen), and white (hydrogen).
Name
the following binary molecular compounds: (a) NCI
3
,
(b) IF
7
, (c) P406, (d)
S2C12.
67
Write chemical formulas for the following molecular compounds:
(a) phosphorus tribromide, (b) dinitrogen tetrafluoride, (c) xenon
tetroxide, (d) selenium trioxide.
Write the molecular formulas and names
of
the following
compounds.
s
(a) (b)
(c)
Write the molecular formulas and names
of
the following
compounds.
(a) (b)
(c)
Section 2.7: Ions and Ionic Compounds
Review Questions
2.65
2.66
2.67
2.68
Give an example
of
each
of
the following: (a) a monatomic
cation, (b) a monatomic anion, (c) a poly atomic cation, (d) a
poly atomic anion.
What
is an ionic compound? How is electrical neutrality
maintained in an ionic compound?
Explain why the chemical formulas
of
ionic compounds are
usually the same
as
their empirical formulas.
What
is the Stock system? What are its advantages over the older
system
of
naming cations?
68
CHAPTER
2 Atoms, Molecules, and Ions
Problems
2.69 Give the number
of
protons and electrons in each
of
the following
. + C 2+ 1
3
+ F
' + 1- F- S2- 0
2
- N
3
-
common
IOns:
Na,
a , A , e- , , , , , .
2.70 Give the number
of
pr
otons and electrons in each
of
the following
. +
2+
F 3+ B - M 2+ C
4
- C
2+
common
IOns:
K ,
Mg
, e ,
r,
n , , u .
2.71 Write the formulas for the following ionic compounds:
(a) sodium oxide, (b) iron sulfide (containing the
Fe
2
+ ion),
(c) cobalt sulfate (containing the
C0
3
+ and
SO
~
-
ions),
(d) barium fluoride.
2.72 Write the formulas for the following ionic compounds: (a)
copper bromide (containing the Cu+ ion), (b) manganese oxide
(containing the
Mn
3+
ion), (c) mercury iodide (containing the
Hgi
+ ion
),
(d) magnesium phosphate (containing the
PO
~
-
ion
).
2.73 Which
of
the following compounds are likely to
be
ionic? Which
are likely to be molecular? SiCI
4
, LiF,
BaCl
b
B
2
H
6
,
KCl, C
2
H
4
2.74 Which
of
the following compounds are likely to
be
ionic? Which
are likely to
be
molecular?
CH
4
, NaBr,
BaF
b
CCI
4
, ICl, CsCl,
NF3
2.75
Name
the following compounds: (a)
KH
2
P0
4
, (b)
K
1
HP0
4
,
(c)
HBr
(gas), (d)
HBr
(in water), (e) Li
2
C0
3
,
(f)
K2
Cr
20
7'
(g)
NH
4
NO
b
(h)
m0
3
,
(i)
PF
s
,
G)
P
4
0
6
,
(k)
CdI
z
, (I)
SrS0
4,
(m) Al(OH)3'
2.76
Name
the following compounds: (a) KCIO, (b)
Ag
2
C0
3
,
(c)
HNO
z
, (d) KMn04, (e)
CsCI0
3
,
(f) KNH
4
S0
4
,
(g) FeO,
(h) Fez03' (i) TiCI
4
,
(j) NaH, (k) Li3N,
(1)
Na
2
0,
(m)
Na20
Z'
2.77 Write the formulas for the following compounds: (a) rubidium
nitrite, (b) potassium sulfide, (c) sod
ium
hydrogen sulfide,
(d) magnesium phosphate, (e) calcium hydrogen phosphate,
(f)
potassium dihydrogen phosphate, (g) iodine heptafluoride,
(h) ammonium sulfate, (i) silver perchlorate,
(j) boron trichloride.
2.78 Write the formulas for the following compounds: (a) copper(I)
cyanide, (b) strontium chlorite, (c) perbromic acid, (d)
hydroiodic acid, (e) disodium ammonium phosphate,
(f) lead(II)
carbonate, (g) tin(II) fluoride, (h) tetraphosphorus decasulfide,
(i) mercury(II) oxide,
G)
mercury(I) iodide, (k)
se
lenium
hexafluoride.
2.79 In the diagrams shown here, match each
of
the drawings with
the following ionic compounds:
A1
2
0
3
,
LiH,
Na
2S,
Mg(N0
3
h.
(Green spheres represent cations and red spheres represent
anions.)
(a) (b) (c) (d)
2.80 Given the formulas for the ionic compounds, draw the correct
ratio
of
cations to anions as shown
in
Problem 2.79: (a)
BaS04,
(b)
CaF
2
, (c)
Mg
3
N
2
, (d)
K
2
0.
Additional Problems
2.81 Define the following terms: acids, bases, oxoacids, oxoanions,
and hydrates.
2.82 A sample
of
a uranium compound is found to
be
losing mass
gradually. Explain what is happening to the sample.
2.83 In which one
of
the following pairs do the two species resemble
each other
mo
st closely in chemical properties: (a) :
Hand:
H
+,
(b) l
iN
and Ij N
3
- , (c)
I
~C
and
I~C?
Explain.
2.84
One isotope
of
a metallic element has mass number 65 and 35
neutrons in the nucleus.
The
cation derived from the isotope has
28 electrons. Write the symbol for this cation.
2.85
One isotope
of
a nonmetallic element has mass number 127 and
74 neutrons in the nucleu
s.
The
anion derived from the isotope
has 54 electrons. Write the symbol for this anion.
2.86 The following table gives numbers
of
electrons, protons, and
neutrons in atoms
or
ions
of
a number
of
elements. Answer the
following: (a) Which
of
the species are neutral? (b) Which are
negatively charged? (c) Which are positively charged? (d)
What
are the conventional symbols for all the species?
Atom
or
Ion
of
Element
A
Number
of
electrons 5
Number
of
protons 5
Number
of
neutrons 5
B
10
7
7
c
18
19
20
D
28
30
36
E
36
35
46
2.87
What
is wrong with
or
ambiguous about the phrase
"four
molecules
of
NaCI"?
F
5
5
6
G
9
9
10
2.88 The following phosphorus sulfides are known: P
4
S
3
, P
4
S
7
, and
P
4
S
IO
.
Do
these compounds obey the law
of
mUltiple proportions?
2.89 Which
of
the following are elements, which are molecules but
not compounds, which are compounds but not molecules, and
which are both compounds and molecules? (a)
SOb
(b)
S8,
(c) Cs, (d) N
2
0
S
'
(e)
0,
(f)
O
z,
(g) 0
3
, (h)
CH
4
,
(i)
KEr, ( j) S,
(k) P
4,
(1)
LiF
2.90
What
is wrong with the name (given in parentheses
or
brackets)
for each
of
the following compounds: (a) BaCl
z
(barium
dichloride),
(b)
Fe20
3 [iron(
ll)
oxide], (c)
CsN0
2
(cesium
nitrate), (d)
Mg(HC0
3
h [magnesium(ll) bicarbonate]?
2.91 Discuss the significance
of
assigning an atomic mass
of
exactly
12 amu to the carbon-12 isotope.
2.92 Determine what is wrong with the chemical formula and
write the correct chemical formula for each
of
the following
compounds: (a) (NH
3
)
2C03 (a
mmonium
carbonate), (b)
CaOH
(calcium hydroxide), (c)
CdS0
3
(cadmium sulfide), (d)
ZnCr04
(z
inc dichromate).
2.93 Fill in the blanks in the table:
Symbol
~~
Fe
2+
Protons 5
79 86
Neutrons 6 16 117 136
Electrons 5
18
79
Net charge
-3
0
2.94 (a) Which elements are most likely to form ionic compounds?
(b) Which metallic elements are most likely to form cations with
different charges?
2.95 Write the formula
of
the common ion derived from each
of
the
following: (a) Li, (b)
S, (c) I, (d) N, (e) AI, (f) Cs, (g) Mg.
2.96 Which
of
the following symbols provides more information about
the atom:
23
Na
or
IINa? Explain.
2.97
2.98
2.99
Write the chemical formulas and names
of
the binary acids
and oxoacids that contain Group 7 A elements.
Do
the same for
elements
in
Groups 3A, 4A,
SA,
and 6A.
Determine the molecular and empirical formulas
of
the
compounds shown here. (Black spheres are carbon, and white
spheres are hydrogen.)
(a) (b) (c)
(d)
For the noble gases (the Group 8A elements) i He, igNe,
i~Ar,
~~Kr,
and
1
~~
Xe,
(a) determine the number
of
protons and
neutrons
in
the nucleus
of
each atom, and (b) determine the ratio
of
neutrons to protons in the nucleus
of
each atom.
De
scribe any
general trend you discover
in
the way this ratio changes with
increasing
atomic number.
2.100 List the elements that exist as gases at room temperature.
(Hint:
Most
of
these elements can be found in Groups SA, 6A, 7 A,
and 8A.)
2.101
The
Group
IB
metals, Cu, Ag, and Au, are called coinage metals.
What
chemical properties make them especially suitable for
making coins and jewelry?
2.102
The
elements in Group 8A
of
the periodic table are called noble
gases. Can you suggest what
"noble" means in this context?
2.103
The
formula for calcium oxide is CaO.
What
are the formulas for
magnesium oxide and strontium oxide?
2.104 A common mineral
of
barium is barytes,
or
barium sulfate
(BaS04). Because elements in the same periodic group have
similar
chemical properties,
we
might expect to find so
me
radium sulfate (RaS04) mixed with barytes since radium is the
last member
of
Group 2A. However, the only source
of
radium
compounds in nature is in uranium minerals.
Why?
2.105 List five elements each that are (a) named after places, (b) named
after people, (c) named after a color. (Consult http://www.
Google.com, ,
or
http://www.
Webelements.com.)
2.
106
Name
the only country that
is
named after an element. (
Hint:
This country is in South America.)
2.107 Fluorine reacts with hydrogen (H) and deuterium
(D) to form
hydrogen fluoride (HF) and deuterium fluoride (DF), where
deuterium
(TH)
is an isotope
of
hydrogen. Would a given anlOunt
of
fluorine react with different masses
of
the two hydrogen
isotopes? Does
this violate the law
of
definite proportion? Explain.
2.108 Predict the formula and name
of
a binary compound formed from
the following elements: (a) Na and H, (b)
Band
0,
(c)
Na
and S,
(d)
Al
and
F,
(e) F and 0 , (f)
Sr
and Cl.
2.109 Identify each
of
the following elements: (a) a halogen whose
anion contains 36 electrons, (b) a radioactive noble gas with
86 protons, (c) a Group 6A element whose anion contains 36
electrons, (d) an alkali metal cation that contains 36 electrons,
(e) a Group
4A
cation that contains 80 electrons.
QUESTIONS
AND
PROBLEMS
69
2.110 Show the locations
of
(a) alkali metals, (b) alkaline earth metals,
(c) the halogens, and (d) the noble gases in the given outline
of
a periodic table. Also draw dividing lines between metals and
metalloids and between metalloids and nonmetals.
lA
8A
D2A
3A
4A
SA
6A 7A
3B
4B
SB
6B 7B
I
8B
liB
2B
2.111 Fill in the blanks in the table.
Cation Anion Formula
Name
Magnesium bicarbonate
SrCl
2
Fe
H
N0
2
Manganese(II) chlorate
SnBr4
Co
2+
PO
~
-
Hcr
2
+
",2
r
CU2C03
Lithium nitride
AI
H
S2-
2.112
Some
compounds are better known by their common names than
by their systematic chemical names. Give the
chemical formulas
of
the following substances: (a) Dry ice, (b) salt, (c) laughing gas,
(d) marble (chalk, limestone), (e) baking soda,
(f)
ammonia,
(g) water, (h)
milk
of
magnesia, (i) epsom salt.
2.113
On page 36 it was pointed out that mass and energy are alternate
aspects
of
a single entity called mass-energy.
The
relationship
between these two physical quantities is Einstein's equation,
E = me
2
,
where E is energy, m
is
mass, and e is the speed
of
light. In a combustion experiment, it was found that 12.096 g
of
hydrogen molecules combined with 96.000 g
of
oxygen
molecules to form water and released l.71S
X 10
3
kJ
of
heat.
Use Einstein's equation to calculate the corresponding mass
change in this process, and comment on whether
or
not the law
of
conservation
of
mass holds for ordinary chemical processes.
2.114 (a) Describe Rutherford's experiment and how the results
revealed the nuclear structure
of
the atom. (b) Consider the
23
Na
atom. Given that the radius and mass
of
the nucleus are
3.04 X
10
-
15
m and 3.82 X 10-
23
g, respectively, calculate the
density
of
the nucleus in g/cm
3
.
The
radius
of
a 23
Na
atom is
186 pm. Calculate the density
of
the space occupied by the
electrons outside the nucleus
in
the sodium atom.
Do
your results
support Rutherford
's
model
of
an atom? [The volume
of
a sphere
of
radius r is 1
'ITr
3
.]
2.115 Draw all possible structural formulas
of
the following
hydrocarbons: CH
4
,
C
Z
H
6
,
C
3
H
s
, C
4
H
IO
, and C
S
H
I2
•
70
CHAPTER
2 Atoms, Molecules, and Ions
2.116 Draw two different structural formulas based
on
the molecular
formula C
2
H
6
0.
Is the fact that you can have more than
one
compound with the same molecular formula consistent with
Dalton's atomic theory?
2.117 Ethane and acetylene are two gaseous hydrocarbons. Chemical
analyses show that in one sample
of
ethane, 2.65 g
of
carbon
are combined with 0.665 g
of
hydrogen, and in
one
sample
of
acetylene, 4.56 g
of
carbon are combined with 0.383 g
of
hydrogen. (a) Are these results consistent with the law
of
multiple
proportions? (b) Write reasonable molecular formulas for these
compounds.
2.118 A
cube
made
of
platinum (Pt) has an edge length
of
1.0 cm.
(a) Calculate the number
of
Pt
atoms in the cube. (b) Atoms are
spherical in shape. Therefore, the
Pt
atoms in the cube cannot fill
all the available space.
If
only
74
percent
of
the space inside the
cube is taken up by
Pt
atoms, calculate the radius in picometers
of
a
Pt
atom.
The
density Pt is 21.45 g/
cm
3, and the mass
of
a
single
Pt
atom is 3.240 X
10
-
22
g. [The volume
of
a sphere
of
radius r is
~1Tr3.l
2.119 A monatomic ion has a charge
of
+ 2.
The
nucleus
of
the parent
atom has a mass number
of
55.
If
the number
of
neutrons in the
nucleus is 1.2 times that
of
the number
of
protons, what is the
name and symbol
of
the element?
2.120 In the following 2
X 2 crossword, each letter must be correct
in four ways: horizontally, vertically, diagonally, and by itself.
When
the puzzle is complete, the four spaces will contain the
overlapping symbols
of
10 elements. Use capital letters for each
square. There is only
one
correct solution.
1
2
3
4
Horizontal
1-2: Two-letter symbol for a metal used in ancient times
3-4:
Two-letter symbol for a metal that bums in air and is
found in Group
5A
Vertical
1- 3:
2-4:
Two-letter symbol for a metalloid
Two-letter symbol for a metal used in
U.S. coins
Single Square
1:
A colorful nonmetal
2:
3:
4:
A colorless gaseous nonmetal
An
element that makes fireworks green
An
element that has medicinal uses
Diagonal
1-4:
Two-letter symbol for an element used in electronics
2-3:
Two-letter symbol for a metal used with
Zr
to make
wires for superconducting magnets
2.121
Name
the given acids.
PRE-PROFESSIONAL PRACTICE
EXAM
PROBLEMS:
PHYSICAL
AND
BIOLOGICAL SCIENCES
Carbon-14, a radioactive isotope
of
carbon, is used to determine the ages
of
fossils in a technique called carbon dating. Carbon-14 is produced in
the upper atmosphere when nitrogen-14 atoms are bombarded by neutrons
from cosmic rays.
14C
undergoes a process called
f3
emission in which a
neutron in the nucleus decays to form a proton and an electron.
The
elec-
tron,
or
f3
particle, is ejected from the nucleus. Because the production and
decay
of
14C
occur simultaneously, the total amount
of
14C
in the atmo-
sphere is constant.
Plants absorb
14C
in the form
of
CO
2
and animals con-
sume plants and other animals. Thus,
all living things contain a constant
ratio
of
12C
to
14c.
When
a living thing dies, the
14C
it contains continues
to decay but because replenishment ceases, the ratio
of
12C to
14C
changes
over time. Scientists use the
12C
to
14C
ratio to determine the age
of
mate-
rial that was once living.
1.
If
atmospheric conditions were to change such that
14C
were
produced at twice the current rate,
a) the world's supply
of
14N
would
be
consumed completely.
b) the
12C
to
14C
ratio in living things would increase.
c) the 12C to
14C
ratio in living things would decrease.
d) the
12C
to
14C
ratio in living things would
not
change.
2.
When
a
14N
nucleus is bombarded by a neutron to produce a
14C
nucleus, what else is produced?
a) Nothing
b) Another neutron
c) An electron
d) A proton
3.
Based
on
the description
of
f3
emission
in
the passage, what nucleus
results from the decay
of
a 1
4C
nucleus by
f3
emission?
a)
14N
b) 13
N
c)
12C
d)
13C
4.
The accuracy
of
carbon dating depends on the assumption that
a)
14C
is the only radioactive species
in
the material being tested.
b) the rate
of
decay
of
14C
is
constant.
c)
12C
and
14C
undergo radioactive decay at the same rate.
d) each
14C
nucleus decays to give a 12C nucleus.
ANSWERS TO IN-CHAPTER MATERIALS
71
ANSWERS
TO
IN-CHAPTER
MATERIALS
Practice Problems
l.lA
(a) p = 5, n = 5, e = 5. (b) p = 18, n = 18, e = 18. (c) p = 38,
D = 47, e = 38. (d) P = 6, n = 5, e = 6. 2.1B (a)
~Be,
(b)
~j
v,
c)
1~!Xe,
(d)
~iGe
.
2.2A 63.54 amu. 2.2B 99.64%
14N
, 0.36%
IS
N
.
23A
CHCI
3
. 2.3B C3H60.
2.4A
(a) dichlorine
monoxide
, (b) silicon
tetrachloride. 2.4B (a) chlorine dioxide, (b) carbon tetrabromide. 2.5
a) CS
2
,
(b) N
2
0
3
. 2.6 (a) C
4
H
s
N
2
0,
(b) C
2
H
s
,
(c) C
2
H
s
N0
2
.
2.7A (a)
hypobromous acid, (b) hydrogen
sulfate ion, (c) oxalic acid. 2.7B (a)
iod
ic
acid, (b) hydrogen chromate ion, (c) hydrogen oxalate ion. 2.8A
HB
r0
4
. 2.8B
H2Cr04' 2.9A (a) sodium sulfate, (b) copper(lI) nitrate, (c)
ir
on(IlI) carbonate. 2.9B (a) potassium dichromate, (b) lithium oxalate,
c) copper(I) nitrate.
2.10A
(a) PbCl
z,
(b)
MgC0
3
, (c)
(~)
3
P0
4
'
2.10B (a) FezS3, (b)
Hg(N0
3
)z, (c) K
2
S0
3
.
Checkpoints
2.3.1 d. 2.3.2 c. 2.4.1 c. 2.4.2
b.
2.5.1 b. 2.5.2 c. 2.6.1 b. 2.6.2 c. 2.6.3 c.
2.6.4 e. 2.7.1 c. 2.7.2
d.
2.7.3
d.
2.7.4 c. 2.7.5 c. 2.7.6
a.
Applying
What
You've
learned
a)
There
are
54
-
26
= 28 neutrons
in
the
s4
Fe
nucleus,
30
neutrons in
the
S6
Fe
nucleus,
31
neutrons
in
the
s7Fe
nucleus, and 32 neutrons in the
S8
Fe
nucleus.
b)
The
average atomic mass
of
iron is 55.845 amu. c)
The
molecular formula for ascorbic acid is C
6
H
8
0
6
.
d)
The
empirical formula
for ascorbic acid is C
3
H
4
0
3
.
e)
The
formula for ferrous sulfate is
FeS04'
•
•
tOIC
lometr
Ratios
of
Combination
3.1 Molecular
and
Formula
Masses
3.2 Percent Composition
of
Compounds
3.3 Chemical Equations
•
Interpreting
and
Writing
Chemical Equations
•
Balancing Chemical
Equations
3.4
The
Mole
and
Molar
Masses
•
The Mole
•
Detennining
Molar
Mass
•
Interconverting Mass, Moles,
and
Numbers
of
Particles
•
Empirical
Fonnula
from
Percent Composition
3.5 Combustion Analysis
•
Detennination
of
Empirical
Fonnula
•
Detennination
of
Molecular
Fonnula
3.6 Calculations with
Balanced Chemical
Equations
•
Moles
of
Reactants
and
Products
•
Mass
of
Reactants
and
Products
3.7
Limiting Reactants
•
Detennining
the
Limiting
Reactant
•
Reaction Yield
•
Chemical
Reactions
and
Chemotherapy
One
of
cancer chemotherapy's greatest success stories began with an accidental discov-
ery. In 1964, Barnett Rosenberg and his research group at Michigan State University
were studying the effect
of
an electric field on the growth
of
bacteria. Using platinum
electrodes, they passed an electric current through a bacterial culture.
To
their surprise,
the cells in the culture stopped dividing. The researchers determined that cisplatin,
Pt(NH3)2CI2, a compound containing platinum from the electrodes, was responsible.
Furthermore, they reasoned that because cancer is the result
of
the uncontrolled division
of
abnormal cells, the compound might be useful as an anticancer drug.
Platinol, the name under which cisplatin is marketed, was approved by the FDA in 1978
for the treatment
of
metastatic testicular and ovarian cancers. Today it is one
of
the most
widely prescribed cancer drugs being used also for cancers
of
the bladder, lung, and
stomach and is probably best known for the role it played
in
seven-time Tour de France
winner Lance Armstrong's battle with testicular cancer. Cisplatin works by attaching
itself to the DNA
of
cancer cells and preventing their replication. The damaged cells are
then destroyed by the body's immune system. Unfortunately, cisplatin can cause seri-
ous side effects, including severe kidney damage. Ongoing research efforts are directed
toward finding related compounds that are less toxic.
In 1964, cisplatin was produced accidentally when platinum electrodes reacted with
ammonia molecules and chloride ions that were present in a bacterial culture. Today,
manufacturers use the principles
of
stoichiometry to produce cisplatin in the most effi-
cient, economical way possible.
•
- -
~,£ ~~
. -
- -
":
In This Chapter, You Will Learn how we use chemical equations to represent chemical reactions.
You
will also
learn how balanced chemical equations are used to solve a variety
of
problems.
Before you begin, you should review
• Average atomic mass
[~
.
Section
2.5]
• Molecular formulas
[~
.
Section
2.6]
Lance
Armstrong,
seven-time
winner
of
the prestigious Tour
de France race, was diagnosed
with
advanced testicular cancer .
in 1996. His successful course
of
treatment included the drug
Platinol
or
cisplatin.
Media
Player/
MPEG
Content
Chapter
in
Review
73
74
CHAPTER
3
Stoichiometry:
Ratios
of
Combination
Caffeine
Think
About
It Double-check that
you have counted the number
of
atoms correctly for each compound
and that you have used the proper
atomic masses from the periodic
table.
Citric
acid
Molecular and Formula Masses
Using
atomic
masses
from
the
periodic
table
and
a
molecular
fOImula,
we
can
determine
the
molecular mass,
which
is
the
mass
in
atomic
mass
units
(amu)
of
an
individual
molecule.
The
molecular
mass
is
simply
the
sum
of
the
atomic
masses
of
the
atoms
that
make
up
the
molecule.
We
multiply
the
atomic
mass
of
each
element
by
the
number
of
atoms
of
that
element
in
the
mol-
ecule
and
then
sum
the
masses
for
each
element
present.
For
example,
molecular
mass
of
H
2
0 = 2(
atomic
mass
of
H)
+
atomic
mass
of
0
= 2(l.008
amu)
+ 16.00
amu
= 18.02
amu
Because
the
atomic
masses
on
the
periodic
table
are
average
atomic
masses,
the
result
of
such
a
deteI
Illination
is
an
average
molecular
mass,
sometimes
referred
to
as
the
molecular weight.
As
with
the
term
atomic
mass,
we
will
use
the
teIm
molecular
mass
in
this
text.
Although
an
ionic
compound
does
not
have
a
molecular
mass,
we
can
use
its
empirical
for-
mula
to
deteImine
its
formula mass
(the
mass
of
a
"formula
unit"),
sometimes
called
the
formula
weight.
Sample
Problem
3.1
illustrates
how
to
deteImine
molecular
mass
and
fOIlilula
mass.
Calculate the molecular mass or the formula mass, as appropriate, for each
of
the following compounds:
(a) propane, C
3
H
8
,
(b) lithium hydroxide, LiOH, and (c) barium acetate, Ba(C
2
H
3
0
2
h.
Strategy
Determine the molecular mass (for each molecular compound)
or
formula mass (for each
ionic compound) by summing all the
atomic masses.
Setup
Using the formula for each compound, determine the number
of
atoms
of
each element present.
A molecule
of
propane contains three C atoms and eight H atoms.
The
compounds in parts (b) and
(c) are ionic and will therefore have formula masses rather than molecular masses. A formula unit
of
lithium hydroxide contains one Li atom, one 0 atom, and one H atom. A formula unit
of
barium
acetate contains one
Ba
atom, four C atoms, six H atoms, and four 0 atoms. (Remember that the
subscript after the parentheses means that there are two acetate ions, each
of
which contains two C
atoms, three H atoms, and two
0 atoms.)
Solution
For
each compound, multiply the number
of
atoms by the atomic mass
of
each element
and then sum the calculated values.
(a) The molecular mass
of
propane is 3(12.01 amu) + 8(1.008 amu) = 44.09 amu.
(b) The formula mass
of
lithium hydroxide is 6.941 amu + 16.00 amu + 1.008 amu = 23.95 amu.
(c)
The
formula mass
of
barium acetate is 137.3 amu + 4(12.01 amu) , + 6(1.008 amu) +
4(16.00 amu) = 255.4 amu.
Practice
Problem
A Calculate the molecular
or
formula mass
of
each
of
the following compounds:
(a) magnesium chloride (MgCI
2
),
(b) sulfuric acid (H
2
S0
4
),
and (c) ibuprofen (C
13
H
18
0
2
).
Practice
Problem
B Calculate the molecular
or
formula mass
of
each
of
the following compounds:
(a) calcium carbonate
(CaC0
3
),
(b) nitrous acid
(HN0
2
),
and (c) caffeine (C
8
H
iO
N
4
0
2
).
Checkpoint 3.1
Molecular and Formula Masses
3.1.1
What
is the molecular mass
of
citric
3.1.2
What
is
the formula mass
of
calcium
acid (H
3
C
6
H
s
0
7
)?
citrate [Ca3(C6Hs07)2]?
a)
192.12 amu a)
309.34 amu
b)
189.10 amu
b) 69.10 amu
c) 132.07 amu
c)
229.18 amu
d)
29.02 amu d)
498.44 amu
e)
89.07 amu
e)
418.28 amu
SECTION
3.2 Percent
Composition
of
Compounds 75
Percent Composition
of
Compounds
The fOimula
of
a compound indicates the number
of
atoms
of
each element in a unit
of
the com-
pound. From a molecular or empirical formula, we can calculate what percent
of
the total mass
is contributed by each element in a compound. A list
of
the percent by mass
of
each element in a
compound is known as the compound's
percent composition
by
mass. One way that the purity
of
a compound can be verified is by comparing its percent composition by mass, determined experi-
mentally, with its calculated percent composition. Percent composition is calculated by dividing
th
e mass
of
each element in a unit
of
the compound by the molecular or fOimula mass
of
the com-
pound and then multiplying by
100 percent. Mathematically, the percent by mass
of
an element in
a compound is expressed as
t b f I t
n X atomic mass
of
element X
loo
m
percen y mass 0 an e emen =
70
molecular or formula mass
of
compound
Equation
3.l
where n is the number
of
atoms
of
the element in a molecule or formula unit
of
the compound. For
ex
ample, in a molecule
of
hydrogen peroxide
(H
2
0
2
),
there are two H atoms and two 0 atoms. The
atomic masses
of
Hand
0 are 1.008 and 16.00 amu, respectively, so the molecular mass
of
H
2
0
2
i 34.02 amu. Therefore, the percent composition
of
H
2
0
2
is calculated as follows:
%H
= 2 X 1.008 amu H X 100% = 5.926%
34.02 amu H
2
0
2
%0
= 2 X 16.00 amu 0 X 100% = 94.06%
34.02
amu H
2
0
2
The sum
of
percentages is 5.296% + 94.06% = 99.99%. The small discrepancy from 100 percent
is
due to rounding
of
the atomic masses
of
the elements.
We
could equally well have used the
empirical fOimula
of
hydrogen peroxide (HO) for the calculation.
In
this case, we would have used
th
e empirical formula mass, 17.01 amu, in place
of
the molecular mass.
%H
= 1.008 amu H X 100% = 5.926%
17
.
01
amu
%0
= 16.00 amu 0 X 100% = 94.06%
17.01
amu
Be
cause both the molecular fonllula and the empirical
fOJ
mula tell us the composition
of
the com-
pound, they both give the same percent composition by mass. Sample Problem 3.2 shows how
to
calculate percent composition by mass.
Lithium carbonate, Li
2
C0
3
,
was the first "mood-stabilizing"
drug
approved
by
the
FDA
for
the
treatment
of
mania
and
manic-depressive illness, also known as bipolar disorder. Calculate the
percent composition
by
mass
of
lithium
carbonate.
Strategy
Use
Equation
3.1 to determine
the
percent
by
mass contributed
by
each
element
in
the
compound.
Setup Lithium carbonate is an ionic compound that contains Li, C, and O.
In
a formula unit, there
are two
Li
atoms,
one
C atom, and three 0 atoms with atomic masses 6.941, 12.01, and 16.00 amu,
respectively.
The
formula mass
of
Li
2
C0
3
is 2(6.941 amu) + 12.01 amu + 3(16.
00
amu) = 73.89 amu.
Solution
.
For
each
element, multiply the
number
of
atoms by the atomic mass, divide
by
the
formula mass, and
multiply
by
100 percent.
%Li
= 2 X 6.941
amu
Li
X 100% = 18.79%
73.89
amu
Li
2
C0
3
%C = 12.01 amu C X 100% = 16.25%
73.
89
amu
Li
2
C0
3
%0
= 3 X 16.00
a~u
0 X 100% =
64.96
%
73.89
amu
L1
2
C0
3
•
(Continued)
Think
About
It
Make
sure that
the
percent composition results
for
a
compound
sum
to approximately
100. (In this case,
the
results sum
to exactly
100 percent- 18.79% +
16.25% +
64.96%
= 100.00%-
but
remember
that
because
of
rounding,
the
percentages
may
sum
to very slightly more
or
very
slightly less.)
76 CHAPTER 3 Stoichiometry: Ratios
of
Combination
Aspirin
Practice Problem A Determine the percent composition by mass
of
acetaminophen (CgH
9
N0
2
),
the
active ingredient in over-the-counter pain relievers such as Tylenol (Figure 3.1).
Practice Problem B Determine the percent composition by mass
of
the artificial sweetener
aspartame
(CI4HlgN20S)'
"-"
,,
Ill READ
THE
LABEL
."H\
N
OC
036l-Clll$-
,S
I
· ~
.
~
Acetaminophen
Figure
3.1
Acetaminophen is the active ingredient in Tylenol, a nonaspirin pain reliever.
Checkpoint 3.2
Percent
Composition
of
Compounds
3.2.1
What
is the percent composition by
mass
of
aspirin (C
9
H
g
0
4
)?
a)
41.4%
C, 3.5% H, 55.1 % 0
b)
60.0% C, 4.5% H, 35.5% 0
c) 82.1% C, 3
.5
% H, 14.4% 0
d) 53.2% C, 16.4% H, 30.4% 0
e) 42.9% C, 38.1% H, 19.0% 0
Chemical Equations
3.2.2
What is the percent composition
by mass
of
sodium bicarbonate
(NaHC0
3
)?
a)
44.2%
Na, 1.9% H, 23.1 % C,
30.8% 0
b) 24.2% Na, 1.1%
H,
13.6% C,
, ,
61.1 %
0'
c) 31.1% Na, 0.8% H, 14.2% C,
53.9%
0
d) 37.1% Na, 2.9%
H,
24.6% C,
35.4%
0
e) 27.3% Na, 1.2% H, 14.3% C,
57.2%
0
A chemical reaction, as described in the third hypothesis
of
Dalton's atomic theory
[
~~
Section
2.
1]
, is the rearrangement
of
atoms in a sample
of
matter. Examples include the rusting
of
iron and
the explosive combination
of
hydrogen and oxygen gases to produce water. A chemical equation
uses chemical symbols to denote what occurs in a chemical reaction. We have seen how chemists
represent elements and compounds using chemical symbols. Now we will look at how chemists rep-
resent chemical reactions using chemical equations.
Interpreting and Writing Chemical Equations
A chemical equation represents a chemical statement. When you encounter a chemical equation,
you may find
it
useful to read
it
as though
it
were a sentence.