Chemistry part 23, Julia Burdge,2e (2009) potx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (13.59 MB, 26 trang )
552
CHAPTER
14 Chemical Kinetics
Recall
t
ha
t when
an
exponent
is
1,
it
need
not
be
shown
[
~
Section
3.3].
we
mu
st compare two experiments in which one reactant concentration changes, and the other
remains constant.
For
example, we first compare the data
from
experiments 1 and
3:
Experiment [F
2
]
(M)
[Cl0
2
]
(M)
Initial Rate (Mis)
1 0.10 0.010 1.2 X 10-
3
2
3
[F, I doubles 0.10
0.20
ICIO,] unchanged 0.040
0.010
Rate doubles 4.8 X 10-
3
2.4 X 10-
3
When
the concentration
of
fluorine doubles, with the chlorine dioxide concentration held constant,
the rate doubles.
[F2h = 0.20 M = 2
[F
2
] 1
O.lOM
rate3 2.4 X 10-
3
Mis
= 2
rate I 1.2 X 10-
3
Mis
This indicates that the rate is directly proportional to the concentration
of
fluorine and the value
of
x is
1.
rate = k [F
2
]
[Cl0
2
Y
Similarl
y,
we
can compare experiments 1 and
2:
Experiment
1
2
3
[F
2
]
(M)
[F, I unchanged { 0.10
0.10
0.20
[CI0
2
]
(M)
[CIO,] quadruples
{
0.01O
0.040
0.010
Initial Rate (Mis)
{
1.2
X 10-
3
Rate quadruples 3
4.8 X 10-
2.4
X 10-
3
We find that the rate quadruples when the concentration
of
chlorine dioxide is quadrupled, but the
fluorine concentration is held
con
stant.
[Cl0
2
h = 0.040 M = 4
[Cl0
2
]l
0.010M
4.8 X 10-
3
Mis
= 4
1.2
X
10-
3
Mis
This indicates that the rate is also directly proportional to the concentration
of
chlorine dioxide, so
the value
of
y is also
1.
Thus,
we
can write the rate law as follows:
rate
=
k[F
2
][Cl0
2
]
Because the concentrations
of
F2
and
Cl0
2
are
each
raised to the first power, we say that the reac-
tion is first order in F2 and first order in
Cl0
2
.
The
reaction is second order overall.
Knowing the rate law,
we
can then
use
the data from
anyone
of
the experiments to calculate
the rate constant.
Using the data for the first experiment in Table 14.2, we can write
k = rate 1.2 X 10-
3
Mis
=
12M-I.
- I
[F
2
]
[Cl0
2
]
(0.10 M)(0.01O
M)'
s
Table
14.3 contains initial rate data for the hypothetical reaction
aA
+ bB
+.
cC + dD
which has the general rate law
rate
=
k[A
Y
[BY
Comparing experiments 1 and 2, we see that when [A] doubles, with [B] unchanged, the rate also
doubles.
Experiment
1
2
3
Thus, x =
1.
Experiment
1
2
3
[A]
(M)
[A] doubles {
OJ
0
0.20
0.10
[A]
(M)
0.10
0.20
0.10
[B]
(M)
[B] unchanged {0.o15
0.015
0.010
[B]
(M)
0.015
0.015
0.030
Initial Rate (Mis)
{
1.2
X 10-
3
Rate doubles 3
4.8 X 10-
2.4
X
10-
3
Initial
Rate
(Mis)
2.1 X 10-
4
4.2 X
10-
4
8.4 X
10-
4
SECTION 14.2
Dependence
of
Reaction Rate
on
Reactant
Concentration
553
Overall
Reaction
Order
Sample
Rate
Law
Units
of
k
o rate = k
1 rate =
k[A]
or rate = k[B]
2 rate = k[A]2, rate =
k[Bf,
or rate =
k[A][B]
3* rate = k[A]2[B]
orrate
=
k[A][Bf
M
- 1
. S
S-1
M
-1
-I
. S
~2
.
S-
1
*
Another
possibility
for
a
third-
or
der
reaction
is
rate
= k [Aj(Bj(C],
although
such reactions are very rare.
Comparing experiments 1 and 3, when [B] doubles with [A] unchanged, the rate quadru-
pie
s.
Experiment
[A]
(M)
[B]
(M)
Initial Rate (Mis)
1 0.10
2
[A
I
unchanged
0.20
3 0.10
0.015
I
BI
double
s 0.015
0.030
2.1 X 10-
4
Rate
quadrupl
es
4.2 X 10-
4
8.4 X
10-
4
Thus, the rate is n
ot
directly proportional to [B] to the first power, but rather it is directly propor-
tional to [B] to the second power (i.e., y = 2):
7
rate
ex
[B]-
The overall rate law is
rate
=
k[A][Bf
This reaction is therefore first order
in
A, second order in B, and third order overall.
Once again, knowing the rate law, we can use data from any
of
the experiments in the table
to calculate the rate constant. Using the data from experiment
1,
we get
rate
k=
-
[A][Bf
2.1
X
10-
4
Mis = 9.3 M -
2
.
S- 1
(0.10 M)(0.015
M)2
Note that the units
of
this rate constant are different from those for the rate constant
we
calculated
for the
F
T
CI0
2
reaction and the bromine reaction. In fact, the units
of
a rate constant depend on
the
overall
order
of
the reaction. Table 14.4 compares the units
of
the rate constant for reactions
that are zeroth-, first-,
secondo, and third-order overall.
The following are three important things to remember about the rate law:
1.
The exponents in a rate law must be determined from a table
of
experimental
data-in
general, they are not related to the stoichiometric coefficients in the balanced chemical
equation.
2.
Comparing changes in individual reactant concentrations with changes in rate shows how
the rate depends on each reactant concentration.
3.
Reaction order is always defined in terms
of
reactant concentration
s,
never product
concentrations.
Sample Problem 14.3 shows how to use initial rate data to determine a rate law.
The
gas-phase reaction
of
nitric oxide with hydrogen at 1280°C is
2NO(g) +
2H
ig
)
N
2
(g) + 2H
2
0(g)
From the following data collected at 1280°C, determine (a) the rate law, (b) the rate constant,
including unit
s,
and (c) the rate
of
the reaction when [NOl = 4.8 X 10-
3
M and
[H2l
= 6.2 X 10-
3
M.
Experiment
1
2
3
[NOl
(M)
5.0 X
10
-
3
1.0 X
10
-
2
1.0 X 10-
2
[H2l
(M)
2.0 X 10-
3
2.0 X 10-
3
4.0 X
10-
3
Initial
Rate
(Mis)
1.3 X
10-
5
5.0 X
10-
5
1.0 X 10-
4
(Continued)
554 CHAPTER
14
Chemical Kinetics
A quotient of
numbers,
each
raised
to
the
same
power,
is
equal
to the quotient
raised
to
that power:
xn
/y
n =
{xly)
n
Think
About
It
The exponent for
the concentration
of
Hz in the rate
law is
1,
whereas the coefficient for
Hz
in
the balanced equation is
2.
It
is a common error to
try to write a
rate law using the stoichiometric
coefficients as the exponents.
Remember that, in general, the
exponents in the rate law are not
related to the coefficients in the
balanced equation. Rate laws must
be determined by examining a table
of
experimental data.
Strategy
Compare two experiments at a time to determine how the rate depends on the
concentration
of
each reactant.
Setup
The rate law is rate = k[NOn H
zY
Comparing experiments 1 and 2, we see that the rate
increases by approximately a factor
of
4 when [NO] is doubled but [Hz] is held constant. Comparing
experiments 2 and 3 shows that the rate doubles when
[H
z] doubles but [NO] is held constant.
Solution
(a) Dividing the rate from experiment 2 by the rate from experiment 1, we get
rat~
= 5.0 X
10
-
5
~
= 4 = k(1.0 X
10
-
2
M)"(2.0 X
10-
3
M)Y
ratel
1.3
X
10
-
5
~
k(5
.0 X
10
-
3
M)X(2
.0 X
10-
3
M)Y
.
. . . . . . .
.
Canceling identical terms in the numerator and denominator gives
(1.0
X 10-
2
M)x .
-'
= 2" = 4
(5.0 X 10-
3
M)x
Therefore, x = 2. The reaction is second order in NO.
Dividing the rate from experiment 3 by the rate from experiment 2, we get
rate3
= 1.0 X 10-
4
~
= 2 =
k(
1.0
X
1O
-
2
.M/(
4.0 X
10
-
3
M)Y
ratez 5.0 X
10
-
5
~
k(1.0 X
lO
-
z
M)X(2
.0 X
1O
-
3
.M)Y
Canceling identical terms in the numerator and denominator gives
(4.0
X 10-
3
M)Y ,
::
= 2) = 2
(2.0 X
10
-
3
M)Y
Therefore, y =
1.
The reaction is first order in Hz. The overall rate law is
(b) We can use data from any
of
the experiments to calculate the
va
lue and units
of
k. Using the data
from experiment
1 gives
k = rate
[NO
f[
H
2
J
1.3 X
10
-
5
Mis = 2.6 X 10
2
M -
2
• S-I
(5.0 X
10-
3
M)
2(2
.0 X
1O
-
3
.M)
(c) Using the rate constant determined in part (b) and the concentrations
of
NO and Hz given in the
problem statement, we can determine the reaction rate as follows:
rate
= (2.6 X
10
2
M -
2
• s-I)(4.8 X
10
-
3
M)\6.2
X
10
-
3
M)
= 3.7 X
10
-
5
Mis
Practice Problem A The reaction
of
peroxydisulfate ion (SzO
t)
with iodide ion (1-) is
From the following data collected at a certain temperature, determine the rate law and calculate the
rate constant, including its units.
Experiment
[
S20~
-
J
(M)
WJ
(M)
Initial Rate (Mis)
1
0.080 0.034
2.2 X
10
-
4
2 0.080
0.017
1.1
X
10
-
4
3 0.16 0.017
2.2 X
10
-
4
Practice Problem B For the following general reaction, rate =
k[Af
and k = 1.3 X lO-
z
M -
1
• S
-I
:
A + B
·2C
Use this information
to
fill
in
the mi
ss
ing table entries.
Experiment
1
2
3
[AJ
(M)
0.013
0.026
[BJ
(M)
0.250
0.250
0.500
Initial Rate (Mis)
2.20 X
10
-
6
2.20 X 10-
6
SECTION
14
.3 Dependence
of
Reactant
Concentrat
ion
on
Ti
me 555
Dependence
of
Reaction Rate on Reactant
Concentration
Answer questions 14.2.1 through 14.2.4 using the
table
of
initial rate data for the reaction
Ex~eriment
[A](M)
[B](M)
A + 2B
• 2C + D
1
0.12 0.010
2 0.36
0.010
3 0.12 0.020
Initial
Rate
(Mis
)
2.2 X 10-
3
6.6 X 10-
3
2.2 X 10-
3
14.2.1
What
is the rate law for the reaction?
14.2.3 What is the overall order
of
the reac
ti
on?
a)
rate
=
k[A][B]
2
a)
a
b)
rate
=
k[A]
2[
B]
b)
1
c)
rate
=
k[A
]3
c)
2
d)
rate
=
k[A]
2
d)
3
e) rate
= k[A]
e)
4
14.2.2 Calculate the rate constant.
14.2.4 Determine the rate when [A] = 0.50 M
a)
0.15 M-
1
.
S-
l
and [B] = 0.25
M.
b)
0.15M·s-
1
a)
9.2
X 10-
3
Mis
c)
0.15 S- I
b)
2.3 X 10-
3
M
is
d)
0.018
S-
I
c)
4.5
X 10-
3
M
is
e)
0.018 M-
1
. S- l
d)
5.0 X 10-
3
Mis
e)
1.3
X 10-
2
Mis
Dependence
of
Reactant Concentration on Time
We can use the rate law to determine the rate
of
a reaction using the rate constant and the reactant
concentrations:
Rate law
1
I J
rate =
k[Ay[BF
! \
Rate Rate constant
A rate law can also be used to determine the remaining concentration
of
a reactant at a specific
time during a reaction. We will illustrate this use
of
rate laws using reactions that are first order
overall and reactions that are second order overall.
First-Order Reactions
Afirst-order reaction is a reaction whose rate depends on the concentration
of
one
of
the reac-
tants raised to the first power. Two examples are the decomposition
of
ethane (C
2
H
6
)
into highly
reactive fragments
ca
lled methyl radicals
('CH
3
),
and the decomposition
of
dinitrogen pentoxide
(N
2
0
S
)
into nitrogen dioxide
(N0
2
)
and molecular oxygen
(0
2
):
C
2
H
6
-
_.
2
·CH
3
2N
2
0
S
(g) -
_.
4N0
2
(g)
+ 0 2(g)
In
a first-order reaction
of
the type
A
• product
rate
= k[C?H
6
J
rate = k[N
2
0 SJ
the rate can be expressed as the rate
of
change in reactant concentration,
as well as in the form
of
the rate law:
Ll[A
J
rate = - - -
I1t
rate = k[AJ
556
CHAPTER
14 Chemical Kinetics
It
is
not
necessary
for
you
to
be
ab
le to do
the
calculus
required
to
arrive
at
Equation
14.3,
but
it is
very
important that
you
know how to
use
Equation
14.3.
The
inverse
of
In
x
is
f!
[
~
Appendix
1) .
Think
About
It
Don't
forget
the minus sign in Equation 14.3.
If
you calculate a concentration
at time
t that is greater than the
concentration at time
0 (or
if
you
get a negative time required for the
concentration to drop to a specified
level), check your solution for this
common error.
Becau
se p
res
su
re
is
proportional to
concentration, for
gaseous
reactions
[
H4
Section
11.4)
Equat
i
ons
14.3
and
14.4
can
be
w
ri
tten
as
and
Pi
In-
= -
kt
Po
In
P, = -
kt
+
In
Po
re
spectively,
where
Po
and
P,
are
the
pressures
of
reactant
A at
times
0
and
1.
respectively.
Setting these two expressions
of
the rate equal to each other we get
_
,1[AJ
= k[A]
,1t
Applying calculus to the preceding equation,
we
can show that
Equation 14.3
1 [A]t =
-kt
n
[AJo
where
In
is the natural logarithm, and [A]o and [A]t are the concentrations
of
A at times 0 and
t,
respectively. In general, time 0 refers to any specified time during a
reaction-not
necessarily the
beginning
of
the reaction. Time t refers to any specified time after time
O.
Equation 14.3 is some-
times called the
integrated rate law.
In Sample Problem 14.4 we apply Equation 14.3 to a specific reaction.
Sample
The
decomposition
of
hydrogen peroxide is first order in HzOz.
The
rate constant for this reaction at 20
0
e is 1.8 X 10-
5
s -
I.
If
the starting concentration
of
H
2
0
2
is
0.75
M,
determine (a) the concentration
of
HzOz remaining after 3 h and (b) how long it will take for
the
H
2
0
2
concentration to drop to 0.10
M.
-
Strategy
Use Equation 14.3 to find
[HzOzJ
" where t = 3 h, and then solve Equation 14.3 for t to
determine how much time must pass for
[HzOzJ, to equal 0.10
M.
Setup [H
2
0
2
JO = 0.75
M;
time t for part (a) is
(3
h)(60 minJh)(60 s/min) = 10,800
s.
Solution
(a) In [HzOzJ, =
-k
t
[HzO
zJo
In
[HzOzJ, =
-(1.8
X
10-
5
s-I)(10,800
s)
=
-0.1944
0.75 M
Take the inverse natural logarithm
of
both sides
of
the equation to get
[H
2
0
2
J, =
e-
O
.
1944
= 0.823
0.75
M
[HzOzJ, = (0.823)(0.75 M) = 0.62 M
The
concentration
of
HzOz after 3
his
0.62 M.
(b)
In
(
~.1O
M)
=
-2.015
=
-(1.8
X
10-
5
S-I)t
.75M
2.015.
=t=1.12
X 10
5
s
1.8 X 10
-)
S-1
The
time required for the peroxide concentration to drop to 0.10 M is 1.1 X
105
s or about
31
h.
Practice Problem A The rate constant for the reaction 2A • B
is
7.5 X
10-
3
S-1
at 110°C. The
reaction is first order in
A. How long (in seconds) will it take for
[AJ
to decrease from 1.25 M to 0.71
M?
Practice Problem B Refer again to the reaction 2A • B, for which k = 7.5 X 10-
3
S- I at
llO
o
e.
With a starting concentration
of
[AJ
= 2.25 M, what will
[AJ
be
after 2.0 min?
Equation 14.3 can be rearranged as follows:
Equation 14.4
In
[AJt =
-kt
+ In
[AJ
o
Equation 14.4 has the form
of
the linear equation y =
mx
+ b:
In
[AJt
=
(-k)(t)
+
In
[AJo
y m x + b
SECTION 14.3 Dependence
of
Reactant
Concentration
on
Time
557
k ln
[Ala
'-< slope =
-k
t
(a) (b)
Figure 14.8(a) shows the decrease in concentration
of
reactant A during the course
of
the reaction.
As we saw in Section 14.1, the plot
of
reactant concentration
as
a function
of
time
is
not a straight
line. For a first-order reaction, however, we do get a straight line
if
we plot the natural log
of
reac-
tant concentration (In
[A]t) versus time (y versus x). The slope
of
the line is equal to
-k
[Figure
14.8(b)], so we can determine the rate constant from the slope
of
this plot.
Sample Problem 14.5 shows how a rate constant can be determined from experimental
data.
I
!
The rate
of
decomposition
of
azomethane is studied by monitoring the partial pressure
of
the reactant
as a function
of
time:
The data obtained at
300°C are listed in the following table:
Time (s)
Pazomethane
(mmHg)
0
284
100 220
150
193
200
170
250
150
300
132
Determine the rate constant
of
the reaction at this temperature.
Strategy
We can use Equation 14.3 only for first-order reactions, so we must first determine
if
the
decomposition
of
azomethane is first order. We do this by plotting
In
P against time.
If
the reaction is
first order, we can use Equation 14.3 and the data at any two
of
the times in the table to determine the
rate constant.
Setup
The
table expressed as
In
P is
Time
(s)
o
100
150
200
250
300
InP
5.649
5.394
5.263
5.136
5.011
4.883
Plotting these data gives a straight line, indicating that the reaction is indeed first order. Thus, we can
use Equation 14.3 expressed in terms
of
pressure.
PI .
In-
=
-kt
Po
P
t
and
Po
can be pressures at any two times during the experiment.
Po
need not be the pressure at
Os-it
need only
be
at the earlier
of
the two times.
(Continued)
Figure 14.8 First-order reaction
characteristics: (a) Decrease
of
reactant
concentration with time. (b) A plot
of
In [All versus
t.
The slope
of
the line is
equal
to
-k.
Th
is
grap
h
ica
l determin
at
i
on
is
an
alternative to
using
the
method of initial
rates
to determi
ne
the
value
of
k.
558 CHAPTER
14
Chemical Kinetics
Think About
It
We could equally
well have determined the rate
constant by calculating the slope
of
the plot
of
In
P versus t. Using
the two points labeled on the plot,
we get
I 5.011 - 5.394
s ope
= 250 - 100
=
-2.55
X
10-
3
S-I
Remember that slope =
-k,
so
k = 2.55 X 10-
3
S-I
.
Solution Using data from times 100
sand
250 s
of
the original table
(Pazomethane
versus t), we get
l50mmHg
In
220
mmHg
=
-k(150
s)
In
0.682 =
-k(1
50
s)
k = 2.55 X 10-
3
S-
I
Practice Problem A Ethyl iodide (C2HsI) decomposes at a certain temperature in the gas phase as
follows:
From the following data, determine the rate constant
of
this reaction.
Time
(min)
[C~HsI]
(M)
0
0.36
15
0.30
30
0.25
48
0.19
75 0.
13
Practice Problem B Use the calculated k from Practice Problem A to fill in the missing values in the
following table:
Time (min)
o
10
20
30
40
0.45
We often describe the rate
of
a reaction using the half-life. The half-life
(t1/2)
is the time
required for the reactant concentration to drop to
half
its original value. We obtain an expression
for
tll2 for a first-order reaction as follows:
According to the definition
of
half-life, t =
t1/2
when [A], = i
[A]o,
so
1 [A]o
t
1/2
= - In
., ~
k
i[A]o
Because
[A]of
i [A]o = 2, and
In
2 = 0.693, the expression for t ll2 simplifies to
Equation 14.5
t
-
0.693
1I2
-
k
According to Equation 14.5, the half-life
of
a first-order reaction is independent
of
the initial
concentration
of
the reactant. Thus, it takes the same time for the concentration
of
the reactant to
decrease from
1.0 M to 0.50 M as it does for the concentration to decrease from 0.10 M to 0.050 M
(Figure 14.9). Measuring the half-life
of
a reaction is one way to determine the rate constant
of
a
first-order reaction.
The half-life
of
a first-order reaction is inversely proportional to its rate constant, so a sh
ort
half-life corresponds to a large rate constant. Consider, for example, two radioactive isotopes used
in
nuclear medicine:
24Na
(t1/2
= 14.7 h) and 60
CO
(t1/2
= 5.3 yr). Sodium-24, with the shorter
half-life, decays faster.
If
we started with an equal number
of
moles
of
each isotope,
most
of
the
sodium-24 would be gone in a week whereas most
of
the cobalt-60 would remain unchanged.
SECTION 14.3 Dependence
of
Reactant
Concentration
on Time 559
[Ala
[Al
a
12
-t+-
'
[Ala/
4
I·
[Ala/
8
O
~ ~~~~~
~~~~
' r r ~
o 1 2
3
4
Time (min)
Sample Problem 14.6 shows how to calculate the half-life
of
a first-order reaction, given the
rate constant.
The
decomposition
of
ethane (C
2
H
6
)
to
meth
yl radicals (
CH
3
)
is a first-order reaction with a rate
constant
of
5.36 X
10-
4
S- 1 at 7
00
°C:
Calculate the half-life
of
the reaction
in
minutes.
Strategy
Use Equation 14.5 to calculate t
1/2
in
seconds, and then conv
elt
to minutes.
Setup
Solution
seconds X (1 minute/60 seconds) =
minut
es
0.693
tl/2 = k
1293 s X 1 min = 21.5 min
60 s
The half-life
of
ethane decomposition at 700°C is 21.5 min.
Practice Problem A Calculate the half-life of the decomposition
of
azomethane, discuss
ed
in
Sample Problem 14.5.
Practice Problem B Calculate the rate constant for the first-order
deca
y
of
24Na (t
1/2
= 14.7 h).
Figure 14.9 A plot
of
[AJ versus
time for the first -order reaction
A
• products.
The
half-life
ofthe
reaction is 1 min.
The
concentration
of
A is halv
ed
ev
er
y half-life.
Think
About
It
Half-lives and rate
constants can
be
express
ed
using
any units
of
time and reciprocal
time, respectively. Track units
carefully w
hen
you convert from
one
unit
of
time to another.
,
560
CHAPTER
14
Chemical Kinetics
•
Th
is
equa
tion
can
also
be
wri
tt
en
as
2
1(
g) • I,(g).
Second-Order Reactions
A second-order reaction is a reaction whose rate depends on the concentration
of
one reactant
raised to the second power or on the product
of
the concentrations
of
two different reactants (each
raised to the first power). For simplicity, we will consider only the first type
of
reaction:
where the rate can be expressed as
or as
A
• product
MA]
rate = -
I::: t
rate =
k[A]
2
As before, we can combine the two expre
ss
ions
of
the rate:
_
I::: [A]
=
k[Af
I::: t
Again, using calculus, we obtain the following integrated rate law:
Equation 14.6
1 = kt + 1
[A],
[A]
o
As before, we can obtain the expression for the half-life by setting
[A]
, = i
[A]o
in Equation 14.6.
Solving for
tl/2, we obtain
Equation
14,7
1 1
-, '
'
= kt
112
+
:: ":::-
i
[A]
o
[A]o
t - 1
112
-
k[A]
o
Note that unlike the half-life
of
a first-order reaction, which is independent
of
the starting con-
centration, the halfclife
of
a second-order reaction
is
inversely proportional to the initial reactant
concentration. Determining the half-life at several different initial concentrations is one way to
distinguish between first-order and second-order reactions,
Sample Problem 14.7 shows how to use Equations
14,6 and 14,7 to calculate reactant con-
centrations and the half-life
of
a second-order reaction,
Iodine atoms combine to form molecular iodine in the gas phase:
•
I(g) + I(g)
+.
1
2
(g)
This reaction is second order and has a rate constant
of7.0
X
10
9
M-
1
,
S-1
at
23
°
C.
(a)
If
the initial
concentration of I is
0.086 M, calculate the concentration after 2.0 min. (b) Calculate the half-life of
the reaction when the initial concentration of I
is
0.60 M and when the initial concentration
of
I is
0.42 M.
Strategy
Use Equation 14.6 to detenrune
[I]
, at t = 2,0 min; use Equation 14.7 to determine
t1/2
when
[1]0
= 0.60 M and when
[1]
0 = 0.42
M.
Setup
t = (2.0 min)(60 s/
min)
= 120
s.
I
I
SECTION
14.3
Dependence
of
Reactant
Concentration
on
Time 561
Solution
()
I_k+l
a [All - t [Alo
=
(7.0 X 10
9
M -
I
•
s-I)(120
s) +
0.0~6
M
= 8.4 X
1011
M-
I
[All = 1 = 1.2 X
10
-
12
M
8.4 X
1011
M-
I
The concentration
of
atomic iodine after 2 min is 1.2 X
10-
12
M.
(b) When
[110
= 0.60
M,
t - 1
l iZ - k[Alo
When
[110
= 0.42 M,
t - 1
1/2 - k[Alo
1
=2.4
X
lO
-
lO
s
(7.0 X
10
9
M-
I
•
sl)(0.60
M)
•
___
-:-
_ :.1_-:-
___
= 3.4 X
10
-
10
s
(7.0 X
10
9
M -
I
•
s- I)(0.42
M)
Practice
Problem
The reaction 2A • B is second order in A with a rate constant
of
32
M-
I
•
S- I at 25°C. (a) Starting with [Alo = 0.0075
M,
how long will it take for the concentration
of
A to drop to 0.0018
M?
(b) Calculate the half-life
of
the reaction for [Alo = 0.0075 M and for
[Alo =
0.0025
M.
First- and second-order reactions are the most
common
reaction types. Reactions
of
overall
order zero exist but are relatively rare.
For
a zeroth-order reaction
A
• product
the rate law is given
by
rate =
k[AJ
o
= k
Thus, the rate
of
a zeroth-order
reaction
is a constant, independent
of
reactant concentration. Third-
order and higher-order reactions are quite rare and too complex to
be
covered
in
this book. Table 14.5
summarizes the kinetics for first-order and second-order reactions
of
the type A • product.
Order
Rate
Law
o
rate = k
1
rate =
k[AJ
2
rate
=
k[AJ2
Kinetics
ReaCtions
Integrated
Rate
Law
[AJI
=
-kt
+
[AJo
[AJt
In
[AJo
=
-kt
1
=kt+
1
[AJI
[AJo
Half-Life
[AJo
2k
0.693
k
1
k[AJo
Think
About
It (a) Iodine, like the
other halogens, exists as diatomic
molecules at room temperature.
It
makes sense, therefore, that
atomic iodine would react quickly,
and essentially completely, to
form
I
z
at room temperature. The
very low remaining concentration
of
I after 2 min makes sense.
(b) As expected, the half-life
of
this second-order reaction is not
constant. (A constant half-life
is a characteristic
of
first-order
reactions
.)
562
CHAPTER
14 Chemical Kinetics
Cookbooks
sometimes
give
alternate
directions
for
cooking
at
high
altitude
s,
where
the
lower
atmospheric
pressure
re
su
lts
in
wa
ter boiling
at
a
lower
tem
pe
ra
ture
[
~.
Section
12.6]
.
Kinetic
energy
is
the
result
of motion of
the
whole
molecule,
relative
to
its
surroundings.
Vibrational
energy
is
the
resu
lt of motion of
the
atoms
in
a
molecule,
relati
ve
to
one
another.
Checkpoint 14.3
Dependence
of
Reactant Concentration
on
Time
The first-order decomposition
of
dinitrogen
pentoxide
(NzOs) is represented by
Use the table
of
data to answer questions
14
.3.1
and 14.3.2:
14
.3.1
Wh
at is the rate constant for the
decomposition
of
N20
S?
a)
9 X
1O
-
4
s-
1
b)
4 X 10-
4
S- I
c)
6 X 1O-
4
s-
1
d)
5 X 10-
3
S-
I
e)
l X
lO
-
3
s-
1
14
.3.2 Approximately how long will it take
for
[N
2
0 SJ to fall from 0.62 M to
0.10
M?
a)
1000 s
b)
2000 s
c)
3000 s
d)
4000 s
e)
5000 s
14
.3.3
14
.3.4
t(s)
o
300
600
1200
3000
0.91
0.75
0.64
0.44
0.16
Calculate the half-life
of
a first-order
reaction for which the rate constant is
2.5 X 10-
2
s - I.
a) 28 s
b)
0.017 s
c)
361 s
d)
58 s
e) 1.4 X
10
3
s
A
• 2B is a second-order reaction
forwhichk
= 5.3 X 10-
1
M-
1
,
S- I,
Calculate t
1/2
when [Alo = 0.55
M.
a) 1.3 s
b)
0.29 s
c) 0.77 s
d)
15
s
e) 3.4 s
Dependence
of
Reaction Rate on Temperature
Nearly all reactions happen faster at higher temperatures.
For
example, the time required to
cook
. . . . . . . . . . . . . . . . . . . .
.
food depends largely on the boiling point
of
water.
The
reaction involved in hard-boiling an egg
happens faster at
100°C (about 10 min) than at 80°C (about
30
min),
The
dependence
ofreaction
rate on temperature is the reason
we
keep food in a
refrigerator-and
why food keeps even longer
in a freezer.
The
lower the temperature, the slower the processes that cause food to spoiL
Collision Theory
Chemical reactions generally occur
as
a result
of
collisions between reacting molecules. A greater fre-
quency
of
collisions usually leads
to
a higher reaction rate, According to the collision theory
of
chemi-
cal kinetic
s,
the reaction rate is directly proportional to the number
of
molecular collisions per second:
t
number
of
collisions
ra
e
ex
- -' '- -' ': '-" '-
s
Consider the reaction
of
A molecules with B molecules to form some product. Suppose that
each
product molecule is formed by the direct combination
of
an A molecule and a B molecule,
If
we
doubled the concentration
of
A, then the
number
of
A-B collisions would also double, because
there would be twice as many A molecules that could collide with B molecules in any given vol-
ume. Consequently, the rate would increase by a factor
of
2. Similarly, doubling the concentration
of B molecules would increase the rate twofold.
Thu
s, we can express the rate law as
rate
=
k[A][B]
The
reaction is first order in both A and B and is second order overalL
This view
of
collision theory is something
of
a simplification, though, because not every
collision between molecules
re
sults
in
a reaction. A collision that does result in a reaction is called
an
effective collision. A molecule in motion possesses kinetic energy; the faster it is moving, the
greater its kinetic energy,
When
molecules collide, part
of
their kinetic energy is converted to
vibrational energy.
If
the initial kinetic energies are large, then the colliding molecules will vibrate
-
Is
the
Shroud
of
Turin
Really
the
Burial
Cloth
,
of
Christ?
Scientists can determine the ages
of
such artifacts as the Shroud
of
Turin using radiocarbon dating. Earth's atmosphere is con-
stantly being bombarded by cosmic rays
of
extremely high pene-
trating power. These rays, which originate in outer space, consist
of
electrons, neutrons, and atomic nuclei.
One
of
the important
reactions between the atmosphere and cosmic rays is the capture
of
neutrons by atmospheric nitrogen (nitrogen-14) to produce the
radioactive carbon-14 isotope and hydrogen:
The carbon-14 atoms eventually
find their way into 14CO
z,
which
mixes with the ordinary carbon dioxide (
12
C0
2
) in the atmosphere.
As the carbon-14 isotope decays, it emits
{3
particles (electrons).
The
rate
of
decay (as measured by the number
of
electrons emit-
ted
per
second) obeys first-order kinetics. It is customary in the
study
of
radioactive decay to write the rate law as
rate
=
kN
where k is the first-order rate constant and N is the number
of
14C
nuclei present. The half-life
of
the decay, t l/2, is 5715 yr, so rear-
ranging Equation 14.5, we write
k = 0.693 = 1.21 X 10-
4
yr-
I
5715 yr
Carbon-14 enters the biosphere when carbon dioxide is taken up
in plant photosynthesis. The
14C
lost by radioactive decay is con-
stantly replenished by the production
of
new
14
C in the atmosphere.
In this decay-replenishment process, a dynamic equilibrium is
established whereby the ratio
of
1
4C
to
12C
remains constant
in
living matte
r.
But
when an individual plant
or
animal dies, the car-
bon-14 in it is no longer replenished, so the ratio decreases as
14C
decays. This same change occurs when carbon atoms are trapped
in coal, petroleum, or wood preserved underground, and,
of
course, in any object that
wa
s once living including the Shroud
of
Turin, which is linen, a cloth made from the flax plant.
In 1955, American chemist Willard
F.
Libby suggested
that the 1
4C:
12C
ratio could be used to estimate the length
of
time
the carbon-14 isotope in a particular specimen has been decay-
ing without replenishment. Rearranging Equation 14.3, we
can
write
No
InN=kt
I
where
No
and
Nt
are the number
of
14C nuclei present at t = 0 and
t
=
t,
respectively. Because the rate
of
decay is directly propor-
tional to the number
of
14
C nuclei present, the preceding equation
can be rewritten as
1
No
t = - In
-::-:-
k NI
1 decay rate at t = 0
ln
1.21 X
10-
4
yr
-I
decay rate at t = t
1 decay rate
of
fresh sample
~
ln
1.21 X 10-
4
yr-
1
decay rate
of
old sample
Knowing
k and the decay rates for the fresh sample and the old
sa
mple,
we
can calculate
t,
which is the age
of
the old sample.
This ingenious technique is based
on
a remarkably simple idea.
Its success depends on how accurately we can measure the rate
of
decay. In a fresh sample, the ratio 14
C/
!?
C is about
1110
12
,
so the
equipment used to monitor the radioactive decay must be very
sensitive.
Precision is more difficult with older samples because
they contain even fewer 1
4C
nuclei. Nevertheless, radiocarbon
dating has become an extremely valuable tool for estimating the
age
of
archaeological artifacts, paintings, and other objects dat-
ing back
1000 to 50,000 years.
In 1988 three laboratories in
Europe
and the United States,
working
on
samples
of
less than 50
mg
of
the shroud, inde-
pendently s
howed
by carbon-14 dating that the shroud dates
from between
A.D. 1260 and A.D. 1390. Thus, the shroud
could not
ha
ve been the burial cloth
of
Christ. Libby received
the Nobel
Prize in Chemistry in 1960 for
hi
s work on radiocar-
bon dating.
so strongly as to break some
of
the chemical bonds. This bond
br
eaking is the first step toward
product formation.
If
the initial kinetic energies are small, the molecules will merely bounce off
of
each other intact. There is a minimum amount
of
energy, the activation energy (Ea), required to
initiate a chemical reaction. Without this minimum amount
of
energy at impact, a collision will be
ineffe
ct
ive; that is, it will not result in a reaction.
When molecules react (as opposed to when
atoms react), having sufficient kinetic energy is not
the only requirement for a collision to be effective. Molecules must also be oriented in a way that favors
reaction. The reaction between chlorine atoms and nitrosyl chloride (NOCl) illustrates this point:
Cl
+ NOCl
_.
Cl
2
+
NO
This reaction is most favorable when a free Cl atom collides directly with the Cl atom in the NOCI
molecule [Figure 14. 10 (a)]. Otherwise, the reactants simply bounce off
of
each other and no reac-
tion occurs [Figure l4.1O(b)].
563
564
CHAPTER
14 Chemical Kinetics
Figure 14.10 (a) In
orderfor
an
effective collision to take place, the free
Cl atom must collide directly with the
Cl atom in
NOCI. (b) Otherwise, the
reactants bounce off
of
one another and
the collision is
ineffective-no
reaction
takes place.
Figure 14.
11
Energy profile
for the reaction
of
Cl with NOCI. In
addition to being oriented properly,
reactant molecules must possess
sufficient energy to overcome the
activation energy.
Ab
solu
te
te
mperature
is
ex
presse
d in ke
lvins
[
H~
Section
1.3).
Before co
lli
sion Collision
After collision
(a)
Before coilision Collision
After co
lli
sion
(b)
>,
OJ)
~
"
~
Ea
-
"
~
'"
~
c
"
~
0
p
+
Reaction pathway
When molecules collide (in
an
effective collision), they form an activated complex (also
called the
transition state), a temporary species formed by the reactant molecules
as
a result
of
the
collision. Figure 14.11 shows a potential energy profile for the reaction between Cl and
NOCl.
We can think
of
the activation energy as an energy barrier that prevents less energetic mol-
ecules from reacting. Because the number
of
reactant molecules in an ordinary reaction is very
large, the speeds, and therefore also the kinetic energies
of
the molecules, vary greatly. Normally,
only a small fraction
of
the colliding molecules
-the
fastest-moving ones have sufficient kinetic
energy to exceed the activation energy. These molecules can therefore take part in the reaction. The
relationship between rate and temperature should now make sense. According
to
kinetic molecular
theory, the average kinetic energy of a sample
of
molecules increases
as
the temperature increases
[
~~
Section
11.6,
Figure
11
.19]. Thu
s,
a higher percentage
of
the molecules in the sample have
sufficient kinetic energy
to
exceed the activation energy, and the reaction rate increases.
The Arrhenius Equation
The dependence
of
the rate constant
of
a reaction on temperature can be expressed by the Arrhe-
nius equation,
Equation 14.8
k =
Ae
- E
,I
RT
where Ea is the activation energy
of
the reaction (in kJ/mol), R
is
the gas constant (8.314
J/K
.
mol), T is the absolute temperature, and e is the base
of
the natural logarithm
[
~~
Appendix
]]
.
The quantity
A represents the collision frequency and is called the frequency factor.
It
can be
treated as a constant for a given reaction over a reasonably wide temperature range. Equation
14.8 shows that the rate constant decreases with increasing activation energy and increases with
increasing temperature. This equation can be expressed in a more useful form by taking the natural
logarithm
of
both sides:
In
k =
In
Ae
-E"I
RT
or
Ea
In
k =
InA
-
RT
SECTION
14.4
D
ependence
of
R
eactio
n Rat e
on
Te
mp
erat
ure 565
Equation 14.9
which can be rearranged to give the following linear equation:
In
k = Equation 14.10
y = m
x + b
Thus, a
plot
of
In k versus
liT
gives a straight line whose slope is equal to -
E/R
and whose y
intercept (b) is equal to In A.
Sample
Problem 14.8 demonstrates a graphical method for determining the activation energy
of
a reaction.
Sample
Problem,
14:8
'
Rate constants for the reaction
cO(
g) +
N0
2
(g) -
_.
CO
2
(g) + NO(g)
were measured at four different temperature
s.
The data are shown in the table. Plot
In
k versus l
iT,
and determine the activation energy (in
kJ
/mo]) for the reaction.
k
(M
-
1
• S-
I)
T(K)
0.052]
288
0.101
298
0.184
308
0.332 318
Strategy Plot In k versus liT, and determine the slope
of
the resulting line. According to Equation
14.10, slope
=
-EalR.
Setup R = 8.314 J/mol . K. Taking the natural log
of
each value
of
k and the inverse
of
each value
of
T gives
Ink
- 2.95
-2.29
- 1.69
-1.10
3.47 X
10
-
3
3.36 X
10
-
3
3.25 X
10
-
3
3.14 X
10
-
3
Solution A plot
of
these data yields the fo
ll
owing graph:
0.0
0.5
-l.0
~
-
l.5
s
- 2.0
-2.5
-3.0
(3.2 X
]0
-
3
,
-1.4)
/
(3.4 X
10-
3
,
-2.5)
/
-3.5
-'
- - -
'
liT
(K-l)
The slope
is
determined using the x and y coordinates
of
any two points on the line. Using the points
that are labeled on the graph gives
-1.4-(
- 2.5) 3
slope = = 5.5 X
10
' K
3.2
X
10
-
3
K-
1
-
3.4 X
10
-
3
K-
1
(Continued)
566 CHAPTER
14
Chemical Kinetics
Think
About
It
Note
that while k
has units
of
M-
1
•
S-
I,
In k has no
units.
No
te
that for two different
temperatures,
the
on
ly thing
in
Equ
ation
14.9
that
changes
is
k.
The
oth
er
varia
bles
, A
and
fa (
and
of
cou
r
se
R),
are
constant.
Equations
14.8
through
14.11
are
all
"Ar
rhe
nius
equations," but
Equation
14.11
is
the
form
you
will
use
most often to
solve
kinetics
problems.
The
value
of
the slope is
-5
.5
X 10
3
K.
Because slope =
-E
aI
R,
Ea
=
-(slope)
(R)
=
-(-5
.5
X
10
3
K)(8.314 J/K . mol)
= 4.6 X
10
4
J/mol or 46 kJ/mol
The
activation energy is 46 kJ/mo!.
Practice Problem
The
second-order rate
co
nstant for the decomposition
of
nitrous oxide to nitrogen
molecules and oxygen atoms has been determined at various temperatures:
k
(M
-
1
.
S-I)
T(
°C)
1.87 X
10
-
3
600
0.0113 650
0.0569 700
0.244
750
Determine the activation energy graphically.
We can derive an even more useful form
of
the Arrhenius equation starting with Equation
.
'"
. . .
.
.,
. . . . . . . . .
.,
. . . .
14.9 written for two different temperatures,
T]
and T
2
:
Ea
In
k2
=
In
A -
RT2
Subtracting
In
k2
from
In
k]
gives
Equation 14.11
Equation 14.11 enables us to do two things:
1 1
Ea
In
A -
RT
2
1.
If
we know the rate constant at two different temperatures,
we
can calculate the activation
energy.
2.
If
we know the activation energy and the rate constant at one temperature, we can determine
the value
of
the rate constant at any other temperature.
Sample Problems 14.9 and
14.10 show how to use Equation 14.11.
Sample Problem 14.9
The
rate constant for a particular first-order reaction is given for three different temperature
s:
T(K)
400
450
500
2.9 X
10-
3
6.1 X
10
-
2
7.0 X
10
-
1
Us
ing these data, calculate the activation energy
of
the reactio
n.
Strategy
Use Equation 14.
11
to solve for Ea.
SECTION
14.4
Dependence
of
Reaction Rate
on
Temperature 567
Setup Solving Equation 14.11 for Ea gives
kl
In-
k2
E = R
=-
a 1
1
Solution Using the rate constants for
400
K (T
I
)
and
450
K (T
2)
'
we
get
E =
8.314J
a
K.
mol
In 2.9
X
10-
3
S- I
6.1 X
10-
2
S- I
1 1
450
K
400
K
=
91
,173 J/
mol
=
91
kJ/
mol
The
activation energy
of
the reaction is
91
kJ
/
mol.
•
•
Practice Problem A
Use
the data
in
the following table to determine the activation
ene
rgy of the
reaction:
T(K)
625
635
645
1.1
X
10
-
4
1.5 X
10-
4
2.0 X
10
-
4
Practice Problem B
Ba
s
ed
on the data shown in Practice Problem A, what will be the value
of
k
at
655
K?
~I
"
A certain first-order reaction has an activation energy
of
83 kJ/
mol.
If
the rate constant for this
reaction is 2.1 X
10
-
2
s
-I
at 150°C, what is the rate constant at
300
°C?
Strategy
Use
Equation 14.11 to solve for k
2
•
Pa
y particular attention to units in this ty
pe
of
problem.
Setup Solving Equation 14.
11
for
k2
gives
k =
2
· . .
·
•
·
•
·
•
•
•
•
•
•
•
•
.
, .
Ea
= 8.3 X 10
4
J,
TI = 423 K, T2 = 573 K, R = 8.314 J/
mol
. K, a
nd
kl = 2. 1 X
10-
2
S
-1
Solution
k =
2
The
rate constant at
300
°C is
lO
s
-I.
2.1
X
10-
2
S
-1
4
8.3 x
10
J/mol
(1
1 )
8.314 J
IK
. mol
S73
K - 423 K
e
1 0 10
1 - 1
.
Xs
Practice Problem A Calculate the rate
con
stant at 200°C for a reaction that has a rate constant
of
8.1 X
10-
4
S-
I at 90°C and an activation energy o
f99
kJ/mo!.
Practice Problem B Calculate the rate
con
stant at 200°C for a reaction that has a rate constant
of
8.1 X
10-
4
s - I at 90°C and an activation energy
of
59 kJ/mol.
Think
About
It
A good way to
check
y
our
work is to use the value
of
Ea that you calculated (and
Equation 14.11) to determine the
rate constant at
500
K.
Make sure it
agrees with the value in the table .
Note that E,
is
co
nverted to jou
les
so
that the
units will
cancel
p
ro
per
ly.
Altern
atively,
the R
cou
ld
be
exp
r
essed
as
0.008314
kJ/K
. m
ol.
Al
so
note
aga
in
that T must
be
expr
es
se
d in
ke
lvins.
Think
About
It
Make sure that
the rate constant you calculate at a
higher temperature is in fact higher
than the original rate constant.
According to the Arrhenius
equation, the rate constant
always increases with increasing
temperature.
If
you get a smaller k
at a higher temperature,
check
y
our
solution for mathematical errors.
568
CHAPTER
14 Chemical Kinetics
Checkpoint 14.4
Dependence
of
Reaction Rate
on
Temperature
Use the table
of
data collected for a first-order
reaction to answer questions 14.4.1 and 14.4.2:
T(K)
300
310
320
3.9 X
10
-
2
1.1
X
10
-
1
2.8 X
10
-
1
14.4.1
What is the activation energy
of
the
14
.4.2
What
is the rate constant at 80°C?
reaction?
a) 5.8 X 1O-
4
0s
-
1
a)
88 kJ
b)
4.5
S- 1
b)
8.8
X
10
4
kJ
c) 2.8
8-
1
c) 8.0 X 10
4
kJ
d) 5.2 X
10-
3
8-
1
-
d)
8.8
X
10
-'
kJ
e)
l.7
X 10
2
S- 1
e)
80 kJ
•
Reaction Mechanisms
A balanced chemical equation does not tell us much about
how
a reaction actually takes place. In
many cases, the balanced equation is simply the
sum
of
a series
of
steps. Consider the following
hypothetical example. In the first step
of
a reaction, a molecule
of
reactant A combines with a
molecule
of
reactant B to form a molecule
of
C:
A + B • C
In the second step, the molecule
of
C combines with another molecule
of
B to produce D:
C
+ B • D
The
overall balanced equation is the
sum
of
these two equations:
Step
1:
Step
2:
A+B
-
-+.$Z'
.e'+
B • D
A +
2B
.D
The
sequence
of
steps that
sum
to give the overall reaction is called the reaction mechanism. A
reaction mechanism is comparable to the
route traveled during a trip, whereas the overall balanced
chemical equation specifies only the
origin and the destination.
For
a specific example
of
a reaction mechanism, we consider the reaction between nitric
oxide and oxygen:
We know that
NO
? does not form as the result
of
a single collision between two
NO
molecules and
one
O
2
molecule because N
2
0
2
is detected during the course
of
the reaction. We can envision the
reaction taking place via the following two steps:
2NO(g) -
-+.
N
2
0
2
(g)
N
2
0ig)
+ 02(g) •
2N0
2
(g)
In the first step, two
NO
molecules collide to form an N
2
0
2
molecule. This is followed by the step
in which
N
2
0
2
and O
2
combine to give two molecules
of
N0
2
.
The
net chemical equation, which
represents the overall change, is given by the sum
of
the first and second steps:
Step
1:
Step
2:
Overall reaction:
NO
+
NO
-
_.
N
2
0
2
N
2
0
2
+ O
2
•
2N0
2
2NO(g) +
Oig)
•
2N0
2
(g)
Species
such
as N
2
0
2
(and C in the hypothetical equation) are called intermediates because they
appear in the mechanism
of
the reaction but not in the overall balanced equation.
An
intermediate
is produced in an early step in the reaction and consumed in a later step.
Elementary Reactions
Each step
in
a reaction mechanism represents an elementary reaction, one that occurs in a single
collision
of
the reactant molecules.
The
molecularity
of
an elementary reaction is essentially the
SECTION
14.5 Reaction Mechanisms 569
number
of
reactant molecules involved in the collision. Elementary reactions may be unimolecu-
. . . . . . . .
.
lar (one reactant molecule), bimolecular (two reactant molecules), or termolecular (three reactant
molecules).
The
se molecules may be
of
the same or different types. Each
of
the elementary steps
in the formation
of
N0
2
from
NO
and O
2
is bimolecular, because there are two reactant mol-
ecules in each step. Likewise, each
of
the steps in the hypothetical A + 2B • D reaction is
. . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . .
.
.
.
bimolecular.
Knowing the steps
of
a reaction enables us to deduce the rate law. Suppose we have the fol-
lowing elementary reaction:
A
• products
Because there is only
one
molecule present, this is a unimolecular reactio
n,
It
follows that the
larger the number
of
A molecules
pr
esent, the faster the rate
of
product formation. Thus, the rate
of
a unimolecular reaction is directly proportional to the concentration of A the reaction is first
order in
A:
rate =
k[A]
For
a bimolecular elementary reaction involving A and B molecule
s,
A + B
+.
products
the rate
of
pr
oduct formation depends on how frequently A and B collide, which in
tum
depends
on the concentrations
of
A and B. Thus, we can express the rate as
rate
=
k[A
]
[B]
Therefore, this is a second-order reactio
n.
Similarly, for a bimolecular elementary reaction
of
the
type
A
+ A -
_.
products
or
2A
• products
the rate
becom
es
rate =
k[A
f
which is also a second-order reaction.
The
preceding examples show that the reaction order for
. . . . .
each reactant in an elementary reaction is equal to its stoichiometric coefficient in the chemical
equation for that step. In general,
we
cannot tell
ju
st by looking at the balanced equation whether
the reaction occurs as shown or in a series
of
steps. This determination must
be
made using data
obtained experimentally.
Rate-Determining Step
In a reaction mechanism consisting
of
mor
e than
one
elementary step, the rate law for the overall
process is given by the
rate-determining step, which is the slowest step in the sequence. An anal-
ogy for a process in which there is a rate-determining step is the
am
ount of time required to buy
stamps at the post office when there is a long line
of
customers.
The
pr
ocess consists
of
several
steps: waiting in line, requesting the stamp
s,
receiving the stamps, and paying for the stamps. At
a time when the line is very long, the amount
of
time spent waiting in line (step 1) largely deter-
mines how much time the overall process takes.
To study reaction mechanism
s,
we first do a series
of
experiments to establish initial rates
at various reactant concentrations. We then analyze the data to determine the rate constant and
overall order
of
the reaction, and we write the rate law. Finall
y,
we propose a plausible mechanism
for the reaction in terms
of
logical elementary steps.
The
steps of the proposed mechanism must
satis
fy
two requirements:
1.
The
s
um
of
the elementary reactions must be the overall balanced equation for the reaction.
2.
The
rate-determining step must have the same rate law as that determined from the experi-
mental data.
The
decomposition
of
hydrogen peroxide can
be
facilitated by iodide ions (Figure 14.12).
The overall reaction is
• • •
Termolecular
react
i
ons
actually
are quite
rare
simply
because
the
simu
lt
aneous
encounter of
three
molecules
is
far
less
likely
than that of two
molecules.
The
overall
equ
ati
on
ha
s t
hr
ee
reactant
molecu
l
es
.
Be
cau
se
it
is
not an el
eme
nt
ary
reaction,
thoug
h,
we do not
classify
it
as
a termolecular r
ea
ction. Rat
her,
it
is
the
combination of two
bimolecular
reactions
(
steps
1
and
2).
It
is
important to
remem
ber that t
his
is
not
the
ca
se
in
gene
r
al.
It only appl
ies
to
el
eme
ntary
reactions
-
and
whether
or
not a
react
i
on
is
el
ementary
mus
t
be
d
ete
rmi
ned
ex
perimenta
ll
y.
570 CHAPTER
14
Chemical Kinetics
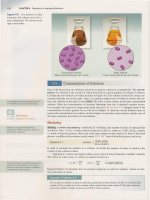
Figure 14.12 The decomposition
of
hydrogen peroxide is catalyzed by
the addition
of
an iodide salt. Some
of
the iodide ions are oxidized to
molecular iodine, which then reacts
with iodide ions to form the brown
triiodide ion
(13)'
Figure 14.13 Potential energy
profile for a two-step reaction in which
the first step is rate determining.
Rand
P represent reactants and products,
respectively.
o
o
+
By experiment, we find the rate law to be
Thu
s,
the reaction is
fir
st order with respect
to
both H
2
0
2
and
1
The decomposition
of
H
2
0
2
is not an elementary reaction, because it does not occur in a
single step.
If
it did, the reaction would be second order in H
2
0
2
(as a result of the collision
of
two
H
2
0
2
molecules
).
What's more, the r- ion, which is not even part of the overall equation, would
not appear in the rate law expre
ss
ion. How can we reconcile these facts? First, we can account for
the observed rate law by assuming that the reaction takes place in two separate elementary steps,
each
of
which is bimolecular.
Step 2: H
2
0
2
+
10
-
k2
. H
2
0 + O
2
+ 1-
If
we further assume that step 1 is the rate-determining step, then the rate of the reaction can be
determined from the first step alone:
where
k[
=
k.
The 10- ion is an intermediate because it
is
produced in the first step and consumed
in the second step. It does not appear in the overall balanced equation. The
1- ion also does not
appear in the overall equation, but it is consumed in the first step and then produced in the second
step. In other word
s,
it is present at the start
of
the reaction, and it is present at the end. The r- ion
is a
catalyst, and its function is to s
pe
ed up the reaction. Catalysts are discussed
in
greater detail in
Section 14.6. Figure 14.13 shows the potential energy profile for a reaction like the decomposition
-
Inte
ml
ediate
(Step 1)
(
St
ep 2)
Reaction progress
E'
a
p
SECTION 14.5 Reaction Mechanisms
571
of
H
Z
0
2
.
The
first step, which
is
the rate-determining step, has a larger activation energy than the
second step.
The
intermediate, although stable enough to
be
observed, reacts quickly to form the
products. Its existence is only fleeting.
A common reaction mechanism is one that involves at least two elementary steps, the first
of
which is very rapid (compared
to
the second step) in both the forward and reverse directions. An exam-
ple
is
the reaction between molecular hydrogen and molecular iodine to produce hydrogen iodide:
Hz(g) + I
2
(g)
-~
.
2HI(g
)
Experimentally, the rate law is found to
be
rate = k[HzJ
[H
For
many years it was thought that the reaction occurred
just
as written that i
s,
as a bimolecular
reaction involving a hydrogen molecule and an iodine molecule, as implied
by
the balanced equa-
tion. In the 1960s, however, chemists found that the actual mechanism is
more
complicated.
The
following two-step mechanism was proposed:
Step
1:
'
Step
2:
H2 +
21
k
2
•
2HI
where kb k
-b
and k
z
are the rate constants for the reactions.
The
I atoms are the intermediate in
this reaction.
When
the reaction begins, there are very few I atoms present. As 12 dissociates, though,
the concentration
of
I
z
decreases while that
of
I increases. Therefore, the forward rate
of
step 1
decreases and the reverse rate increases. Soon the two rates
become
equal, and a chemical equi-
librium is established. Because the elementary reactions (forward and reverse) in step 1 are much
faster than the one in step 2, equilibrium is reached before any significant reaction with hydrogen
occurs, and this state
of
equilibrium persists throughout the reaction.
In the equilibrium condition
of
step 1, the forward rate is equal to the reverse rate; that i
s,
or
k[[12J
= k_[[
IJ
2
[If
=
kl
[1
2
J
L[
The
rate
of
the reaction is given
by
the slow, rate-determining step, which is step
2:
?
rate = k
z
[H
2
J[IJ-
Substituting the expression for
[If
into this rate law,
we
obtain
klk2
rate = L 1 [Hz
][l
zJ
= k[H
2
J[H
where k = k[ k
z/L
I'
This two-step mechanism also gives the correct rate law for the reaction. This
agreement, along with the experimental observation
of
intermediate I atoms, provides strong evi-
dence that the mechanism is con"ect.
Finally
we
note that not all reactions have a single rate-determining step. A reaction may
have two
or
more
comparably slow steps.
The
kinetic analysis
of
s
uch
reactions is so
mewhat
more
complicated than what is presented in this book.
Sample
Problem
14.11 illustrates the mechanistic study
of
a relatively simple reaction.
SampieProblem14.11'
The gas-phase decomposition
of
nitrous oxide
(N
2
0)
is
believed
to
occur in two steps:
Step
1:
N
2
0 k, . N2 + 0
Step
2:
N
2
0 + 0 k2 . N 2 + O
2
Experimentally the rate law is found to be rate =
k[N
2
0].
(a) Write the equation
fo
r the overall
reaction. (b) Identify the intermediate(s). (c) Identify the rate-determining step.
(Continued)
•
572 CHAPTER
14
Chemical Kinetics
Think
About
It
A species tha gets
canceled when steps are added may
be
an
intermediate or a catalyst. In
this case, the canceled species is
an intermediate because it was first
generated and then consumed. A
species that is first consumed and
then generated, but does
n't
appear
in the overall equation, is a catalyst.
Strategy
Add the two equations, canceling identical terms on opposite sides
of
the arrow,
to
obtain
the overall reaction. The canceled terms will be the intermediates
if
they were first generated and then
consumed. Write rate laws for each elementary step; the one that matches the experimental rate law
will be the rate-determining step.
Setup
Intennediates are species that are generated in an earlier step and consumed in a later step.
We
can write rate laws for elementary reactions simply by using the stoichiometric coefficient for
each species as its exponent in the rate law.
O
kJ
0
Step
1:
N2 •
N2
+
Step 2: N
2
0 + 0 k
2
•
N2
+ O
2
(b) 0 (atomic oxygen) is the intermediate.
rate
==
k[N
2
0]
rate
==
k[N
2
0][0]
(c) Step I is the rate-determining step because its rate law is the same as the experimental rate law:
rate
==
k[N
2
0]
Practice Problem A The reaction between
N0
2
and CO
to
produce NO and CO
2
is thought to occur
in
two step
s:
Step 1:
N0
2
+
N0
2
k
J
•
NO
+
N0
3
Step 2:
N0
3
+ CO
k2
.
N0
2
+
CO
2
The experimental rate law is rate
==
k[N0
2
f (a) Write the equation for the overall reaction.
(b) Identify the intermediate(s). (c) Identify the rate-determining step.
Practice Problem B Propose a plausible mechanism for the reaction A +
2B
-_.
C + D given
that the rate law for the reaction is rate
==
k[A][B].
Experimental Support for Reaction Mechanisms
When you propose a mechanism based on experimental rate data, and your mechanism satisfies
the two requirements
li
sted on page 569, you can say that the proposed mechanism is
plausible-
not that it is necessarily correct.
It
is
not possible to prove that a mechanism is correct using rate
data alone.
To
determine whether or not a proposed reaction mechanism is actua
ll
y correct, we
must conduct other experiments. In the case
of
hydrogen peroxide decomposition, we might try to
detect the presence
of
the
10
- ions.
If
we can detect them, it will support the proposed mechanism.
Similarly, for the hydrogen iodide reaction, detection
of
iodine atoms would lend support to the
proposed two-step mechanism. For example,
12 dissociates into atoms when it is ilTadiated with
visible light. Thus, we
might predict that the formation
of
HI
from
H2
and
12
would speed up as the
intensity
of
light is increased because that would increase the concentration
of
I atoms. Indeed,
this
is
just
what
is
observed.
In one case, chemists wanted to know which
C
-O
bond was broken in the reaction between
methyl acetate and water to better understand the reaction
•
The two possibilities
of
bond breaking are
~
1
CH
3
-C-
-0-
CH
3
~
1
CH
-c-o-
-CH
3 3
and
(a)
(b)
To
distinguish between schemes (a) and (b), chemists used water containing the oxygen-I8 iso-
tope instead
of
ordinary water, which contains the oxygen-16 isotope.
When
the
18
0 water was
used, only the acetic acid formed contained
18
0:
o
II
18
CH3-C-
O-H
,
Thus, the reaction must have occurred via bond-breaking scheme (a), because the product formed
via scheme (b) would have retained both
of
its original oxygen atoms and would contain no
18
0.
Another example is photosynthesis, the process by which green plants produce glucose from
carbon dioxide and water:
A question that arose early in the studies
of
photosynthesis was whether the molecular oxygen
produced
came
from the water, the carbon dioxide, or both.
By
using water containing only the
oxygen-I8
isotope, it was concluded that all the oxygen produced by photosynthesis
came
from
the water and none
came
from the carbon dioxide, because the 0
0
produced contained only
18
0.
These examples illustrate how creative chemists must
be
in order to study reaction
mechanisms.
Checkpoint 14.5
Reaction Mechanisms
SECTION 14.6 Catalysis 573
Use
the following information to
answer
questions 14.5.1 to 14.5.3:
For
the reaction
14
.5.3
Which
of
the following is a plaus
ible
mechanism for the
reaction?
A+B +'C
+ D
the
experimental
rate law is
rate
= k[B]2
14.5.1
What
is the
order
of
the reaction with
re
s
pect
to A and B,
respectively?
a)
0 and 1
b) 1 and 0
c) 0 and 2
d) 2 and
1
e) 2
and
0
a)
b)
c)
d)
e)
Step
1:
A + B
• C + E (slow)
Step 2: E + A • D (fast)
Step
1:
B + B • C + E (slow)
Step
2:
E + A • D + B (fast)
Step
1:
A + A • B + D (slow)
Step
2: B + B
• A + C (fas
t)
Step
1:
B + B
• C + E (slow)
Step
2:
C + A • D + B (fast)
Step
1:
A + B
• D + E (slow)
Step 2: E + A
• C (fast)
14.5.2
What
is the overall
order
of
the reaction?
14.5.4 A plausible
mechanism
for
the reaction
a) 0
H2 + 2ICI -
-+.
12 +
2HCl
is the following:
b) 1
c) 2
Step
1:
H2 + ICI
k,
.
HI
+
HCl
(slow)
d) 3
Step 2:
HI
+ ICI k
2
•
12 + HCI (fas
t)
.
e)
4
Select
the
rate
law that will be
determined
experimentally.
?
a) rate = k[H
2
]-
?
b) rate = k[H
2
][ICl]-
?
c) rate = k[ICl]-
d) rate
= k[H
2
][ICl]
e) rate
= k[HI][ICI]
Catalysis
Recall from Section 14.5 that the reaction rate for the decomposition
of
hydrogen peroxide
depends on the concentration
of
iodide ion
s,
even though I- does not appear in the overall equa-
tion. Instead, I- acts as a catalyst for the reaction. A
catalyst is a substance that increases the rate
of
a chemical reaction without itself being consumed.
The
catalyst
ma
y react to form an intermedi-
ate, but it is regenerated in a subsequent step
of
the reaction.
Molecular oxygen is prepared in the laboratory by heating potassium chlorate.
The
reaction
•
IS
2KCl0
3
(s)
+
2KC1(s) +
30ig)
However, this thermal decomposition process is very slow in the absence
of
a catalyst.
The
rate
of
decomposition can
be
increased dramatically by adding a small amount
of
manganese
(N)
dioxide
.
_
~
. .
M
ultimedia
Catalysis.
574
CHAPTER
14 Chemical Kinetics
Figure 14.14 Comparison
of
the
activation energy barriers
of
(a) an
uncatalyzed reaction and (b) the same
reaction with a catalyst. A catalyst
lowers the energy barrier but does not
affect the energies
of
the reactants or
products. Although the reactants and
products are the same in both cases, the
reaction mechanisms and rate laws are
different in (a) and (b).
•
>,
E a
>,
e n
e n
"
"
E'
0:
"
"
"
a
-
-
'"
'"
.
~
~
~
"
A+ B
-
A+B
-
B
"
~
0
0
0 0
C + D
C+D
Reaction progress Reaction progress
(a)
(b)
(Mn0
2) a black powdery substance. All the
Mn02
can be recovered at the end
of
the reaction,
just
as all the
1-
ions remain following the decomposition
of
H
2
0
2
.
A catalyst speeds up a reaction by providing a set
of
elementary steps with more favorable
kinetics than those that exist in its absence. From Equation 14.8 we know that the rate constant
k
(and hence the rate)
of
a reaction depends on the frequency factor (A) and the activation energy
(Ea) the larger the value
of
A (or the smaller the value
of
E
a
),
the greater the rate. In many cases,
a catalyst increases the rate by lowering the activation energy for the reaction.
Let's
assume that the following reaction has a certain rate constant k and an activation
energy
Ea.
A+B
k
.C+D
In the presence
of
a catalyst, however, the rate constant is ke,
ca
lled the catalytic rate constant.
By the definition
of
a catalyst,
rate
ea
tal
yzed
>
rateun
e
at
al
yze
d
Figure 14.14 shows the potential energy profiles for both reactions.
The
total energies
of
the reac-
tants (A and B) and those
of
the products (C and D) are unaffected by the catalyst; the only differ-
ence between the two is a lowering
of
the activation energy from Ea to
E~.
Because the activati
on
energy for the reverse reaction is also lowered, a catalyst enhances the rates
of
the forward and
reverse reactions equally.
There are three general types
of
catalysis, depending on the nature
of
the rate-increasing
substance: heterogeneous catalysis, homogeneous catalysis, and enzyme catalysis.
Heterogeneous Catalysis
In heterogeneous catalysis, the reactants and the catalyst are in different phases.
The
catalyst is
usually a solid, and the reactants are either gases
or
liquids. Heterogeneous catalysis is by far the
most important type
of
catalysis in industrial chemistry, especially in the synthesis
of
many impor-
tant chemicals. Heterogeneous catalysis is also used in the catalytic converters in automobiles.
At high temperatures inside a car's engine, nitrogen and oxygen gases react to form nitric
oxide:
When
released into the atmosphere,
NO
rapidly combines with O
2
to form
N0
2
.
Nitrogen dioxide
and other gases emitted by automobiles, such as carbon monoxide
(CO) and various unburned
hydrocarbons, make automobile exhaust a major source
of
air pollution.
Most
new cars are equipped with catalytic converters [Figure 14.1S(a)]. An efficient cata-
lytic converter serves two purposes:
It
oxidizes
CO
and unburned hydrocarbons to
CO
2
and H
2
0,
and it converts
NO
and
N0
2
to
N2
and O
2
,
Hot
exhaust gases into which air has been injected are
passed through the first chamber
of
one converter to accelerate the complete burning
of
hydrocar-
bons and to decrease
CO
emission
s.
Because high temperatures increase
NO
produ
ct
ion, however,
a second chamber containing a different catalyst (a transition metal or a transition metal oxide
such as
CuO or
Cr
20 3) and operating at a lower temperature is required to dissociate
NO
into
N2
and O
2
before the exhaust is discharged through the tailpipe [Figure 14.1S(b)].
Internal combustion engine
Exhaust
Air compressor
Homogeneous Catalysis
(a)
(b)
Catalytic
converters
Tailpipe
In homogeneous catalysis the reactants and the catalyst are dispersed in a single phase, usually
liquid. Acid and base catalyses are the most important types
of
homogeneous catalysis in liquid
solution. For example, the reaction
of
ethyl acetate with water
to
form acetic acid and ethanol nor-
mally occurs too slowly to be measured.
In the absence
of
the catalyst, the rate law is given by
The reaction, however, can be catalyzed by an acid.
Often a catalyst is shown above the arrow in
a chemical equation:
In the presence of acid, the rate is faster and the rate law is given by
rate
= k
c
[CH
3
COOC
2
H
5
][H
+]
Because
kc
> k in magnitude, the rate is determined solely by the catalyzed portion
of
the
reaction.
Homogeneous catalysis has several advantages over heterogeneous catalysis. For one thing,
the reactions can often be can-ied out under atmospheric conditions, thus reducing production
costs and minimizing the decomposition
of
products at high temperatures. In addition, homo-
geneous catalysts can be designed
to
function selectively for particular types of reactions, and
homogeneous catalysts cost less than the precious metals (e.g., platinum and gold) used in hetero-
geneous catalysis.
Enzymes: Biological Catalysts
Of
all the intricate processes that have evolved in living systems, none is more striking or more
essential than enzyme catalysis.
Enzymes are biological catalysts. The amazing fact about enzymes
is that not only can they increase the rate
of
biochemical reactions by factors ranging from 10
6
to
10
18
,
but they are also highly specific. An enzyme acts only on certain reactant molecules, called
substrates, while leaving the rest
of
the system unaffected.
It
has been estimated that an average
living cell may contain some
3000 different enzymes, each
of
them catalyzing a specific reac-
tion in which a substrate is converted into the appropriate product(s). Enzyme catalysis is usually
homogeneous because the substrate and enzyme are present in aqueous solution.
An enzyme is typically a large protein molecule that contains one or more active sites where
interactions with substrates take place. These sites are structurally compatible with specific sub-
strate molecule
s,
in much the same way that a key fits a particular lock.
In
fact, the notion of a
rigid enzyme structure that binds only to molecules whose shape exactly matches that
of
the active
SECTION 14.6 Catalysis 575
Figure 14.15 (a) A two-stage
catalytic converter for an automobile.
(b) In the second stage, NO molecules
bind to the surface
of
the catalyst. The
N atoms bond to each other and the
0
atoms bond to each other, producing
N2
and O
2
,
respectively.