Chemistry part 7, Julia Burdge,2e (2009) ppt
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (17.88 MB, 26 trang )
136 CHAPTER 4 Reactions in Aqueous Solutions
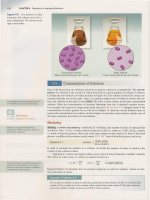
Figure 4.8 Two
so
lutions
of
iodine
in
ben
ze
ne.
The
solution
on
the
left is
more
concentrated.
The
so
lution
on
the
right is
more
dilute.
The
qualitative
terms
concentrated
and
dilute
are
relati
ve
terms,
like
expensive
and
cheap.
r
"'
,
_ :
Multimedia
Solutions-preparation of
so
lutions.
Molarity
can
equally
well
be
defined
as
millimoles
per
milliliter (mmol/mL
),
which
ca
n
simplify
some
calculations.
• •
Students
sometimes
have
difficulty
seeing
how
units
cancel
in
these
equations.
It
may
help
to
write
M
as
mollL unt
il
you
become
completely
comfortable with
these
equations.
Concentrated
solution:
Dilute
solution:
More
solute particles
per
unit
volume
Fewer
so
lute particles
per
unit
volume
Concentration
of
Solutions
One
of
the factors that can influence reactions in aqueous solution is concentration.
The
concen-
tration
of
a solution is
the
amount
of
solute dissolved in a given quantity
of
solvent
or
solution.
Consider
the
two solutions
of
iodine pictured in Figure 4.8.
The
solution
on
the left is
more
con-
centrated than the
one
on
the
right-that
is, it contains a
higher
ratio
of
solute
to
solvent.
By
con-
. . . . . . . . , . . . . . . . . . . . . . . . . . . . . . . . . . . . , . . . . . . . . . . . . . . . . . . . . .
• • • • •
trast,
the
solution
on
the right is
more
dilute.
The
color is
more
intense in
the
more concentrated
solution.
Often the concentrations
of
reactants determine how fast a chemical reaction occurs.
For
example, the reaction
of
magnesium
metal and acid
[
~~
Section
4.4] happens faster
if
the
concentration
of
acid is greater. As
we
will see in Chapter 13, there are several different ways to
express the concentration
of
a solution. In this chapter,
we
introduce only molarity, which is one
of
the
mo
st
commonly
u
se
d units
of
concentration.
Molarity
Molarity,
or
molar concentration, symbolized
M,
is defined as the number
of
moles
of
solute per liter
of
solution. Thu
s,
1 L
of
a 1.5 molar solution
of
glucose (C
6
H
I2
0
6
),
written as 1.5 M C
6
H
12
0
6
,
contains
1.5 moles
of
dissolved glucose.
Half
a liter
of
the same solution would contain 0.75 mole
of
dissolved
glucose, a milliliter
of
the solution would contain 1.5 X 10-
3
mole
of
dissolved glucose, and so on.
Equation
4.1
l
't
moles solute
mo
an
y =
liters solution
In
order
to calculate the molarity
of
a solution,
we
divide the
number
of
moles
of
solute by the
volume
of
the solution
in
liters.
Equation
4.1 can
be
rearranged in three ways to solve
for
any
of
the three variables: molarity
(M), moles
of
solute (mol),
or
volume
of
solution
in
liters (L) .
.
. . .
. . . . .
. . ,
·mol· mel·
.
(1) M = L (2) L = M (3)
mol
= M
XL
Sample
Problem
4.9 illustrates
how
to use these equations to solve for molarity, volume
of
solu-
tion, and moles
of
solute.
Sample Problem 4.9
For
an aqueous solution
of
glucose
(C
6
H
I2
0
6
),
determine
(a) the molarity
of
2.00 L
of
a
so
lution that
contains
50.0 g
of
glucose, (b) the
volume
of
this
so
lution
that
would
co
ntain 0.250
mole
of
glucose,
and
(c) the
number
of
mole
s
of
glucose in 0.500 L
of
this solution.
SECTION 4.5
Concentration
of
Solutions 137
Strategy
Convert the mass
of
glucose given
to
mole
s,
and use the equations for interconversions
of
M,
liter
s,
and moles
to
calculate the answers.
Setup
The molar mass
of
glucose is
180.2
g.
50.0
g
moles
of
glucose =
80
I =
0.277
mol
1 .2 glmo
.
: A common way to
state
the
concentration of
:
this
solution
is
to
say,
"This
solution
is
0.
139
M
·
in
glucose."
•
•
•
•
•
•
•
•
•
•
Solution
0.277
mol C6H'206
:
(a) molarity
=
200
L l ' =
0.139
M
Think
About
It
Check
to
see that
the magnitudes
of
your answers are
logical. For example, the mass given
in
the problem corresponds
to
0.277
mole of solute.
If
you are asked,
.
so
utlOn
0.250
mol C
6
H
p
0
6
(b)
volume = 0 9 - =
1.
80
L
.13 M
(c)
moles
of
C
6
H
12
0
6
in 0.500 L = 0.500 L X
0.139
M =
0.0695
mol
Practice Problem A For
an
aqueous
so
lution
of
sucrose
(C
12
H
22
0
11
),
determine (a) the molarity
of
5.00
L
of
a solution that contains
235
g
of
sucrose, (
b)
the
vo
lume
of
this
so
lution that would contain
1.26
mole
of
sucrose, and (c) the number
of
moles of sucrose in
1.89
L
of
this
so
lution.
Practice Problem B For
an
aqueous solution
of
sodium chloride (NaCl), determine (
a)
the molarity
of
3.75
L
of
a solution that contains
155
g
of
so
dium chloride, (b) the
vo
lume
of
this solution that
would contain
4.58
moles
of
so
dium chloride, and (c) the number
of
moles
of
sodium chloride
in
22.75
L
of
this
so
lution.
~.
_
The
procedure
for
preparing
a
so
lution
of
known
molarity
is s
hown
in
Figure
4.9.
First,
the
solute
is
weighed
accurately
and
tran
s
ferred
,
often
with
a
funnel,
to a
volumetric
flask
of
the
desired
volume.
Next
,
water
is
added
to
the
flask,
which
is
then
sw
irled
to
dissolve
t
he
so
lid.
After
all
the
solid
ha
s
dis
so
lved
,
more
water
is
added
slowly
to
bring
the
level
of
o
lution
exactly
to
the
volume
mark.
Knowing
the
volume
of
the
so
lution
in
the
fla
sk
and
the
. . . . . . . . . . . . . . . . . . . . . . . . . .
q
uantity
of
compound
dissolved
,
we
can
determine
the
molarity
of
the
so
lution
us
ing
Equation
4.
1.
Note
that
this
procedure
doe
s
not
require
that
we
know
the
exact
amount
of
water
added.
Beca
u
se
of
the
way
molarity
is
defined
,
it
is
important
only
that
we
know
the
final
volume
of
the
solution.
Dilution
Co
ncentrated
"stock"
solutions
of
commonly
u
se
d
substances
typicall
y
are
kept
in
the
labora-
to
ry
stockroom.
Often
we
need
to
dilute
these
s
tock
s
olution
s
before
us
ing
them.
Dilution
is
the
p
rocess
of
preparing
a
less
concentrated
sol
ution
from
a
more
co
ncentr
ated
one.
Suppose
th
at
we
want
to
prepare
1.00 L
of
a 0.400 M
KMn04
so
lution
from
a
so
lution
of
1.00 M
KMn0
4'
For
this
purpo
se
we
need
0.400
mole
of
KMn0
4'
Becau
se
ther
e is 1.00
mole
of
KMn0
4
in
1.
00 L
of
a 1.00 M
KMn0
4 s
olution
,
there
is 0.400
mole
of
KMn0
4
in
0.400 L
of
the
same
-o
lution:
1.00
mol
KMn04
0.400
mol
KMn0
4
1.00 L
of
solution
OAOO
L
of
solution
The
refore,
we
must
withdraw
400
mL
from
the
1.00 M
KMn0
4
so
lution
and
dilute
it
to 1.00 L
by
adding
water
(in a 1.00-L
volumetric
fla
sk)
.
This
method
gives us 1.00 L
of
the
desired
0.400 M
KM
n04'
r
In
carrying
out
a dilution
proce
ss,
it
is u
se
ful to
remember
thilt
adding
more
solvent
to
a
giv
en
amount
of
the
stock
solution
changes
(
decrea
ses)
the
concentration
of
the
solution
without
ha
nging
the
number
of
moles
of
so
lute
pre
se
nt
in
the
solution
(F
igure
4.10, p. 140).
moles
of
solute
befor
e dilution =
mole
s
of
solute
after
dilution
Equation
4.2
L ing
arrangement
(3)
of
Equation
4.1 ,
we
can
calculate
the
number
of
mole
s
of
solute:
mol
es
of
so
lute.
.
moles
of
solute = X
hter
s
of
solutlon
liters
of
so
lution
as
in part (b), for the volume that
contains a number
of
moles smaller
than
0.277,
make sure your answer
is smaller than the original volume.
,
•
-
Media
Player/MP
EG
Animation:
Figure
4.
9,
Prepar
i
ng
a
Solution
from
a
So
l
id
,
pp
. 138-139.
,
It
is
important to
remember
that molarity
is
defined
in
terms
of the
volume
of solution, not
the
volume
of
solven
t.
In
many
cases,
these
two
are
not the
same.
Multimedia
Solutions-dilution.
•
!
Figure 4.9
•
138
Weigh
out
the solid
KMn04
(The tare function on a digital
balance automatically subtracts
the mass
of
the
weighing paper.)
The mass likely will not be
exactly the calculated
number
Transfer the weighed
KMn04
to the volumetric flask
Calculate the mass
of
KMn04
necessary
for the target concentration
of
0.1 M.
0.1
tal
X 0.2500 L = 0.02500 mol
158.04
g
0.02500 mol X I = 3.951 g
KMn04
rna
\
•
Add water sufficient to dissolve the
KMn0
4
Fill exactly to the calibration
mark using a wash bottle
Calculate the actual concentration
of
the solution.
1 mol
3.896
g
KMn04
X 158.04 g = 0.024652 mol
0.024652 mol =
009861
M
0.2500 L .
Swirl the flask to dissolve the solid
Add more water
Whatls the point?
The goal
is
to prepare a solution
of
precisely known concentration,
with that concentration being very close to the target concentration
of
0.1
M.
Note that because 0.1 is a specified number, it does not
limit the number
of
significant figures in our calculations.
139
140
CHAPTER
4 Reactions in Aqueous Solutions
Figure 4.10 Dilution
changes
the
concentration
of
a solution; it does
not
change
the
number
of
moles
of
solute
in
the
solution.
Students
sometimes
resist
using
the unit
millimole.
However,
using
the
Me X
mLc
=
Md X
m'-d
form of
Equation
4.3 often
reduces
the number
of
steps
in
a
problem
thereby
reducing
the
number of opportunities to
make
calculation
errors.
It
is
very
important to
note
that, for
safety,
when
di
luting a concentrated
acid,
the
acid
must
be
added
to the wate
r,
and
not the other
way
around.
Think About It
Plug
the
answer
into
Equation
4.3, and
make
s
ure
that
the
product
of
concentration
and
vo
lum
e
on
both sides
of
the
equation
give
the
same
result.
•
Before
dilution:
More
solute
particles
per
unit
vo
lume
Add
solvent
•
After
dilution:
Fewer
so
lute
particles
per
unit
volume
Because the number
of
moles
of
solute before the dilution is the same as that after dilution, we
can write
Equation 4.3
where the subscripts c and d stand for
concentrated and dilute, respectively. Thus, by knowing the
molarity
of
the concentrated stock solution (Me) and the desired final molarity (Md) and volume
(Ld)
of
the dilute solution, we can calculate the
vo
lume
of
stock solution required for the dilution (Le).
Because most
vo
lumes measured in the laboratory are in milliliters rather than liters, it is
worth pointing out that Equation 4.3 can be written with volumes
of
the concentrated and dilute
solutions in milliliters.
.
. . . . . . . . . . . .
. . . . . . . .
.
• • • •
·
•
•
•
•
•
•
·
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
In this form
of
the equation, the product
of
each side is in millimoles (mmol) rather than moles.
We apply Equation 4.3 in Sample
Problem 4.10.
Sample Problem 4.10
What
volume
of
12.0 M
Hel
, a
co
mmon
laboratory
stock
solution,
must
be
u
sed
to
prepare
250.0
mL
of
0.125
M
He]?
Strategy
Use
Equation
4.3 to
determine
the vo
lume
of
12.0 M
Hel
required
for
the
dilution.
Because
the
desired final vo
lum
e is given in milliliters, it will
be
convenient
to use
the
form
of
Equation
4.3
that
include
s milliliters.
Setup
Me
= 12.0
M,
Md
= 0.125
M,
mLd =
250.0
mL.
Solution
12.0 M X
mL
e =
0.l25
M X
250.0
mL
. . . . . . . . . . . . . .
.
~
=
0.l25
M X
250.0
mL =
2.60
mL
e 12.0 M
Prac.tic.e
Problem A
What
vo
lume
of
6.0
M H
2
S0
4
is n
ee
ded
to
prepare
500.0
mL
of
a solution
that
is 0.25 M in H
2
S0
4
?
Practice
·Problem B
What
vo
lum
e
of
0.20
M H
2
S0
4
ca
n
be
prepared
by
diluting 125
mL
of
6.0
M
H
2
S0
4
?
SECTION 4.5
Concentration
of
Sol
utions
141
Solution Stoichiometry
Soluble ionic compounds such as
KMn04
are strong electrolytes, so they undergo complete
dissociation upon dissolution and exist in solution entirely as ions.
KMn04
dissociates, for
example, to give 1 mole
of
potassium ion and 1 mole
of
permanganate ion for every mole
of
potassium permanganate. Thus, a 0.400 M solution
of
KMn04
will be 0.400 M in K+ and-
0.400 M in MnO 4 .
In the case
of
a soluble ionic compound with other than a
1:
1 combination
of
constituent
ions, we must use the subscripts in the chemical formula to determine the concentration
of
each
ion in solution. Sodium sulfate
(Na
1
S04) dissociates, for example, to give twice as many sodium
ions as sulfate ions.
Na2S0is)
H
2
0,
2Na+(aq) +
SO
~-(
aq)
Therefore, a solution that is 0.35
Min
Na2S04 is actually 0.70 M in
Na
+ and 0.35 M in
SO
~-
.
Frequently, molar concentrations
of
dissolved species are expressed using square brackets. Thus,
the concentrations
of
species in a 0.35 M solution
of
Na2S04 can be expressed as follows: [Na +] .
2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
.
~
. . . . . . . . . . . "
0.70 M and [SO 4 - ] = 0.35
M.
Sample Problem 4.11 lets you practice relating concentrations
of
:
•
compounds and concentrations
of
individual ions using solution stoichiometry. :
Using square-bracket notation, express the concentration
of
(a) chloride ion in a solution that is
1.02 M in AICI
3
, (b) nitrate ion
in
a solution that is 0.451
Min
Ca(N0
3
)2,
and (c)
Na
2
C0
3
in
a
solution
in
which [Na +] = 0.124
M.
Strategy
Use
the concentration given
in
each case and the stoichiometry indicated in the
conesponding
chemical
fOlTllula
to determine the concentration
of
the specified ion
or
compound.
Setup (a) There are 3 moles
of
Cl-
ion
for every 1
mole
of
AlCl
3
,
so the concentration
of
Cl-
will
be
three times the concentration
of
AlCl
3
.
•
(b) There are 2 moles
of
nitrate ion for every 1 mole
of
Ca(N0
3
)2,
Ca(N0
3
Ms)
H2
0,
Ca
2+
(aq) +
2NO](aq)
so [NO] ] will
be
twice
[Ca(N0
3
h
].
(c)
There
is 1 mole
of
Na
2
C0
3 for every 2 moles
of
sodium ion,
Na
2C03(S) H
2
0,
2Na
+(aq) +
CO
~
-
(aq)
so [Na
2C
0
3]
will
be
half
of
[
Na
+]. (Ass
ume
that
Na2C0
3 is the only source
of
a +
ion
s in this
solution.)
Solution
(a)
[CI-]
=
[AICH
X 3 mol Cl-
o 1 mol AICl
3
~1~.0~2~~~i
3
X 3 mol Cl-
L 1
3.06
mol
Cl-
L
= 3.06 M
3
_ 2 mol
NO]
(b)
[N0
3
]
=
[Ca(N0
3
)2]
X 1
mol
Ca(N0
3
)2
= 0.451 mol
Ca(H0
3
);
X 2 mol
NO
] _
L 1 _mol
Ca(N0
3
h
0.902 mol
NO]
L
= 0.902 M
(Continued)
•
•
•
'
Square
brackets
around
a
chemical
species
can
be
read
as
"the concentration
of"
that
species
.
For
example,
[Na
+]
is
read
as
"the concentration
of
sodium
ion."
If
we
only
need
to
express
the
concentration
of
the
compound,
rather
than
the
concentrations
of
the
individual
ions,
we
could
express
the
concentration
of
this
solution
as
[Na,S04]
= 0.35 M
•
142
CHAPTER
4
Reac
t ions in Aqueous
So
l
utions
Think About It
Make
sure that
units cancel properly.
Remember
that the concentration
of
an ion can
never be less than the concentration
of
its dissolved parent compound.
It
will always
be
the concentration
of
the parent
compound
time
s
it
s
stoichiometric subscript in the
chemical formula.
Ex
per
i
ments
t
ha
t me
as
ur
e the amount of
a s
ubstan
ce pres
en
t
ar
e
ca
ll
ed
qu
a
ntitative
a
na
l
ys
i
s.
Ac
co
rdin
g to the
in
formation
in
T
ab
le
4
.2,
Ag
el
is an
inso
l
ub
le
exce
ption to the
chlo
r
ides,
w
hi
ch
ty
pica
lly
are
so
lubl
e.
_ 0.124
~
X 1 mol
Na
2
C0
3
L
2
~
0.0620 mol
Na
2
C0
3
L
=
0.0620M
Practice Problem A Using the square-bracket notation, express the concentrations
of
ions in a
solution that is
0.
750
M
in
aluminum sulfate [AI
2
(
S0
4)3
].
Practice Problem B Using the square-bracket notation,
expr
ess the concentration
of
chloride ions
in
a solution that is 0.250 M in s
odium
chloride (NaCl) and 0.25 M in magnesium chloride (MgCI
2
).
Checkpoint 4.5
Concentration
of
Solutions
4.
5.1
Calculate the
molar
concentration
of
a
solution prepared by dissolving 58
.5
g
NaOH
in
enough
water to yield 1.25 L
of
solution.
a)
1.46M
b)
46.8 M
c)
2.14
X
10-
2
M
d)
1.l7M
e) 0.855 M
4.5.2
What
ma
ss
of
gl
uco
se (C
6
H
12
0
6
)
in
grams must
be
used in order to prepare
500
mL
of a solution that is 2.50
Min
glucose?
a)
225 g
b)
125 g
c)
200 g
d)
1.25 g
e)
625 g
4.5.3
4.5.4
What
volume in
mL
of
a 1.20 M
HC
I
solution
must
be diluted
in
order to
prepare
1.00 L
of
0.0150 M HCI?
a)
15.0
mL
b) 12.5
mL
c) 12.0
mL
d) 85.0
mL
e)
ll5
mL
A solution that is 0.18
Min
Na
2
C0
3 is
also . (Choose
all that apply.)
a)
0.18MinCO
~-
b) 0.18
Min
Na
+ .
c)
0.09 M in Na+
d)
0.09 M
in
CO
~-
e)
0.36
Min
Na
+
Aqueous Reactions and Chemical Analysis
.
. . . . . . . . .
. . . . . . . . .
.
Certain aqueous reactions are useful for determining how much
of
a particular substance is pres-
ent in a sample. For example,
if
we want to know the concentration
of
lead in a sample
of
water,
or
if
we need to know the concentration
of
an acid, knowledge
of
precipitation reactions, acid-
base reactions, and solution stoichiometry will
be
useful. Two common types
of
such quantitative
analyses are
gravimetric analysis and acid-base titration.
Gravimetric Analysis
Gravimetric analysis is an analytical technique based on the measurement
of
mass. One type
of
gravimetric analysis experiment involves the formation and isolation
of
a precipitate, such as
AgCI(s):
AgN0
3
(aq) + NaCI(aq) - -+.
NaN0
3
(aq) + AgCI(s)
SECTION
4.6 Aqueous Reactions and Chemical Analysis 143
This reaction is often used in gravimetric analysis because the reactants can be obtained in pure
form. The net ionic equation is
Ag+(aq)
+
Cl
- (aq)
AgCI(s)
Suppose, for example, that we wanted to test the purity
of
a sample
of
NaCI by determining the
percent by mass
of
Cl. First, we would accurately weigh out some NaCl and dissolve it
in
water.
To
this mixture, we would add enough
AgN0
3
solution to cause the precipitation
of
all the Cl-
ions present in solution as AgCl. (In this procedure NaCI is the limiting reagent and
AgN0
3
is the
excess reagent.) We would then separate, dry, and weigh the AgCl precipitate. From the measured
mass
of
AgCl, we would be able to calculate the mass
of
CI using the percent by mass
of
CI
in
AgCl. Because all the CI in the precipitate came from the dissolved NaCl, the amount
of
Cl that
we calculate is the amount that was present in the original NaCI sample. We could then calculate
the percent by mass
of
CI in the NaCI and compare it to the known composition
of
NaCl to deter-
mine its purity.
Gravimetric analysis is a highly accurate technique, because the mass
of
a sample can be
measured accurately. However, this procedure is applicable only to reactions that go to comple-
tion
or
have nearly 100 percent yield. In addition,
if
AgCI were soluble to any significant degree,
it would not be possible to remove all the
CI-
ions from the original solution, and the subsequent
calculation would be in error. Sample
Problem 4.12 shows the calculations involved in a gravimet-
ric experiment.
A 0.8633-g sample
of
an ionic. compound containing chloride ions and an unknown metal cation is
dissolved in water and treated with an excess
of
AgN0
3
.
If
1.5615 g
of
AgCl precipitate forms, what
is the percent by mass
of
Cl
in the original compound?
Strategy
Using the mass
of
AgCI precipitate and the percent composition
of
AgCI, determine
what mass
of
chloride the precipitate contains.
The
chloride in the precipitate was originally in the
unknown compound. Using the mass
of
chloride and the mass
of
the original sample, determine the
percent
Cl
in the compound.
Setup To determine the percent Cl in AgCl, divide the molar mass
of
CI by the
molar
mass
of
AgCI:
35.45 g
-:-::-:::-:-:
::
-c-=:-:::-:: :- X 100 % = 24.72 %
(35.45 g + 107.9 g) .
The
mass
of
Cl
in the precipitate is 0.2472 X 1.5615 g = 0.3860
g.
Solution
The
percent
Cl
in the unknown compound is the mass
of
Cl in the precipitate divided by
the mass
of
the original sample:
0.3860 g
c:-::-=-::-
- X 100% = 44.71 % Cl
0.8633 g
Practice Problem A A 0.5620-g sample
of
an ionic compound containing the bromide ion (Br- )
is dissolved in water and treated with an excess
of
AgN0
3
.
If
the mass
of
the
AgBr
precipitate that
forms is 0.8868 g, what is the percent by mass
of
Br
in the original compound?
. Practice Problem B A sample that is 63.9 percent chloride by mass is dissolved in water and treated
with an excess
of
AgN0
3
.
If
the mass
of
the AgCI precipitate that forms is 1.085 g, what was the
mass
of
the original sample?
~,
_
Gravimetric analysis is a quantitative method,
not
a qualitative one, so it does not establish
:he identity
of
the unknown substance. Thus, the results in Sample Problem 4.12 do
not
identify
:he cation. However, knowing the percent by mass
of
CI greatly helps us narrow the possibili-
ties. Because no two compounds containing the same anion (or cation) have the same percent
;:o
mposition by mass, comparison
of
the percent
by
mass obtained from gravimetric analysis
":\i
th that calculated from a series
of
known compounds could reveal the identity
of
the unknown
~
o
mpounds.
Think
About
It
Pay close attention
to which numbers correspond
to which quantities.
It
is easy in
this type
of
problem to lose track
of
which mass is the precipitate
and which is the original sample.
Dividing by the wrong mass at
the end will result in an incorrect
answer.
144
CHAPTER
4 Reactions in Aqueous Solutions
•
Multimedia
Chemical reactions- titrations.
Standardization
in
this
context
is
the
meticulous
determination
of
concentration.
Note
that
KHP
is
a
monoprotic
acid,
so
it
reacts
in
a
1:
1 ratio with
hydroxide
ion
.
The
endpoint
in
a titration
is
used
to
approximate the
equivalence
point. A
careful
choice
of
indicators,
which
we
will
discuss
in
Chapter
16,
helps
make
this
approximation
reasonable.
Phenolphthalein,
although
very
common,
is
not appropriate for
every
acid-base
titration.
Figure 4.11 Apparatus for titration.
Acid-Base Titrations
Quantitative studies
of
acid-base neutralization reactions are most conveniently carried out using
a technique known as titration. In
titration, a solution
of
accurately known concentration, called
a
standard solution, is added gradually to another solution
of
unknown concentration, until the
chemical reaction between the two solutions is complete, as shown in Figure 4.11.
If
we
know the
volumes
of
the standard and unknown solutions used in the titration, along with the concentration
of
the standard solution, we can calculate the concentration
of
the unknown solution.
A solution
of
the strong base sodium hydroxide can
be
used as the standard solution in a
titration, but it must first be
standardized, because sodium hydroxide in solution reacts with car-
hon'
dIOXIde
'
In
' the'
aiT:
iiiabng
'
Its
'con"centratloiiliiistable 'over'
dill
·
e.
We
'can' standardize the sodium
hydroxide solution by titrating
it
against an acid solution
of
accurately known concentration.
The
acid often chosen for this task is a monoprotic acid called potassium hydrogen phthalate (KHP),
for which the molecular formula is KHC
s
H
4
0
4
.
KHP
is a white, soluble solid that is commercially
available in highly pure form.
The
reaction between KHP and sodium hydroxide is
<
.
.
. . .
.
.
.
.
.
KHC
s
H
4
0iaq)
+ NaOH(aq) • KNaC
s
H
4
04(aq)
+ H
2
0(l)
and the net ionic equation is
To standardize a solution
of
NaOH
with KHP, a known amount
of
KHP
is transferred to
an
Erlenmeyer flask and
some
distilled water is added to make up a solution. Next,
NaOH
solution is
carefully added to the
KHP
solution from a buret until all the acid has reacted with base. This point
in the titration, where the acid has been completely neutralized, is called the
equivalence point.
It
.
,
,
is usually signaled
by
the endpoint, where an indicator causes a sharp change in the color
of
the
solution. In acid-base titrations,
indicators are substances that have distinctly different colors in
acidic and basic media.
One
commonly used indicator is phenolphthalein, which is colorless in
acidic and neutral solutions
but
reddish
pink
in basic solutions. At the equivalence point, all the
KHP present has
been
neutralized
by
the added
NaOH
and the solution is still colorless. However,
if
we
add
just
one more drop
of
NaOH
solution from the buret, the solution will
be
basic and will
immediately turn pink.
Sample
Problem
4.13 illustrates
just
such a titration.
'Y
" «
"
'.
r
-
~
L-
.;'
i
\
I'ItENOlPHTHAWH
INDICATOR
~
._
,,
_J
,.
I·
1.
~
. 0
•
I
I
,
t
«
SECTION
4.6
Aqueous Reactions and Chemical Analysis 145
In
a titration experiment, a student finds that 25.49
mL
of
an
NaOH
solution is needed to
ne
utralize
0.7137 g
of
KHP.
What
is the concentration (in M)
of
the N
aOH
solution?
Strategy
Using
the
ma
ss given
and
the
molar
ma
ss
of
KHP,
determine
the
number
of
moles of KHP.
Recognize
that
the
number
of
moles
of
N
aOH
in the vo
lume
giv
en
is equal to the
numb
er
of
mole
s
of
KHP.
Divide
mole
s
of
NaOH
by v
olume
(in liters) to get molarity.
Setup
The
molar
mas
s
of
KHP
(
KHC
8
H
4
0
4
)
= [39.1 g + 5(
l.008
g) + 8(12.01 g) + 4(16.00 g)] =
204.2 g/mol.
Solution
0.7
13
7 g
mole
s
of
KHP
= 204.2 g/
mol
=
0.003495
mol
Because
moles
of
KHP
=
mole
s
of
N
aOH
, then
mo
les of
Na
OH
= 0.003495 mol.
1
't
f N
OH
0.00
3495 mol
01371
M
mo
an
y 0 a = 0.
02549
L = .
Practice Problem A
How
man
y
gram
s
of
KHP
are n
ee
ded to neutralize
22
.36
mL
of
a 0.1205 M
NaOH
solution?
Practice Problem B
What
volume (in
mL
)
of
a
0.2550
M N
aOH
solution can
be
neutraliz
ed
by
10.75 g
of
KHP?
LI
__________________________________________________________________________
III
The reaction between NaOH and KHP is a relatively simple acid-base neutralization. Sup-
pose, though, that instead
of
KHP, we wanted to use a diprotic acid such as H
2
S0
4
for the titration.
The reaction
is
represented by
Because
2 mol NaOH
"'"
1 mol H
2
S0
4
, we need twice as much
NaOH to react completely with an
H,
S0
4 solution
of
the same molar concentration and volume as a monoprotic acid such as HCl.
On the other hand, we would need twice the amount
of
HCI to neutralize a
Ba
(
OH
)2
solution com-
pared to
an
NaOH solution having the same concentration and volume because 1 mole
of
Ba
(
OH
)2
yields 2 moles
of
OH
- ions:
2HCI(aq) + Ba(OH
hC
aq)
+
. Ba
CI
2
(
aq
) +
2H
,O(l)
In
any acid-base titration, regardless
of
what acid and base are reacting, the total number
of
moles
of H+ ions that have reacted at the equivalence point must be equal to the total number
of
moles
of
OH- ions that have reacted. Sample Problem 4.
14
explores the titration
of
an
NaOH solution
with a diprotic acid.
~,,'"
*'
·T
§!l
mp
~~J.!!l,,~ii~
What
volume
(in
mL)
of
a 0.203 M N
aOH
solution is n
ee
ded to neutralize 25.0
mL
of
a
0.1
88 M
H
2
S0
4
solution?
Strategy
First, write
and
balance
the
chemical
equation that corres
pond
s to the neutralization
reaction:
The
base
and
the
diprotic
acid
combine
in a 2: 1 ratio: 2N
aOH
"" H
2
S0
4
,
Use
th
e mo
larit
y
and
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
the
volume
given
to
determine
the
number
of
millimole
s
of
H
2
S0
4
,
Us
e the
numb
er
of
millim
oles
of
H
2
S0
4
to
determine
the
number
of
millimole
s of N
aOH.
Us
ing
millim
o
le
s
of
N
aOH
and
th
e
concentration
given,
determine
the
v
olume
of
N
aOH
that
will
contain
the c
orr
ect
numb
er
of
millimole
s.
( Continued)
Think
About
It
Remember
that
molarit
y
can
also
be
defined as
mrnolimL. Try solving the
problem
again us
ing
millimoles and
make
s
ure
you g
et
the s
ame
answer.
0.003495
mol
= 3.495 X
10-
3
mol
= 3.495 mrnol
and
3.495 mrnol = 0 1371 M
25.49
mL
.
Re
member:
molarity x
mL
= mil
lim
ol
es.
This
saves
st
e
ps
in
titrat
ion
problems
.
•
146 CHAPTER 4 Reactions in Aqueous Solutions
Think
About
It
Notice
that the
two concentrations
0.203 M and
0.188 M are similar.
Both
round
to the
same
value
(-0
.
20
M) to
two significant figures.
Therefore
,
the titration
of
a diprotic
acid
with
a
monobasic
base
of
roughly
equal concentration
should
require
roughly
twice
as
much
base
as the
beginning
volume
of
acid:
2
X
25.0
mL
= 46.3
mL.
Think
About
It
In
order
for this
technique
to work,
we
must
know
whether
the acid is
monoprotic,
diprotic,
or
polyprotic. A diprotic
acid, for example,
would
combine
in a 1:2 ratio with the base, and
the result
would
have
been
a
molar
mass
twice
as large.
Setup
The
necessary
conversion factors are:
.
2rnmol
NaOH
From
the
balanced
equatIOn: I H
SO
1 rnmo 2 4
From
the molarity
of
the
NaOH
given:
0.2~3~~~~~OH
Solution
rnillimoles
of
H
2
S0
4
= 0.188 M X 25.0
mL
=
4.70
rnmol
millimoles
of
NaOH
required = 4.70
.!JUDo}
II
2
S0
4
- X 2 rnmol
Na~~
_
=
9.40
rnmol
NaOH
l.mm
o1
I12
4
- 1
mLNaOH
vo
lume
of
0.
203
M
NaOH
=
9.40
!DIDgl
NaOH
X 0 03
oFi
= 46.3
mL
.2 .mmol
Na
Practice Problem A
How
many
milliliters
of
a 1.42 M H
2
S0
4
solution are
needed
to
neutrali
ze
95.5
mL
of
a
0.336
M
KOH
solution?
Practice Problem B
How
many
milliliters
of
a 0.211 M
HCl
solution are
needed
to neutralize
275
mL
of
a
0.0350
M
Ba(OH
h
so
lution
?
Sample Problem 4.15 shows how titration with a standard base can be used to determine the
molar mass
of
an unknown acid.
•
Sample Problem 4.15
A
0.1216-g
sample
of
a
monoprotic
acid
is dissolved
in
25
mL
water, and
the
resulting solution is
titrated
with
0.1104
M
NaOH
so
lution. A
12.5-mL
volume
of
the
ba
se
is
required
to neutralize the
acid.
Calculate
the
molar
mass
of
the
acid.
Strategy
Using
the
concentration
and
volume
of
the
ba
se,
we
can
determine
the
number
of
moles
of
ba
se
required
to neutrali
ze
the acid.
We
then
determine
the
number
of
moles
of
acid and divide the
mass
of
the
acid
by
the
number
of
moles
to
get
molar
mass
.
Setup
Because
the
acid
is
monoprotic,
it
will
react
in
a
1:
1
ratio
with
the
base; therefore, the
number
of
moles
of
acid
will
be
equal
to the
number
of
moles
of
base.
The
volume
of
base
in
liters
is
0.0125
L.
Solution
0.0125
L
moles
of
base
= =
0.00138
mol
0.1104
mol/L
Because
moles
of
ba
se
= moles
of
acid, the
moles
of
acid =
0.00138
mol.
Therefore,
0.1216
g
molar
mass
of
the
acid
= = 88.1
g/mol
0.00138
mol
Practice Problem A
What
is the
molar
mass
of
a
monoprotic
acid
if
28.1
mL
of
0.0788
M
NaOH
is
required
to
neutralize
a
0.205-g
sample?
Practice Problem B
What
is the
molar
mass
of
a diprotic acid
if
30.5
mL
of
0.1112
M
NaOH
is
required
to neutralize a
0.1365-g
s
ample?
4.6 Aqueous Reactions and Chemical Analysis 147
Checkpoint
4.6
Aqueous Reactions and Chemical Analysis
4.6.1
4.6.2
4.6.3
What
ma
ss
of
AgCl will be recovered
if
a solution containing 5.00 g
of
NaCl
is treated with enough
AgN0
3
to
precipitate all the chloride ion?
a)
12.3 g
b)
5.00 g
c)
3.03 g
d)
9.23 g
e)
10.0 g
A
1O.0-g sample
of
an unknown
ionic compound is dissolved, and the
solution is treated with enough
AgN0
3
to precipitate all the chloride ion.
If
30.1 g
of
AgCl are recovered, which
of
the following compounds could be the
unknown?
a)
NaCI
b)
NaN0
3
c)
BaCl
2
d)
MgCl
2
e) KCl
What
volume
of
0.110
MNaOH
is required to neutralize 75.5
mL
of
a solution that is 0.0798 M
in
HCl
?
a)
66.3
mL
b) 54.8
mL
c)
104mL
d)
95.4
mL
e) 75.5
mL
4.6.4
4.6.5
4.6.6
If
25.0
mL
of
an H
2
S0
4
solution
requires 39.9
mL
of
0.228 M NaOH to
neutralize, what is the concentration
of
the H
2
S0
4
solution?
a)
0.728 M
b)
0.364M
c)
0.182 M
d)
0.228M
e) 0.91OM
What
volume
of
0.144 M H
2
S0
4
is required to neutralize 25.0 mL
of
0.0415
MBa(OHh?
a)
7.20
mL
b) 3.60
mL
c) 14.4
mL
d) 50.0
mL
e) 12.5
mL
What
volume of 0.110 M HCl
is required to neutralize
25 .0 mL
of
0.0415
MBa(OH)
2?
a) 7.20 mL
b)
3.60
mL
c) 14.4
mL
d) 50.0 mL
e) 12.5
mL
/ -
148
CHAPTER
4 Reactions in Aqueous Solutions
Applying
What
You've Learned
The balanced overall equation for the Breathalyzer reaction is
3CH
3
CH
2
0H(g)
+ 2K2Cr207(aq) + 8H
2
SOiaq)
•
3HC
2
H
3
0
2
(aq) + 2Cr2(S04Maq) +
2K
2
SOiaq)
+ 11H
2
0(I)
Problems:
a)
Classify each
of
the species in the Breathalyzer reaction as a strong electrolyte,
weak electrolyte, or nonelectrolyte.
[
~.
Sample
Problem
4.2]
b)
Write the ionic and net ionic equations for the Breathalyzer reaction.
[
~.
Sample
Problem
4.4]
c)
Determine the oxidation number
of
each element in the overall equation.
[
~.
Sample
Problem
4.6]
d)
One manufacturer
of
Breathalyzers specifies a potassium dichromate concentration
of
0.025 percent weight per volume (0.025 g K2Cr2
07
per 100
mL
of
solution).
Express this concentration in terms
of
molarity.
[
~.
Sample
Problem
4.9]
e) What volume
of
0.014 M stock solution
of
K2Cr207 would have to be diluted to
250
mL
to make a solution
of
the specified concentration?
[
~.
Sample
Problem
4.10]
f) Using square-bracket notation, express the molarity
of
each ion in a K2Cr207
solution
of
the specified concentration.
[
~.
Sample
Problem
4.11]
CHAPTER SUMMARY
Section 4.1
• A solution is a homogeneous mixture
con
sisting
of
a solvent and one
or
more
dissolved species called solutes.
• An electrolyte is a
compound
that dissolves in water to give an
electrically conducting solution.
Nonelectrolytes dissolve to give
nonconducting solutions. Acids
and
bases are electrolytes.
• Electrolytes may
be
ionic
or
molecular. Ionic electrolytes undergo
dissociation
in
solution; molecular electrolytes undergo ionization.
Strong electrolytes
dissociate (
or
ionize) completely. Weak
electrolytes
ionize only partially.
Section 4.2
• A precipitation reaction results
in
the formation
of
an insoluble
product called a
precipitate.
From
general guidelines about solubilities
of
ionic compounds,
we
can predict whether a precipitate will form
in
a reaction.
• Hydration is the process in which water molecules surround solute
particles.
• Solubility is the
amount
of
solute that will dissolve
in
a specified
amount
of
a given solvent at a specified temperature.
• A
molecular
equation represents a reaction as though
none
of
the
reactants
or
products has dissociated
or
ionized.
• An ionic equation represents the strong electrolytes in a reaction as
•
IOns.
• A spectator
ion
is one that is not involved
in
the reaction. Spectator
ions appear
on
both
sides
of
the ionic equation. A
net
ionic equation
is an ionic equation from which spectator ions have
been
eliminated.
Section 4.3
• The hydrogen ion
in
solution is
more
realistically
repr
esented as the
hydronium
ion
(H
3
0 +
).
The
terms hydrogen ion, hydronium io
n,
and
proton are used interchangeably
in
the context
of
acid-base reactions.
•
Arrhenius
acids ionize
in
water to give H+ ions, whereas
Arrhenius
bases ionize (or dissociate) in water to give
OH
- ions.
Br¢nsted
acids
donate protons (H+ ions), whereas
Br¢nsted
bases accept protons.
• Brliinsted acids may
be
monoprotic, diprotic,
or
triprotic, depending
on the
number
of
ionizable hydrogen atoms they have.
In
general, an
acid with
more
than
one
ionizable hydrogen atom is called polyprotic.
• The reaction
of
an acid and a
ba
se is a neutralization reaction.
The
products
of
a neutralization reaction are water and a salt.
KEyWORDS
Activity series, 130
Arrhenius acid, 123
Arrhenius base, 123
Base, 112
Brliinsted acid, 123
Brliinsted base,
123
Combustion, 134
Concentration,
136
Dilution, 137
Diprotic acid, 123
Di
splacement reaction, 130
Disproportionation reaction, 134
Dissociation, 112
Electrolyte, 112
KEY WORDS
149
Section 4.4
• Oxidation-reduction, or redox, reactions are those in which electrons
are exchanged. Oxidation and reduction always occur simultaneously.
You cannot
ha
ve one without the other.
• Oxidation is the loss
of
electrons; reduction is the gain
of
electrons.
In
a redox reaction, the oxidizing
agent
is the reactant that
get
s reduced
and
the reducing
agent
is the reactant that gets oxidized.
• Oxidation
numbers
or
oxidation states help us keep track
of
charge
distribution and are assigned to all atoms in a compound
or
ion
according to specific rules.
•
Man
y redox reactions can
be
further classified as combination,
decomposition, displacement, hydrogen displacement, combustion,
or disproportionation reactions.
The
activity series can
be
used to
determine whether
or
not a displacement reaction will occur.
• A half-reaction is a
ch
emical equation representing only the oxidation
or
only the reduction
of
an oxidation-reduction reaction. Redox
equations, which
mu
st be balanced for both
ma
ss and charge, can be
balanced using the
half-reaction method.
Section 4.5
•
The
concentration
of
a solution is the amount
of
solute dissolved in
a given amount
of
solution. Molarity (M)
or
molar
concentration
expresses concentration as the number
of
moles
of
solute in 1 L
of
solution .
•
Addin
g a s
ol
ve
nt
to a solution, a process known as dilution, decreases
the concentration (molarity)
of
the solution without changing the total
number
of
mole
s
of
solute pres
ent
in the solution.
Section 4.6
• Gravimetric analysis oft
en
in
volves a precipitation reaction.
• Acid-base titration involves an acid-base reaction. Typically, a
solution
of
known concentration (a standard solution) is added
gradually to a solution
of
unknown concentration with the goal
of
determining the
unkno
wn concentration.
•
The
point at which the reaction
in
the titration is complete is called
the
equivalence point.
An
indicator is a substance that changes color
at
or
near the equivalence point
of
a titration.
The
point at which the
indicator changes color is called the
endpoint
of
the titration.
Endpoint
, 144
Equivalence point, 144
Gravimetric analysis, 142
Half-reaction,
127
Half-reaction method, 131
Hydration, 117
Hydrogen dis
placem
ent,
13
3
,
Hydronium ion, 123
Indicator, 144
Ionic equation,
120
Ionization, 112
Molar concentration, 136
Molarit
y,
136
Molecular equation, 119
\ \ \
\.
150
CHAPTER
4 Reactions in Aqueous Solutions
Monoprotic acid, 123
Net ionic equation,
120
Neutralization reaction, 124
Nonelectrolyte,
ll2
Oxidation, 127
Oxidation number, 127
Oxidation state, 127
KEY EQUATIONS
Oxidation-reduction reaction,
Oxidizing agent, 127
Polyprotic acid, 123
Precipitate, 117
Precipitation reaction, 117
Redox reaction, 126
Reducing agent, 127
126
4.1 I
. ty moles solute
mo
an
= :::=c:c: c:: : :.:=-
liters solution
Reduction,
127
Spectator ion,
120
Salt, 124
Standard solution, 144
Solubility,
ll7
Strong electrolyte, 112
Solute, 112
Titration, 144
Solution, 112
Triprotic acid, 123
Solvent, 112
Weak electrolyte, 112
4.2
4.3
moles of solute before dilution
= moles
of
solute after dilution
Me X Le =
Md
X
Ld
QUESTIONS AND PROBLEMS
Section 4.1: General Properties
of
Aqueous Solutions
Review Que
st
io
ns
4.1 Define solute, solvent, and solution by describing the process
of
dissolving a solid in a liquid.
4.2
What
is the difference between a nonelectrolyte and an
electrolyte? Between a weak electrolyte and a strong electrolyte?
4.3
4.4
What
is the difference between the symbols
+,
and
+.=='
in
chemical equations?
Water is an extremely weak electrolyte and therefore cannot
conduct electricity.
Why
are we often cautioned not to
ope
rate
electrical appliances when
our
hands are wet?
4.5 Lithium fluoride (LiF) is a strong electrolyte.
What
species are
present in
LiF
(aq)?
Problems
4.6
The
aqueous solutions
of
three compounds are shown in the
diagram. Identify each compound as a nonelectrolyte, a
weak
electrolyte,
or
a strong electrolyte.
•
•
(a) (b)
(c)
4.7
4.8
4.9
4.10
4.11
Which
of
the following diagrams best represents the hydration
of
NaC! when dissolved in water?
The
C!- ion is larger in size than
the
Na
+ ion.
•
•
•
(a)
(b) (c)
Identify each
of
the following substances as a strong electrolyte,
weak electrolyte,
or
nonelectrolyte: (a) H
2
0,
(b) KCI, (c)
HN0
3
,
(d)
HC
2
H
3
0
2
,
(e) C
12
H
22
0".
Identify each
of
the following substances as a strong electrolyte,
weak electrolyte,
or
nonelectrolyte: (a)
Ba(N0
3)z
' (b) Ne,
(c)
NH
3
, (d)
NaOH
.
The
passage
of
electricity through an electrolyte solution is
caused by the movement
of
(a) electrons only, (b) cations only,
(c) anions only, (d) both cations and anions .
Predict and explain which
of
the following systems are
electrically conducting: (a) solid
NaC
I, (b)
mo
lten NaCI, (c) an
aqueous soluti
on
of
NaC!.
4.12 You are given a water-soluble compound
X. Describe how you
would determine whether it is an electrolyte
or
a nonelectrolyte.
If
it is an electrolyte, how would you determine whether
it
is
strong
or
weak?
4.13 Explain why a solution
of
HCI in benzene does not conduct
electricity but in water it doe
s.
Section 4.2: Precipitation Reactions
Review Questions
4.14 Describe hydration. What properties
of
water enable its
molecules to interact with ions in solution?
4.15
What
is the difference between an ionic equation and a molecular
equation?
4.16
What
is the advantage
of
writing
net
ionic equations?
Problems
4.17 Two aqueous solutions
of
AgN0
3
and NaCl are mixed. Which
of
the following diagrams best represents the mixture?
4.
18
Na+(aq)
CI
-(
aq)
Ag+(aq)
N0
3
(aq)
(a)
Ag
+(a
q)
CI- (aq)
(b)
Na
+(
aq)
N0
3
(aq)
AgCI(s)
(c)
AgCI(s)
NaN°
3
(s)
(d)
Two aqueous solutions
of
KOH and MgCI2 are mixed. Which
of
the following diagrams best represents the mixture?
KCI(s)
(a)
K
+(a
q)
CI
- (aq)
Mg(OHh(s)
(b)
K
+(a
q)
CI-(aq)
Mg2+(aq)
OH
-(
aq)
(c)
KCI(s)
Mg(OH)z(s)
(d)
4.19 Characterize the following compounds
as
soluble or insoluble in
water: (a)
Ca
3(
P04h,
(b) Mn(OH)z, (c)
AgCI0
3
,
(d) K
2
S.
4.20 Characterize the following compounds
as
soluble or insoluble
in water: (a)
CaC0
3
,
(b) ZnS04, (c)
Hg(N0
3
)2,
(d)
HgS0
4
,
(e) NH
4
CI0
4
.
4.21 Write ionic and net ionic equations for the following reactions:
(a)
AgN0
3
(aq) +
Na2S0iaq)
•
(b) BaCI
2
(aq) + ZnS04(aq) •
(c) (NH4)2C03(aq) + CaClz(aq) •
•
4.22 Write ionic and net ionic equations for the following reactions:
(a) Na2S(aq) +
ZnCI
2
(aq)
•
(b) K
3
P0
4
(aq)
+
3Sr(N0
3
)z(
aq)
+.
(c)
Mg(N0
3
M aq)
+ 2NaOH(aq)
-_.
4.23 Which
of
the following processes will likely result in a
precipitation reaction? (a) Mixing an
NaN0
3
solution with a
CUS04 solution. (b) Mixing a BaCl
2
solution with a K
2
S0
4
solution. Write a net ionic equation for the precipitation reaction.
Section 4.3: Acid-Base Reactions
Review Questions
~.2
4
List the general properties
of
acids and bases.
~.2
5
Give Arrhenius's and Br¢nsted's definitions
of
an acid and a base.
Why
are Br¢nsted's definitions more useful in describing acid-
base properties?
~.
26
Give an example
of
a monoprotic acid, a diprotic acid, and a
triprotic acid.
QUESTIONS
AND
PROBLEMS
151
4.27 What are the characteristics
of
an
acid-base neutralization
reaction?
4.28 What factors qualify a compound as a salt? Specify which
of
the
following compounds are salts: CH
4
, NaP,
NaOH,
CaO
, BaS04,
RN0
3
,
NH
3
,
KEr.
4.29 Identify the following as a weak or strong acid or base: (a) NH
3
,
(b)
H
3
P0
4
,
(c) LiOH, (d)
HCOOH
(formic acid), (e) H
2
S0
4
,
(f)
HF
, (g) Ba(OH)z.
Problems
4.30 Identify each
of
the following species as a Br¢nsted acid, base, or
both: (a) HI, (b)
C
2
H
3
0;-
, (c) H
2
P0
4',
(d)
HS04'.
4.31 Identify each
of
the following species as a Br¢nsted acid, base, or
both: (a)
po
i-,
(b) CIO
;-,
(c)
NHt,
(d)
HCO
}.
4.32 Balance the following equations and write the corresponding
ionic and net ionic equations (if appropriate):
4.33
(a)
HBr
(aq) + NH3(aq) •
(b)
Ba(OHMaq)
+ H
3
POiaq)
•
(c)
HCIOiaq)
+
Mg(OHMs)
•
Balance the following equations and write the corresponding
ionic and net ionic equations
(if
appropriate):
(a)
HC
z
H
3
0
2
(aq)
+
KOH(aq)
•
(b) H
2
C0
3
(aq) + NaOH(aq) •
(c)
HN0
3
(aq)
+
Ba(OHMaq)
•
Section 4.4: Oxidation-Reduction Reactions
Review Questions
4.34 Give an example
of
a combination redox reaction, a
decomposition redox reaction, and a displacement redox reaction.
4.35 All combustion reactions are redox reactions. True or false?
Explain.
4.36
What
is an oxidation number? How is it used to identify redox
reactions? Explain why, except for ionic compounds, the
oxidation number does
not
have any physical significance.
4.37 (a) Without referring to Figure 4.7, give the oxidation numbers
of
the alkali and alkaline earth metals in their compounds. (b) Give
the highest oxidation numbers that the Groups
3A-7
A elements
can have.
4.38 How is the activity series organized? How is it used in the study
of
redox reactions?
4.39
Use the following reaction to define the terms redox reaction,
half-reaction, oxidizing agent,
and reducing agent:
4Na
(s) + Oz(g) •
2Na
z
O(s).
4.40
Is it possible to have a reaction in which oxidation occurs and
reduction does not? Explain.
Problems
4.41
For the complete redox reactions given here, break down each
reaction into its half-reactions, identify the oxidizing agent, and
identify the reducing agent.
(a) 2Sr
+ O
2
-_.
2SrO
(b) 2Li + H2 • 2LiH
(c) 2Cs +
Br
2 • 2CsBr
(d) 3Mg
+ N2 •
Mg
3NZ
152
CHAPTER
4 Reactions in
Aqueous
Solutions
4.42
For
the complete
redox
reactions given here, write the half-
reactions and identify the oxidizing and reducing
agents·:
4.43
4.44
(a)
4Fe
+
30
2
-_
.
2F~0
3
(b) CI
2
+
2NaBr
• 2NaCI +
Br2
(c) Si +
2F2
•
Sif
4
(d) H2
+ Cl
2
• 2HCI
Arrange the following species in order
of
increasing oxidation
number
of
the sulfur atom: (a) H
2
S, (b) S8, (c) H
2
S0
4
,
(d)
S2
- ,
(e) HS - ,
(f)
50
2
,
(g)
50
3
.
Phosphorus
forms many oxoacids. Indicate the oxidation
numb
er
of
phosphorus
in
each
of
the following acids: (a)
HP0
3
,
(b) H
3
PO
Z
'
(c) H
3
P0
3
,
(d) H
3
P0
4
,
(e) H
4
P
2
0
7
, (f) H
S
P
3
0IQ.
4.45 Give the oxidation numbers for the underlined atoms in the
following molecules and ion
s:
(a) CIF, (b)
IF
7
, (c)
CH
4
, (d)
C
2
H
z,
(e) C
Z
H
4
,
(f) K2Cr04, (g) K2Cr207, (h)
KMn02'
(i)
NaHC0
3
,
(j) Li
z,
(k)
NaI0
3
,
(I) KO
z,
(m)
PF
6"
, (n) KAuCI
4
·
4.46 Give the oxidation number for the following species: H
2
,
Se
s, P
4
,
0,
U, As
4
, B
12
.
4.47 Give the oxidation
number
s for the underlined atoms in the
following molecules and ions: (a) Cs
2
0,
(b) CaI
2
, (c)
A1
2
0
3
,
(d) H
3
As0
3
, (e)
Ti0
2
,
(f)
MoO
~-,
(g)
PtC1
~
-
,
(h)
PtCI
~
-
,
(i)
SnF
2
,
G)
CIF
3
,
(k)
SbF
6·
4.48 Give the oxidation numbers for the underlined atoms in the
following molecules and ions: (a)
Mg
3
N
2
, (b)
Cs0
2
, (c)
CaC
2
,
(d)
CO
~
-
,
(e)
C20~
-
,
(f)
ZnO
i- , (g)
NaBH
4
, (h)
WO
~
-
.
4.49 Nitric acid is a strong oxidizing agent. State which
of
the
following species is
least likely to be produced when nitric
acid reacts with a strong reducing agent s
uch
as z
inc
metal, and
explain why:
N
2
0,
NO,
N0
2
,
N
2
0
4
, N
2
0
S
'
NH
r.
4.50
Determine
which
of
the following metals can react with water:
(a) Au, (b) Li, (c) Hg, (d) Ca, (e)
Pt.
4.51
One
of
the following oxides does not react with molecular
oxygen:
NO, N
2
0,
50
2
,
50
3
, P
4
0
6
.
Based on oxidation numbers,
which one is it? Explain.
4.52
Predict the
outcome
of
the reactions represented by the following
equations by using the activity series, and balance the equations.
(a) Cu(s) +
HCI(aq)
•
(b)
Au
(s) +
NaBr(aq)
•
(c)
Mg(s)
+ CuS04(aq) •
(d)
Zn(s
) +
KBr(aq
) •
4.53 Classify the following redox reactions as combination,
decomposition,
or
displacement:
(a)
2H
2
0
2
•
2H
2
0
+ O
2
(b)
Mg
+
2AgN0
3
•
Mg(N0
3
)2
+
2Ag
(c)
NH
4
N0
2
•
N2
+
2H
2
0
(d) H2 +
Br
2 •
2HBr
4.54
Classify the following redox reactions as combination,
decomposition,
or
displacement:
(a)
P
4
+ IOCI
2
• 4PCI
s
(b)
2NO
•
N2
+ O
2
(c)
CI
2
+ 2KI • 2KCI + 12
(d)
3HN0
2
•
RN0
3
+ H
2
0 +
2NO
Section 4.5: Concentration
of
Solutions
Review
Questions
4.55 Write the equation for calculating molarity.
Why
is molarity a
convenient concentration unit in chemistry?
4.56 Describe the steps involved in preparing a solution
of
known
molar concentration using a volumetric
flask.
4.57 Describe the
ba
sic steps involved in diluting a solution
of
known
concentration.
4.58 Write the equation that enables us to calculate the concentration
of
a diluted solution. Give units for all the terms.
Problems
4.59 Calculate the mass
of
KI in grams required to prepare 5.00 X
10
2
mL
of
a 2.80 M solution.
4.60
De
scribe how you would prepare
250
mL
of
a 0.707 M
NaN0
3
solution.
4.61 How many moles
of
MgCI2 are
pre
sent
in
60.0
mL
of
a 0.100 M
MgCI2 solution?
4.62 How many grams
of
KOH
are present in
35.0
mL
of
a
5.50
M
KOH solution?
4.63 Calculate the molarity
of
each
of
the following solutions:
(a)
29.0 g
of
ethanol
(C
2
H
s
OH) in 545 mL
of
solution,
(b) 15.4 g
of
sucrose (C
12
H
22
0
J
J) in
74
.0
mL
of
solution,
(c)
9.00 g
of
sodium chloride (NaCI) in 86.4 mL
of
solution.
4.64 Calculate the molarity
of
each
of
the following solutions:
(a) 6.57 g
of
methanol (CH
3
0H)
in 1.50 X 10
2
mL
of
solution,
(b)
10.4 g
of
calcium chloride (CaCI
2
) in 2.20 X 10
2
mL
of
solution, (c) 7.82 g
of
naphthalene
(CIQH
8
)
in 85.2 mL
of
benzene
solution.
4.65 Calculate the volume in milliliters
of
a solution required to
pro
vi
de the following: (a) 2.14 g
of
so
dium
chloride from a 0.270
M solution, (b) 4.30 g
of
ethanol from
aLSO
M solution, (c) 0.85
g
of
acetic acid (
HC
2
H
3
0
2
) from a
0.30 M solution.
4.66
Det
ermine how many grams
of
each
of
the following solutes
would
be
needed to make 2.50 X 10
2
mL
of
a 0.100 M solution:
(a) cesium iodide (CsI), (b) sulfuric acid (H
2
S0
4
), (c) sodium
carbonate
(N
a
2C0
3), (d) potassium dichromate (K2Cr207),
(e) potassium permanganate
(KMn04)
.
4.67
De
scribe how to prepare 1.00 L
of
a 0.646 M HCI solution,
starting with a
2.00 M HCI solution.
4.68 Water is added to
25.0
mL
of
a 0.866 M
KN0
3
solution until
the volume
of
the solution is exactly
500
mL.
What
is the
concentration
of
the final solution?
4.69 How would you prepare 60.0
mL
of
0.200 M
HN0
3
from a stock
solution
of
4.00 M
HN0
3
?
4.70 You have 505
mL
of
a 0.125 M HCI solution and you want to
dilute it to exactly
0.100
M.
How much water should you add?
4.71 A volume
of
35.2
mL
of
a 1.66 M
KMn04
solution is mixed
with 16.7
mL
of
a 0.892 M
KMn04
solution. Calculate the
concentration
of
the final solution.
4.72 A volume
of
46.2
mL
of
a 0.568 M calcium nitrate
[Ca(N0
3
h]
solution is mixed with 80.5
mL
of
a
l.396
M calcium nitrate
solution. Calculate the concentration
of
the final solution.
4.73
The
general test for Type 2 diabetes is that the blood sugar
(glucose, C
6
H
12
0
6
)
level should be below 120
mg
per
deciliter.
Convert this concentration to molarity.
4.74
The
current maximum level
of
fluoride that the EPA allows in
U.S. drinking water is 4 mglL. Convert this concentration to
molarity.
Section 4.6: Aqueous Reactions and Chemical Analysis
Review Questions
4.75 Describe the basic steps involved in gravimetric analySis. How
does this procedure help us determine the identity
of
a compound
or
the purity
of
a compound
if
its formula is known?
4.76 Distilled water must be used in the gravimetric analysis
of
chlorides.
Why?
4.77 Describe the basic steps involved in an acid-base titration.
Why
is
this technique
of
great practical value?
4.78
How
does an acid-base indicator work?
4.79 A student carried out two titrations using an
NaOH
solution
of
unknown concentration in the buret. In one titration she weighed
out
0.2458 g
of
KHP
(see page 144) and transferred it to an
Erlenmeyer flask.
She then added 20.00
mL
of
distilled water to
dissolve the acid. In the other titration she weighed out
0.2507 g
of
KHP
but added 40.00 mL
of
distilled water to dissolve the
acid. Assuming no experimental error, would she obtain the s
ame
result for the concentration
of
the
NaOH
solution?
4.80 Would the volume
of
a 0.10 M
NaOH
solution needed to titrate
25.0
mL
of
a 0.10 M
HNO
z
(a
weak
acid) solution be different
from that needed to titrate
25.0
mL
of
a 0.10 M
HC1
(a strong
acid) solution?
Problems
4.81
If
30.0
mL
of
0.150 M CaCl
2
is added to 15.0
mL
of
0.100 M
AgN0
3
, what is the mass in grams
of
AgCl precipitate?
4.
82 A sample
of
0.6760 g
of
an unknown compound containing
barium ions (Ba
2+
) is dissolved in water and treated with an
excess
of
Na2S04'
If
the mass
of
the
BaS04
precipitate formed
is
0.4105 g, what is the percent by mass
of
Ba
in the original
unknown compound?
4.83 How many grams
of
NaCl are required to precipitate most
of
the
Ag
ions from 2.50 X 10
2
mL
of
a 0.0113 M
AgN0
3
solution?
Write the net ionic equation for the reaction.
4
.8
4
The
concentration
of
Cu
2+
ions in the water (which also contains
sulfate ions) discharged from a certain industrial plant is
determined by adding excess sodium sulfide
(NazS) solution to
0.800 L
of
the water.
The
molecular equation is
Na2S(aq) +
CuSOiaq)
+.
Na2S04(aq) + CuS(s)
Write the net ionic equation and calculate the molar
concentration
of
Cu
2
+ in the water sample
if
0.0177 g
of
solid
CuS is formed.
4.85 A quantity
of
18.68
mL
of
a KOH solution is needed to neutralize
0.4218 g
of
KHP.
What
is the concentration (in molarity)
of
the
KOH
solution?
QUESTIONS
AND
PROBLEMS 153
4.86 Calculate the concentration (in molarity)
of
an
NaOH
solution
if
25.0
mL
of
the solution is needed to neutralize 17.4
mL
of
a
0.312 M HCl solution.
4.87 Calculate the volume in milliliters
of
a
l.420
M
NaOH
solution
required to titrate the following solutions:
(a)
25.00
mL
of
a 2.430 M
HCl
solution
(b)
25.00
mL
of
a 4.500 M H
2
S0
4
solution
(c)
25.00
mL
of
a 1.500 M H
3
P0
4
solution
4.88
What
volume
of
a 0.500 M
HCl
solution is needed to neutralize
each
of
the following:
(a)
10.0
mL
of
a 0.300 M
NaOH
solution
(b)
10.0
mL
of
a 0.200 M Ba(OH)2 solution
Additional Problems
4.89
Cla
ssify the following reactions according to the types discussed
in the chapter:
(a) Cl
2
+
20H
- •
Cl
- + ClO- + H
2
0
(b)
Ca
2+
+
CO
~-
•
CaC0
3
(c)
NH
3 + H+ •
NH
t
(d) 2CCl
4
+
CrO
~
-
• 2COCl
2
+
Cr0
2Cl2 + 2Cl-
(e) Ca
+ F2 •
CaF
2
(f)
2Li + H2 •
2LiH
(g)
Ba(N0
3
)2
+
Na
2
S0
4 •
2NaN0
3
+
BaS0
4
(h) CuO + H2 • Cu + H
2
0
(i) Zn +
2HCl
•
ZnCl
2
+ H2
(j) 2FeCl
2
+
Cl
2
• 2FeCl
3
4.90 Oxygen
(0
2
) and carbon dioxide
(C0
2
) are colorless and odorless
gases. Suggest two chemical tests that would allow you to
distinguish between these two gases.
4.91
Which
of
the following aqueous solutions would you expect to be
the best conductor
of
electricity at 25°
C?
Explain your answer.
(a)
0.20 M NaCl
(b)
0.60
MHC
2
H
3
0
Z
(c) 0.25 M HCl
(d)
0.20 M
Mg
(
N0
3
)2
4.92 A
5.00 X 10
2
mL
sample
of
2.00 M
HCl
solution is treated with
4.47 g
of
magnesium. Calculate the concentration
of
the acid
solution after all the metal has reacted. Assume that the volume
remains unchanged.
4.93 Calculate the volume
of
a 0.156 M CUS04 solution that would
react with 7.89 g
of
zinc.
4.94 Sodium carbonate
(Na
Z
C0
3
)
is available in very pure form and
can be used to standardize acid solutions.
What
is
the molarity
of
an
HCl
solution
if
28.3
mL
of
the solution is required to react
with
0.256 g
of
Na
2
C0
3?
4.95 Identify each
of
the following compounds as a nonelectrolyte,
a weak electrolyte, or a strong electrolyte: (a) ethanolamine
(C
2
H
5
0NH
2
),
(b
) potass
ium
fluoride (KF), (c) ammonium nitrate
(
NH
4
N0
3
), (d) isopropanol (C
3
H
7
0H).
4.96 Identify each
of
the following compounds as a nonelectrolyte, a
weak electrolyte,
or
a strong electrolyte: (a) lactose (C
12
H
22
0
11
),
(b) lactic acid (
HC
3
H
5
0
3
),
(c) dimethylamine [(CH3
hNH],
(d) barium hydroxide [Ba(OH)2].
4.97 Determine the predominant species (there may be more than one)
in' an aqueous solution for
each
of
the compounds in Problem
4.95.
I
154
CHAPTER
4
Reactions
in
Aqueous
Solutions
4.98
Detennine the predominant species (there may be more than one)
in an aqueous solution for each
of
the compounds in Problem 4.96.
4.99 A 3.664-g sample
of
a monoprotic acid was dissolved in water.
It took
20.27
mL
of
a 0.1578 M NaOH solution to neutralize the
acid. Calculate the molar mass
of
the acid.
4.100 Acetic acid (HC
2
H
3
0
2
) is an important ingredient
of
vinegar. A
sample
of
50.0 mL
of
a commercial vinegar is titrated against a
1.00 M NaOH solution.
What
is the concentration (in
M)
of
acetic
acid present in the vinegar
if
5.75 mL
of
the
ba
se is needed for
the titration?
4.101 A 15.00-mL solution
of
potassium nitrate
(KN0
3
) was diluted
to
125.0 mL, and 25.00
mL
of
this solution was then diluted
to
1.000 X 10
3
mL. The concentration
of
the final solution is
0.00383 M. Calculate the concentration
of
the original solution.
4.102 When
2.50 g
of
a zinc strip was placed in an
AgN0
3
solution,
silver metal formed on the surface
of
the strip. After some time
had
pa
ssed, the strip was removed from the solution, dried, and
weighed.
If
the mass
of
the strip was 3.37 g, calculate the
ma
ss
of
Ag and Zn metals
pr
esent.
4.103 Calculate the mass
of
the precipitate formed when 2.27 L
of
0.0820 M Ba(OH)2 is mixed with 3.06 L
of
0.0664 M Na2S04'
4.104
Calculate the concentration
of
the acid (or base) remaining in
solution when 10.7
mL
of
0.211 M
HN0
3
is added to 16.3
mL
of
0.258 M NaOH.
4.105 (a) Describe a preparation for magnesium hydroxide [Mg(OH)ZJ
and predict its solubility. (b)
Milk
of
magnesia contains mostly
Mg(OH)z
and is effective in treating acid (mostly hydrochloric
acid) indigestion. Calculate the volume
of
a 0.035 M HCI
solution (a typical acid concentration in an upset stomach)
needed to react with two spoonfuls (approximately
10 mL)
of
milk
of
magnesia [at 0.080 g
Mg(OH
)zlmLJ.
4.106 A quantitative definition
of
solubility is the number
of
grams
of
a
solute that will dissolve in a given volume
of
water at a particular
temperature. Describe an experiment that would enable you to
determine the solubility
of
a soluble compound.
4.107 A 60.0-mL 0.513 M glucose (C
6
H
I2
0
6
)
solution is mixed with
120.0
mL
of
a 2.33 M glucose solution.
What
is the concentration
of
the final solution? Assume the volumes are additive.
4.108
An
ionic compound X is only slightly soluble in water.
What
test would you employ to show that the compound does indeed
dissolve in water to a certain extent?
4.109 You are given a colorless liquid. Describe three chemical tests
you would perform on the liquid to show that it is water.
4.110 Using the apparatus shown in Figure 4.1, a student found that
a sulfuric acid solution caused the lightbulb to glow brightly.
However, after the addition
of
a certain amount
of
a barium
hydroxide [Ba(OH)
2J
solution, the light began to dim even
though Ba(OH)2 is also a strong electrolyte. Explain.
4.111 Which
of
the diagrams shown corresponds to the reaction
between AgOH(s) and
HN0
3
(aq)? Write a balanced equation for
the reaction. (For simplicity, water molecules are not shown.)
•
"-,,
- -
• -
• • • •
• ••• •
••••
••
••
••
(a)
'-
~
"
.
'-
(b)
'-
~
-
""::
'It
_I:·
• Ag+
e
N0
3
(c)
4.112 Which
of
the diagrams shown corresponds to the reaction
between
Ba(OHhCaq) and H
2
S0
4
(aq)? Write a balanced equation
for the reaction. (For simplicity, water molecules are not shown.)
~
"
~
(a)
-~
.
'
'-
~.
) ,
•
••
(b)
~
.
,
'
Ba2+
SO~-
(c)
4.113
You
are given a soluble compound
of
an unknown molecular
formula. (a) Describe three tests that would show that the
compound is an acid. (b)
Once you have established that the
compound is an acid, describe how you would determine its
molar mass using an
NaOH
solution
of
known concentration.
(Assume the acid is monoprotic.) (c) How would you find out
whether the acid is weak or strong? You are provided with a
sample
of
N aCI and an apparatus like that shown in Figure 4.1
for comparison.
4.114 You are given two colorless solutions, one containing NaCI and
the other sucrose (C
12
H
22
0
11
).
Suggest a chemical and a physical
test that would allow you to distinguish between these two
solutions.
4.115 The concentration
of
lead ions (Pb
2+
) in a sample
of
polluted
water that also contains nitrate ions
(NO;-) is determined by
adding solid sodium sulfate (Na2S04) to exactly
500
mL
of
the water. (a) Write the molecular and net ionic equations for
the reaction. (b) Calculate the molar concentration
of
Pb
2
+
if
0.00450 g
of
Na
2
S04
was needed for the complete precipitation
of
Pb
2
+ ions as
PbS0
4
.
4.116 Hydrochloric acid is not an oxidizing agent in the sense that
sulfuric acid and nitric acid are. Explain why the chloride ion is
not
a strong oxidizing agent like
SO~
-
and NO
;-
.
4.117 Explain how you would prepare potassium iodide (KI) by means
of
(a) an acid-base reaction and (b) a reaction between an acid
and a carbonate compound.
4.118 Sodium reacts with water to yield hydrogen gas. Why is this
reaction not used in the laboratory preparation
of
hydrogen?
4.119 Describe how you would prepare the following compounds:
(a) Mg(OH)2, (b) AgI, (c)
Ba
3(
P04)z.
4.120
Someone spilled concentrated sulfuric acid on the floor
of
a chemistry laboratory. To neutralize the acid, would it
be
preferable to
pour
concentrated sodium hydroxide solution
or
spray solid sodium bicarbonate over the acid? Explain your
choice and the chemical basis for the action.
4.121 Describe in each case how you would separate the cations
or anions in the following aqueous solutions: (a)
N
aN0
3
and
Ba(N0
3
h,
(b)
Mg(N0
3
h and
KN0
3
,
(c)
KEr
and
KN0
3
,
(d) K
3
P0
4
and
KN0
3
,
(e)
Na
l
C0
3
and NaN0
3
.
4.122
The
following are common household compounds: salt (NaCl),
sugar (sucrose), vinegar (contains acetic acid), baking soda
(NaHC0
3
),
washing soda (NalCO} . lOHlO), boric acid (H
3
B0
3
,
used in eyewash), Epsom salts
(MgS0
4
.
7H
z
O), sodium
hydroxide (used in drain openers), ammonia, milk
of
magnesia
[Mg(OHh
],
and calcium carbonate.
Ba
sed on what you
ha
ve
learned in this chapter, describe tests that would allow you to
identify each
of
these compounds.
4.123 Sulfites (compounds containing the
SO
~-
ions) are used as
preservatives in dried fruits and vegetables and in wine making.
In
an experiment to test the presence
of
sulfite in fruit, a student
first soaked several dried apricots in water overnight and then
filtered the solution to remove all solid particles.
She then treated
the solution with hydrogen peroxide (H
2
0
2
) to oxidize the sulfite
ions to sulfate ions. Finally, the sulfate ions were precipitated
by treating the solution with a few drops
of
a barium chloride
(BaCI
2
) solution. Write a balanced equation for each
of
the
preceding steps.
4.124 A
0.8870-g sample
of
a mixture
of
NaCl and KCl is dissolved in
water, and the solution is then treated with an excess
of
AgN0
3
to yield 1.913 g
of
AgCl. Calculate the percent by
ma
ss
of
each
compound in the mixture.
4.125 Chlorine forms a number
of
oxides with the following oxidation
numbers: +
1,
+ 3,
+4,
+6,
and + 7. Write a formula for each
of
these compounds.
4.126 A useful application
of
oxalic acid is the removal
of
rust (F
ez
0
3)
from, say, bathtub rings according to the reaction
Fe203
(S)
+
6H
z
CP
4(aq) •
2Fe(C
2
0
4)~-(
aq)
+ 3H
z
O +
6H
+(aq)
Calculate the number
of
grams
of
rust that can
be
removed by
5.00 X 10
2
mL
of
a 0.100 M solution
of
oxalic acid.
4.127 Acetylsalicylic acid (HC
9
H
7
0
4
) is a monoprotic acid commonly
known as
"aspirin." A typical aspirin tablet, however, contains
only a small amount
of
the acid.
In
an experiment to determine
its composition, an aspirin tablet was crushed and dissolved in
water. It took 12.25
mL
of
0.1466 M NaOH to neutralize the
solution. Calculate the number
of
grains
of
aspirin in the tablet
(one grain
= 0.0648 g).
4.128 A 0.9157-g mixture
of
CaBr2 and NaBr is dissolved in water, and
AgN0
3 is added to the solution to form AgBr precipitate.
If
the
mass
of
the precipitate is 1.6930 g, what is the percent by
ma
ss
of
NaBr in the original mixture?
4.129 Hydrogen halides (HF, HCl, HBr, HI) are highly reactive
compounds that have many industrial and laboratory uses. (a)
In
the laboratory,
HF
and HCl can be generated by reacting CaF2
and NaCl with concentrated sulfuric acid. Write appropriate
equations for the reactions.
(Hint:
The
se are not redox reactions.)
(b) Why is it that
HBr
and HI cannot
be
prepared similarl
y,
that
is, by reacting NaBr and NaI with concentrated sulfuric acid?
(Hint: H
2
S0
4
is a stronger oxidizing agent than both Brl and 12.)
(c)
HBr
can be prepared by reacting phosphorus tribromide
(PBr}) with water. Write an equation for this reaction.
-i.1
30 A 325-mL sample
of
solution contains 25.3 g
of
CaCl
z
. (a)
Calculate the molar concentration
of
Cl-
in this solution. (b)
How many grams
of
Cl-
are in 0.100 L
of
this solution?
QUESTIONS
AND
PROBLEMS
155
4.131 Phosphoric acid (H
3
P0
4
)
is an important industrial chemical used
in fertilizers, detergents, and the food industry.
It
is produced by
two different methods.
In the electric furnace method elemental
phosphorus
(P
4
) is burned in air
to
form P
4
0
IO
, which is then
combined with water to give
H
3
P0
4
.
In
the wet process the
mineral phosphate rock
[Ca
s(
P0
4)}
F] is combined with sulfuric
acid
to
give H}
P0
4
(and
HF
a
nd
CaS0
4)
. Write equations for
these processes, and classify each step as precipitation, acid-base,
or redox reaction.
4.132 Ammonium nitrate
(
NH
4
NO
})
is one
of
the most important
nitrogen-containing fertilizers. Its purity can
be
analyzed
by titrating a solution
of
NH
4
N0
3
with a standard
NaOH
solution.
In
one experiment a 0.2041-g sample
of
industrially
prepared
NH
4
NO}
required 24.42
mL
of
0.1023 M NaOH for
neutralization. (a) Write a net ionic equation for the reaction. (b)
Wh
at is the percent purity
of
the sample?
4.133 Potassium superoxide (KO
z
) is used in some self-containing
breathing equipment by firefighters.
It
reacts with carbon dioxide
in respired (exhaled) air to form potassium carbonate and
oxygen gas. (a) Write an equation for the reaction. (b) What is
the oxidation number
of
oxygen in the
O
~-
ion? (c) How many
liters
of
respired air can react with 7.00 g
of
K0
2
if
each liter
of
respired air contains 0.063 g
of
CO
z
?
4.134
4.135
4.136
4.137
4.138
4.139
4.140
4.141
Barium sulfate (
BaS0
4)
ha
s important medical uses.
The
dense
salt
ab
sorbs X rays and acts as an opaque barrier. Thus, X-
ray examination
of
a patient who has swallowed an aqueous
suspension of
BaS0
4 particles allows the radiologist to diagnose
an ailment
of
the patient's digestive tract. Given the following
starting compound
s,
describe how you would prepare
BaS0
4
by neutralization and by precipitation:
Ba
(
OH
h BaCl
z
,
BaC0
3
,
H
2
S0
4
, and K
2
S0
4
.
Is the following reaction a redox reaction? Explain.
What is the oxidation number
of
° in HFO?
Dra
w molecular models to represent the following acid-base
reactions:
(a)
OH-
+ H
3
0 +
-_.
2H
z
O
(b) NHd + NH
2"
•
2NH
3
Identify the
Bn
ll
nsted acid and base in each case.
On standing, a concentrated nitric acid gradually turns yellow.
Explain.
(Hi
nt
: Nitric acid slowly decomposes. Nitrogen dioxide
is a colored gas.)
When preparing a solution
of
known concentration, explain why
one must first dissolve the solid completely before adding enough
solvent to fill the volumetric flask to the mark.
Can the following decomposition reaction be characterized as an
acid-base reaction? Explain.
Give a chemical explanation for each
of
the following: (a) When
calcium metal is added to a sulfuric acid solution, hydrogen gas
is generated. After a few minute
s,
the reaction slows down and
eventually stops even though none
of
the reactants is used up.
Explain. (b)
In the activ
it
y series, aluminum is above hydrogen,
yet the metal appears to
be
unreactive toward hydrochloric
acid.
Wh
y? (Hint: Al forms an oxide,
Al
l
0
3
,
on the surface.)
(c) Sodium and potassium lie above copper in the activity series.
Explain why
Cu
2+
ions in a CUS04 solution are not converted to