Chemistry part 25, Julia Burdge,2e (2009) pps
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (16.64 MB, 26 trang )
•
•
Think
About
It It
isn't
nece
ssary
for every species in the reaction
to be a gas only those species
that appear in the equilibrium
.
expressIOn.
which simplifies to
where
D.n
= b -
a.
In general,
Equation
15
.3
D.n
= moles
of
gaseous products - moles
of
gaseous reactants
Because pressures are usually expressed in atmospheres, the gas constant
R is 0.08206 L .
atmlmol . K, and we can write the relationship between
Kp
and
Kc
as
Equation 15.4
Kp
= K
c
[(0
.08206 L . atmlK . mol) X
T]~
n
Kp
is equal to Kc only in the special case where
D.n
= 0, as in the following equilibrium reaction:
Hig)
+ Br2(g) +=. ==:z'
2HBr(g)
In
this case, Equation 15.4
can
be written as
Kp
= Kc [(0.08206 L . atmlK . mol) X
T]0
= K
c
Keep in mind that
Kp
expressions can only be written for reactions in which every species in
the equilibrium expression is a gas. (Remember that solids and pure liquids do not appear
in
the equilibrium expression.)
Sample
Problems 15.5 and 15.6 let you practice writing
Kp
expressions and illustrate the
conversion between
Kc and
Kp
.
Write
Kp
expressions for (a)
PCI
3
(g) + CI
2
(g) ;;: .• =
::!:'
PCl
s(g), (b) 0 2(g) +
2H
2
(g)
:;:.
=
::!:'
2H
2
0(I),
and (c)
Fig)
+ H2(g) • '
2HF(g).
Strategy Write equilibrium expressions for each equation, expressing the concentrations
of
the
gases in partial pressures.
Setup (a) All the species
in
this equation are gases, so they will all appear in the
Kp
expression.
(b)
Only the reactants are gases.
(c) All species are gases.
Solution
Checkpoint
15.3
Equilibrium Expressions
15.
3.1
Select the correct equilibrium expression for the reaction 15.3.2 Select the corr
ec
t equilibrium expression for the reaction
H +(aq) +
OW
(aq) • ' H
2
0(
I)
CaO(s) + CO
2
(g) • '
CaC0
3
(s)
a)
[H
2
O
l
eq
Kc = +
[H
leq[OWl
eq
•
a)
K = 1
c
[C0
2
l
eq
b)
K =
[H+l
eq[
OWl
eq
c
[H
2
O
l
eq
b)
[CaC0
3
l
eq
K=
c [CaOl
eq
[C0
2l
eq
c) Kc =
[H
+l
eq[
OH- l
eq
c)
Kc =
[C0
2
l
eq
d)
K = 1
c + _
[H
l
eq
[OH l
eq
d)
K =
[CaOl
eq
[C0
2l
eq
c
[
CaC0
3
l
eq
e)
Kc = [H+l
eq
[OH-
leq[
H20l
eq
e)
[C0
2
l
eq
K=
c [CaOl
eq
][CaC0
3l
eq
604
15.3.3
•
Practice Problem A Write Kp expressions for (a) 2CO(g) + Oz(g)
:;:,
=~.
2CO
z
(g),
(b)
CaC0
3
(s) • •
CaO
(s) +
CO
z(g), and (c) Nz(g) +
3H
z
(g)
• •
2NH
3
(g).
Practice Problem B Write the equation for the
ga
seous equilibrium corresponding
to
each
of
the
following
Kp
expressions:
(P
NO
/
(a)
Kp
= z
(P
NO
)
(Po)
2 2
The
equilibrium constant, Kc, for the reaction
(PHI
i
(
c) K -
' '
=
-
p - (P
L,
)(P
R
,)
is 4.63 X 10-
3
at 25°C.
What
is the value
of
Kp
at this temperature?
Strategy
Use Equation 15.4 to convert f
rom
Kc to
Kp.
Be
sure to convert temperature in degrees
Celsius to kelvins.
Setup Using Equation 15.3,
T = 298
K.
Solution
Kp
=
[K
c (0.08206 L .
atm
) X
T]
K'mol
= (4.63 X
10-
3
)(0.08206 X 298)
= 0.113
Practice Problem A
Por
the reaction
Kc is 2.3 X
lO
-
z
at 375°C. Calculate
Kp for the reaction at this temperature.
Practice Problem B
Kp
= 2.79 X
10-
5
for the reaction in Practice Problem A at 472°C.
What
is
Kc for this reaction at
472
°
C?
Given the following information,
HP(aq) • • H +(aq) + P- (aq)
15.3.4
Kc for the reaction
Think
About
It
Note that
we
have essentially disregarded
the units
of
Rand
T so that the
resulting equilibrium constant,
K
p
,
is
unitless. Equilibrium constants
commonly are treated as unitless
quantities.
Br
2(g)
:;:.
=~.
2Br
(g)
Kc = 6.8 X 10-
4
(at 25°C)
H
Z
C
Z
0
4
(aq)
:;:.=~'
2H
+(aq) +
C
1
0
~
-
(
aq
)
is 1.1 X
10-
3
at 1280°C. Calculate the value
of
Kp for this
reaction at this temperature.
Kc = 3.8 X
10-
6
(at 25°C)
a)
1.1
X 10-
3
determine the value
of
Kc for the following reaction at 25°C:
b)
18
C
2
0
~
-
+
2HF.
•
2P-
+ H
1
C
2
0
4
c) 0.14
a)
2.6 X
10-
9
d) 9.1 X 10
2
b)
1.8 X
10-
11
e) 8.3 X 10-
6
c)
1.2
X 10-
1
d) 2.6 X 10
5
e)
6.8
X
10-
4
605
I'
I
,
\
\
606
CHAPTER
15
Chemical Equilibrium
Qc is
calcula
t
ed
us
i
ng
the initial concentrations
of
reactants
and
products.
Simil
a
rly,
Q
p
can
be
calcul
ated us
ing
the
initial
pa
rti
al
pr
essu
res
of
re
actants
and
products.
Remem
ber
t
ha
t
calc
u
lati
ng Q
c
is
just
like
ca
lc
ul
ating Kc: prod
ucts
ov
er
r
eact
ants,
each
rais
ed
to the
appro
pr
iate pow
er
excep
t that
the
co
n
ce
n
tra
ti
ons
we
use
to
calcu
la
te
Q
c
are
the starting
conce
n
tra
ti
ons
. To
calculate
Kc
we
must
us
e equilibrium
conce
ntrat
io
n
s.
The
co
m
par
is
on
of Q with K
can
re
f
er
ei
ther to
Q
c
an
d Kc or Qp
an
d
Kp
.
U sing Equilibrium Expressions to Solve Problems
We have already us
ed
equilibrium expressions to determine the value
of
an equilibrium constant
using equilibrium concentrations. In this section, we will learn how to
use
equilibrium expressions
to predict the direction
of
a reaction and to calculate equilibrium concentrations.
Predicting the Direction
of
a Reaction
If
we
start an experiment with only reactants, we know that the reactant concentrations will
decrease and the product concentrations will increase; that is, the reaction
must
proceed in the
forward direction in order for equilibrium to
be
established. Likewise,
if
we start an experiment
with only product
s,
we know that the
product
concentrations will decrease
and
the reactant con-
centrations will increase. In this case, the reaction
mu
st proceed in the reverse direction to achieve
equilibrium,
But
often we
mu
st predict the direction in which a reaction will proceed when we
start with a mixture
of
reactants and products.
For
this situation, we calculate the value
of
the reac-
. . . . . . . . . . . . . . . . . . . . . . . . .
tion quotient, Q
e,
and compare
it
to the value
of
the equilibrium constant, Ke.
The
equilibrium constant, Ke, for the
ga
seous formation
of
hydrogen iodide from molecular
hydrogen and molecular iodine,
is 54.3 at
430
°C.
If
we
were to conduct an experiment starting with a mixture
of
0.243 mole
of
H?, 0.146 mole
of
1
2
, and 1.98 moles
of
III
in a 1.00-L container at
430
°C, would more
HI
form
or would
III
be
consumed and more H2 and 12 form? Using the starting concentrations, we can
.
calC
u
la
te'
the
reaction quotient as follows:
(1.98)2
-:-::-
:: :: , :-::-
-:-: :::-
=
111
(0.243)(0.146)
where the subscript
"i" indicates initial concentration. Because the reaction quotient does not
equal
Ke
(Q
e =
Ill
, Ke = 54.3), the reaction is not at equilibrium.
In
order to establish equilib-
rium, the reaction will pro
cee
d to the left, consuming
III
and producing H2 and 1
2
, decreasing the
value
of
the numerator and increasing the value in the denominator until the value
of
the reaction
quotient equals that of the equilibrium constant.
Thu
s, the reaction proceeds in the reverse direc-
tion (from right to left) in order to reach equilibrium.
.
. . . . . . . . . . . . . . . . . . . . . .
There are three possibilities when we compare Q with K:
Q < K
The
ratio
of
initial concentrations
of
products to reactants is too small. To reach
equilibrium, reactants
mu
st
be
converted to products.
The
system proceeds in the
forward direction (from left to right
).
Q = K
The
initial concentrations are equilibrium concentrations.
The
system is already at
equilibrium, and there will
be
no
net
movement in either direction.
Q
> K
The
ratio of initial concentrations
of
products to reactants is too large. To reach
equilibrium, products
mu
st
be
converted to reactants,
The
system proceeds in the
reverse
dir
ection
(f
rom
right to left).
Sample
Problem 15.7 shows how the value
of
Q is used to determine the direction
of
a reac-
tion that is not at equilibrium.
Sample Problem 15.7
At 375°C, the equilibrium constant for the reaction
is
1.
2. At the start of a reac
ti
on, the concentrations
of
N
2
,
H
z,
and NH3 are 0.071
M,
9.2 X 10-
3
M,
and
1.
83 X 10-
4
M, respec
ti
vel
y.
Determine whether this system is at equilibrium, and
if
not,
determine in which direction it must proceed to establish equilibrium.
Strategy Use the initial concentrations to calculate Qc, and then compare Qc with Kc.
SECTION
15.4
Using
Equilibrium
Expressions
to
Solve Problems 607
Setup
(1.83 X
1O-
4
)z
- -
, ,-
= 0.61
(0.071)(9.2 X 10-
3
)3
Solution The calculated value
of
Qc
is
less than Kc. Therefore, the reaction is not at equilibrium and
must proceed
to
the right to establish eqUilibrium.
Practice Problem A The equilibrium constant, Kc, for the formation
of
nitros
yl
chloride from nitric
oxide and chlorine,
2NO(g) +
Cl
z(g)
+=,
~.
2NOCl(g)
is
6.5 X 10
4
at
35
°
C.
In which direction will the reaction proceed to reach equilibrium
if
the starting
concentrations of
NO,
Clz,
and NOCI are
1.1
X
10
-
3
M,
3.5 X 10-
4
M, and 1.9
M,
respectively?
•
Practice Problem B Calculate
Kp
for the formation
of
nitrosyl chloride from nitric oxide and
chlorine at
35°
C,
and determine whether the reaction will proceed to the right or the left to achieve
equilibrium when the starting pre
ss
ures are P
NO
= 1.01 atm, P
CI
= 0.42 atm, and P
NOCI
= 1.76 atm.
2
Calculating Equilibrium Concentrations
If
we
know
the
equilibrium
constant
for
a
reaction
,
we
can
calculate
the
concentrations
in
the
equi-
1ibrium
mixture
from
the
initial
reactant
concentrations.
Consider
the
following
system
involving
two
organic
compounds,
cis-
and
trans-stilbene:
,
,
cis-Stilbene
H
/
l
=C
H
trans-Stilbene
The
equilibrium
constant
(K
e)
for
this s
ystem
is 24.0
at
200°
C.
If
we
know
that
the
starting
con-
centration
of
cis-stilbene
is
0.850
M,
we
can
use
the
equilibrium
expre
ss
ion
to
determine
the
equilibrium
concentrations
of
both
specie
s.
The
s
toichiometry
of
the
reaction
tells us
that
for
every
mole
of
cis-stilbene
converted,
1
mole
of
trans-stilbene is
produced.
We
will
let x
be
the
equi-
librium
concentration
of
trans-stilbene
in
mollL;
therefore
,
the
equilibrium
concentration
of
cis-
stilbene
mu
st
be
(0.850 - x)
mollL.
It
is us
eful
to
summarize
the
se
change
s
in
concentration
s
in
an
equilibrium
table:
cis-stilbene
+,
=='
trans-stilbene
Initial
concentration
(M):
0.850 o
Change
in
concentration
(M):
-x
+x
Equilibrium
concentration
(M):
0.850 - x
x
We
then
use
the
equilibrium
concentration
s,
defined
in
term
s
of
x,
in
the
equilibrium
expre
ssion:
[trans-stilbene]
Ke = .
[cis-stIlbene]
24.0 = x
0.850 - x
x
= 0.816 M
Think
About
It
In
proceeding
to the right, a reaction consumes
reactants and produces more
produc
ts.
This increases the
numerator in the reaction quotient
and decreases the denominator.
The result
is
an increase in Qc until
it is equal to
Kc, at which point
equilibrium will be established.
608 CHAPTER 15 Chemical Equilibrium
Think
About
It
Always check
your answer by inserting the
calculated concentrations into the
equilibrium expression:
[HIl
~
q
(0.378)2
[H2le
q[I
2
leq
(0.051)2
= 54.9 = Kc
The
small difference between the
calculated
Kc
and the one given
in the problem statement is due to
rounding.
Having
solved
for
x,
we
calculate
the
equilibrium
concentrations
of
cis-
and
trans-stilbene
as
follows:
[cis-stilbene]
= (0.850 - x) M = 0.034 M
[trans-stilbene]
= x M = 0.816 M
A
good
way
to
check
the
answer
to
a
problem
such
as
this
is
to
use
the
calculated
equilibrium
con-
centrations
in
the
equilibrium
expression
and
make
sure
that
we
get
the
correct
Kc
value.
K = 0.816 = 24
c 0.034
Sample
Problems
15.8
and
15.9
provide
additional
examples
of
this
kind
of
problem.
Kc for the reaction
of
hydrogen and iodine to produce hydrogen iodide,
is 54.3 at
430°C.
What
will the concentrations be at equilibrium
if
we start with 0.240 M
concentrations
of
both H2 and 1
2
')
Strategy
Construct an equilibrium table to determine the equilibrium concentration
of
each species
in terms
of
an unknown (x); solve for
x,
and use it to calculate the equilibrium molar concentrations.
Setup Insert the starting concentrations that
we
know into the equilibrium table:
H
2
(g) + I
2
(g)
:;::.
=::!:'
2HI(g)
Initial concentration (M):
0.240 0.240 0
Change in concentration (M):
Equilibrium concentration (M):
Solution We define the change in concentration
of
one
of
the reactants as x. Because there is no
product at the start
of
the reaction, the reactant concentration must decrease; that is, this reaction
must proceed in the forward direction to reach equilibrium. According to the stoichiometry
of
the
chemical reaction, the reactant concentrations will both decrease by the same amount
(x), and the
product concentration will increase by twice that amount
(2x). Combining the initial concentration
and the change in concentration for each species,
we
get expressions (in terms
of
x)
for the
equilibrium concentrations:
+
•
,
2HI(g)
o
Initial concentration
(M):
0.240
~
~
-x
+
2x
Change
in
concentration (M): - x
+ ~
Equilibrium concentration (M): 0.240 - x
0.240 - x
2x
•
Next,
we
insert these expressions for the equilibrium concentrations into the equilibrium expression
and solve for
x.
?
[HIl~q
K = =-:: c=-':
c [H2le
q[I
2leq
(2X)
2
54.3 =
c::
~
, c:
:-::-cc:
(0.240 - x)(0.240 -
x)
"54.3 =
2x
0.240 - x
x
= 0.189
(0.240 -
X)2
Using the calculated value
of
x, we can determine the equilibrium concentration
of
each species as
follows:
[H2leq
= (0.240 - x) M = 0.051 M
[I2leq = (0.240 - x) M = 0.051 M
[HIl
eq
=
2x
= 0.378 M
SECTION 15.4 Using
Equilibrium
Expressions
to
Solve Problems 609
Practice Problem A Calculate the equilibrium concentrations
of
H
2
, 1
2
, and
HI
at 430°C
if
the initial
concentrations are
[H2l;
= [I2li = 0
M,
and [HIli = 0.525 M.
Practice Problem B Calculate the equilibrium concentrations
of
H2o
120
and
HI
at
430°C
if
the initial
concentrations are
[H
2
];
=
[I2l;
= 0.100
M,
and [HIl; = 0.200 M.
For the
same
reaction and temperature as in Sample
Problem
15.8, calculate the equilibrium
concentrations
of
all three species
if
the starting concentrations are as follows: [H
2
l;
= 0.00623 M,
[1
2
];
= 0.00414
M,
and [HIl; = 0.0424 M.
Strategy
Using
the initial concentrations, calculate the reaction quotient, Q
c,
and compare
it
to the
value
of
Kc
(given
in
the
problem
statement
of
Sample Problem 15.8) to determine which direction
the reaction will proceed in order to establish equilibrium. Then, construct an equilibrium table to
determine the equilibrium concentrations. ·
Setup
[HIl!
[H
2
];[I
2
l;
(0.0424i
(0.00623)(0.00414) = 69.7
Therefore,
Qc
>
Kc,
so the system will have to proceed to the left (reverse) to reach equilibrium.
The
equilibrium table is
H2(g) + I2(g)
:;:,
=='
2HI(g)
Initial concentration (M):
0.00623 0.00414 0.0424
Change
in
concentration (M):
Equilibrium concentration (M):
Solution
Because
we
know the reaction must proceed from right to left, we know that the
concentration
of
HI will decrease and the concentrations
of
H2 and 12 will increase. Therefore, the
table should
be
filled
in
as follows:
Initial concentration
(M):
Change
in
concentration (M):
Equilibrium concentration (M):
Hig)
0.00623
+x
0.00623 + x
+
I
2
(g)
0.00414
+x
0.00414 + x
,
,
2HI(g)
0.0424
-2x
0.0424 -
2x
Next,
we
insert these expressions for the equilibrium concentrations into the equilibrium expression
and solve for
x.
(0.0424 -
2xi
5 4 . 3 = c::-::-::-::-' :: , ,-:: ::-::-
-'-: c c-
(0.00623 + x)(0.00414 + x)
It
isn't
possible to solve this equation the way
we
did
in
Practice
Problem
15.8 (by taking the square
root
of
both sides) because the concentrations
of
H2
and 12 are unequal. Instead,
we
have to carry out
the multiplications,
54.3(2.58 X
10-
5
+ 1.04 X
1O
-
2
x + x
2
)
= 1.80 X
10-
3
-
1.70 X 1O-
1
x +
4x
2
Collecting terms
we
get
50.3x
2
+ 0.735x -
4.00
X 10-
4
= 0
This
is
a quadratic equation
of
the form
ax
2
+
bx
+ c =
O.
The
solution for the quadratic equation
(see Appendix 1) is
x=
-b
±
~
b
2
- 4ac
2a
(Continued)
610
CHAPTER
15 Chemical Equilibrium
Think About
It Checking this
result gives
[HI]2
Kc
= [H
2
][12]
(0.0414i
: , :-
:: :c : :-:: :-:: = 54. 3
(0.00676)(0.00467)
If
Q < K the
reaction
will
occur
as
wr
itten. If
Q > K the
reverse
reaction
will
occur.
For
this
reaction,
1M
=
0;
therefore, Kc
is
equal
to
Kp.
Here
we
have a = 50.3, b = 0.735, and c =
-4.00
X
1O~4,
so
-0.735
±
~(0.735)2
4(50.3)(- 4.00 X
1O
~
4)
x =
2(50.3)
x = 5.25 X
1O~4
or x =
-0.0151
Only the first
of
these values, 5.25 X
1O~4,
makes sense because concentration cannot
be
a negative
number. Using the calculated value
of
x,
we
can determine the equilibrium concentration
of
each
species as follows:
[H
2
]e
q =
(0.00623+
x) M = 0.00676 M
[1
2]
eq
= (0.00414 + x) M = 0.00467 M
[HI]eq
= (0.0424 - 2x) = 0.0414 M
Practice Problem
At
1280°C the equilibrium constant
Kc
for the reaction
Br
2(g)
:;::.
=::t, 2Br(g)
is
1.1
X
1O
~3
.
If
the initial concentrations are [Br2] = 6.3 X
1O~2
M and [Br] = 1.2 X
1O~2
M,
calculate the concentrations
of
these two species at equilibrium.
Here is a summary
of
the use of initial reactant concentrations to determine equilibrium
concentrations:
1.
Construct an equilibrium table, and fill in the initial concentrations (including any that are
zero).
2.
Use initial concentrations to calculate the reaction quotient,
Q,
and compare Q to K to deter-
mine
'
the
'
cfirectloi11ll
'
whiCh
'
th
'e '
reacdoil
will
proceed.
3.
Define x
as
the amount
of
a particular species consumed, and use the stoichiometry
of
the
reaction to define (in
terIllS
of
x) the amount of other species consumed or produced.
4. For each species in the equilibrium, add the change in concentration to the initial concentra-
tion to get the equilibrium concentration.
5.
Use the equilibrium concentrations and the equilibrium expression to solve for
x.
6.
Using the calculated value
of
x,
determine the concentrations
of
all species at equilibrium.
7.
Check your work by plugging the calculated equilibrium concentrations into the equilibrium
expression. The result should
be
very close to the
Kc
stated in the problem.
The same procedure applies to
Kp.
Sample Problem 15.10 shows how to solve an equilibrium
problem using partial pressures .
.
Sample Problem 15.10
A mixture
of
5.75 atm
of
H2 and 5.75 atm
OfI
2 is contained in a 1.0-L vessel at 430°C.
The
eqiiiili:iii'uiii con·s·t'ant' (Kp) for the reaction
at this temperature is 54.3. Determine the equilibrium partial pressures
of
Hb
Ib
and
HI.
Strategy Construct an equilibrium table to determine the equilibrium partial pressures.
Setup The equilibrium table is
H
2
(g)
+
12(g)
,
2HI(g)
•
Ini tial partial pressure (atm): 5.75 5.75 0
Change in partial pressure (atm):
- x - x
+2x
Equilibrium partial pressure (atm):
5.75 -
x
5.75 - x
2x
SECTION
15.5
Factors
That
Affect
Chemical Equilibrium 611
Solution
Setting the equilibrium expression equal to K
p
,
(
2X
)2
54.3 =
-
~~
,
(5
.75 - xi
Taking the square root
of
both sides
of
the equation gives
/
54.3 = 2x
5.75 - x
7.369 = 2x
5.75 - x
7.369(5.75 - x) = 2x
42.37 -
7.369x
= 2x
42.37 = 9.369x
x = 4.52
The equilibrium partial pressures are
PH
= PI = 5.75 - 4.52 = 1.23 atm, and Pm = 9.04 atm.
2 2
•
Practice
Problem
A Determine the equilibrium partial pressures
of
H
2
,
1
20
and
HI
if
we begin the
experiment with 1.75
atm
each
of
H2 and 12 at
430
°C.
··················
·
············
·
·······
·
····························
Practice
Problem
B. Determine the equilibrium partial pressures
of
H
2
,
1
2
,
and
HI
if
we
be
gin the
experiment with 2.75
atm
HI
at
430
°C.
Checkpoint 15.4 Using Equilibrium Expressions to Solve Problems
Use the following information to answer questions 15.4.1 and 15.4.2: Kc for the reaction
is 1.7
X
10-
2
at 250°C.
15.4.1
What
will the equilibrium
concentration
of
A, B, and C be at this
temperature
if
[Ali = [Bli = 0.7
50
M
.
([Cl
=
OJ?
a) 6.1 X
10-
3
M,
6.1 X 10-
3
M,
0.092M
b) 0.046 M, 0.046 M, 0.092 M
c) 0.70 M, 0.70 M, 0.046 M
d) 0.70 M, 0.70
M,
0.092 M
e)
0.087
M,
0.087
M,
0.66 M
A +
B.
• 2C
15.4.2
What
will the equilibrium
concentrations
of
A, B, and C
be
at this
temperature
if
[C]i = 0.875 M ([Ali =
[Bl
= O
J?
a) 0.41 M, 0.41 M, 0.82 M
b) 0.41 M, 0.41 M, 0.054 M
c) 0.43 M, 0.43 M, 0.00
74
M
d) 0.43 M, 0.43 M, 0.44 M
e) 0.43 M, 0.43 M, 0.43 M
Factors That Affect Chemical Equilibrium
One
of
the interesting and useful features
of
chemical equilibria is that they can be manipulated in
specific ways to maximize production
of
a desired substance. Consider, for example, the industrial
production
of
ammonia from its constituent elements by the Haber proces
s:
More than 100 million tons
of
ammonia is produced annually by this reaction, with most
of
the
resulting ammonia being used for fertilizers to enhance crop production. Clearly it would be in the
best interest
of
industry to maximize the yield
of
NH
3. In this section, we
willleam
about the vari-
0us ways in which an equilibrium can be manipulated in order to accomplish this goal.
Le
Chiitelier's principle states that when a stress is applied to a system at equilibrium, the
system will respond by
shifting in the direction that minimizes the effect
of
the stress. In this con-
text,
"stress" refers to a disturbance
of
the system at equilibrium by
an
y
of
the following means:
Think
About
It Plugging the
calculated partial pressures into the
equilibrium expression gives
(9.04)2
:c
= 54.0
(1.23)2
The
small difference between this
result a
nd
the equilibrium constant
given
in the problem statement is
due to rounding.
Wh
en
a r
eaction
starts
with re
actan
ts
a
nd
p
ro
du
cts
,
be
su
re to c
al
c
ul
at
e Q a
nd
co
m
pa
re
it
to
K to d
eterm
ine whi
ch
dir
ecti
on
the
re
a
cti
on
will
proceed
to re
ach
equilibri
um
.
612 CHAPTER
15
Chemical Equilibrium
Re
mem
ber
that at
equ
ili
br
iu
m,
the
reactio
n
quoti
ent,
Q" is
eq
ual to the
eq
uilibrium
constan
t, K,.
While
ree
stabli
sh
ing
equilibrium
ca
u
se
s a
decrease
in the N2
conc
en
t
ra
tion, t
he
final
co
nc
entr
at
i
on
will
still
be
h
ig
he
r th
an
that
in
the or
ig
inal
equilib
rium
mixtur
e.
Th
e
system
re
sp
ond
s to t
he
stre
ss of
the
a
dde
d
re
actan
t by
cons
umi
ng
part of
it.
•
The
addition
of
a reactant or product
•
The
removal
of
a reactant or product
• A change in volume
of
the system, resulting in a change in concentration or partial pressure
of
the reactants and products
• A change in temperature
"Shifting" refers to the
occunence
of
either the forward or reverse reaction such that the effect
of
the stress is partially offset as the system reestablis
he
s equiliblium. An equilibrium that shifts
to the right is one in which more products are produced by the forward reaction.
An
equi'librium
that shifts to the left is one in which more reactants are produced
by
the reverse reaction. Using
Le
Chatelier's principle, we can predict the direction in which an equilibrium will shift, given the
specific stress that is applied.
Addition or Removal
of
a Substance
Again using the
Haber
process as an example,
consider a system at
700
K,
in which the equiliblium concentrations are as follows:
. . . . . . . . , . . . . . . . . . . . . .
.
,
Using these concentrations in the reaction quotient expression,
we
can calculate the value
of
Kc for
the reaction at this temperature as follows:
[
NH
3f
[N
2
]
[H
2
] 3
(
1.52)2
=- = 0.297 = Kc
(2.05)(1.56)3
If
we were to apply stress to this system by adding more N
2
, increasing its concentration from
2.05 M to 3.51 M, the system would no longer be at equilibrium.
To
see that this is true, use the
new concentration
of
nitrogen in the reaction quotient expression.
The
new calculated value
of
Qc
(0.173) is no longer equal to the value
of
Kc (0.297).
[
NH
3]2
[N
2
]
[H
2
] 3
(
1.52)2
,.
= 0.173
=I=-
Kc
(3.51)(1.56)3
For this system to reestablish equilibrium, the net reaction will have to shift in such a way that
Qc
is again equal to Ke, which is constant at a given temperature. Recall from Section 15.4 that when
Q is less than
K,
the reaction proceeds to the
light
in order to achieve equilibrium. Likewise, an
equilibrium that is stres
sed
in such a way that Q
becomes
less than K will shift to the right in order
to reestablish equiliblium. This means that the forward reaction, the consumption
of
N2
and
H2
to
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
.
produce
NH
3
, will occur.
The
result will be a net decrease in the concentrations
of
N2
and
H2
(thus
making the denominator
of
the reaction quotient smaller), and a net increase in the concentration
of
NH
3 (thus making the numerator larger).
When
the concentrations
of
all species are such that
Qc is again equal to K
c,
the system will have established a new equilibrium position, meaning that
it
will have shifted in one direction or the other, resulting in a new equilibrium concentration for
each species. Figure 15.5 shows how the concentrations
of
N
2
,
H
2
, and
NH
3 change when
N2
is
added to the original equiliblium mixture.
Conversely,
if
we
were to remove N2 from the original equiliblium mixture, the lower con-
centration in the denominator
of
the reaction quotient would result in Qc being greater than K
e
.
In this case the reaction will shift to the left. That is, the reverse reaction will take place, thereby
increasing the concentrations
of
N2 and H2 and decreasing the concentration
of
NH
3 until Qc is
once again equal to
K
c
.
The
addition or removal
of
NH
3 will cause a shift in the equilibrium, too.
The
addition
of
NH
3 will cause a shift to the left; the removal
of
NH
3 will cause a shift to the right. Figure 15.6(a)
shows the additions and removals that cause this equiliblium to shift to the right. Figure 15.6(b)
shows those that cause it to shift to the left.
In essence, a system at equiliblium will respond to addition
of
a species by consuming some
of
that species, and
it
will respond to the removal
of
a species
by
producing more
of
that species.
It
is important to
remember
that addition or removal
of
a species from an equilibrium mixture does
not change the value
of
the equiliblium constant,
K.
Rather,
it
changes temporarily the value
of
the
reaction quotient,
Q.
Furthermore, in order to cause a shift in the equilibrium, the species added
or removed
must
be
one that appears in the reaction quotient expression. In the case
of
a heteroge-
SECTION 1S.5 Factors
That
Affect
Chemica
l Equilibri
um
613
~
S
c
0
.~
~
'"
l:J
c
"
u
"
0
U
4.0
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0.0
-
-
-
-
-
-
-
-
.
I
. Original equili brium mixture
Addition Addition
~ ~
[
NH
3f
[N
z
l[
H
z
I
3
•
I
Time
(
1.52)z
:;
= 0.173
(3.51)(1.56)3
Immedi atel
y after addition
of
N
z
N
z
NH
3
Hz
I
[NH
Jz
(
164
)z
- - - -
0
= 0.297
[Nz
J[HzI3 (3.45)(1.38
)3
After
eq
uilibrium
has been reestablished
Ad
di
tion
/
Nz(g) + 3H
z
(g) , '
2N
H3(g) Nz(g) + 3H
ig)
+:
,
:::::;
,
:-
2N
H
3
(g)
~
/ /
Remov
al
Removal Rem
ova
l
(a) (b)
neou
s
equilibrium,
altering
the
amount
of
a
so
lid
or
liquid
spec
i
es
does
n
ot
cha
ng
e
the
p
os
iti
on
of
th
e e
quilibrium
b
eca
use
doing
so
does
not
change
the
va
lue
of
Q.
Sample
Problem
15.11 s
how
s
the
effects
of
st
re
ss
on
a
sys
tem
at
equilibrium
.
.
. Sample Problem 15.11
Hydrogen sulfide (Hz
S)
is a contaminant commonly found in natural gas. It is removed by reaction
with oxygen to produce elemental sulfur.
2H
z
S(
g)
+ O
zCg)
:;:,
=='
2S(s
) +
2H
z
O(g)
For each
of
the
fo
llowing scenarios, determine whether the equilibrium will shift to the right, sh
ift
to the left, or neithe
r:
(a) addition
of
Oz
(g),
(b) removal
of
H
2
S(
g),
(c) removal
of
H
zO(g),
and (d)
addition
of
S(s).
Strategy
Use Le Chiitelier's principle to
pr
edi
ct
the direction
of
shift for each case. Remember that
the position
of
th
e equilibrium is o
nl
y changed by the addition or removal
of
a species that appears
in
the reaction quotient expression.
Setup
Begin by writing the reaction quotient expression:
[H
z
O]
z
Qc = ?
[J:lzSnO
z]
Because sulfur is a solid, it does not appear in the expression. Changes in the concentrati
on
of
any
of
the other species will cause a change in the equilibrium position. Addition
of
a reactant or removal
of
a product that appears in the expression for Qc
wi
ll
shift the equilibrium to the right:
Addition Addition
\ \
2H2S(g) + 0 2(g)
2S(s)
+
2H20(g)
,
-
\
Removal
(Continued)
Figure 15.5 Adding more
of
a
reactant to a system at equilibrium
causes the equilibrium position to shift
toward product.
Th
e system responds to
the addition
of
N
z
by consuming some
of
the added N
z
(and some
of
the other
reactant,
H z) to produce more
NH
3
.
Figure 15.6 (a) Addition
of
a
reactant or removal
of
a product will
cause an equilibrium to shift to the
right.
(b) Addition
of
a product or
rem
oval
of
a reactant will cause an
equilibrium to shift to the l
ef
t.
614 CHAPTER 15 Chemical Equilibrium
Think
About
It
In each case,
analyze the effect the change will
have on the va
lue
of
Qc.
In
part (a
),
for example, O
2
is added, so its
concentration increases. Looking at
the reaction quotient expression,
we
can see that a larger concentration
of
oxygen corresponds to a larger
overall
denominator-giving
the
overall fraction a smaller value.
Thus,
Q will temporarily
be
smaller
than
K and the reaction will have to
shift to the right, consuming
some
of
the added O
2
(along with s
ome
of
the H
2
S in the mixture)
in
order
to reestablish equilibrium.
Figure 15.7
The
effect
of
a vo
lume
decrease (pressure increase) on the
N
2
0
4
(g) • •
2N0
2
(g) equilibrium.
When volume is decreased, the
equilibrium is driven toward the side
with the smallest number
of
moles
of
gas.
Removal
of
a reactant
or
addition
of
a product that appears in the expression for Qc will shift the
equilibrium to the left:
Solution
(a) Shift to the right
(b) Shift to the left
(c) Shift to the right
(d) No
change
N2(g)
I
Removal
+
jH2(g)
I
Removal
Addition
I
2NH3(g) •
-
Practice Problem For each change indicated, determine whether the
eq
uilibrium
PCI
3
(g) + CI
2
(g)
:;:.=='
PCls(g)
will shift to the right, shift to the left,
or
neither: (a) addition
of
PCI
3
(g), (b) removal
of
PCI
3
(g),
(c)
remo
val
of
PCIs(g), and (d) removal
of
CI
2
(g).
Changes in Volume and Pressure
If
we
were to start with a gaseous system
at
equilibrium in a cylinder with a movable piston,
we
could
change
the volume
of
the system, thereby changing the concentrations
of
the reactants and
products.
Consider again the equilibrium between
N
2
0
4
and
N0
2
:
At
25°C the equilibrium constant for this reaction is 4.63 X 10-
3
.
Suppose
we
have an equilibrium
mixture
of
0.643 M N
2
0
4
and 0.0547 M
N0
2
in a cylinder fitted with a movable piston.
If
we
push
down
on
the piston, the equilibrium will
be
disturbed and will shift in the direction that minimizes
the effect
of
this disturbance. Consider what happens to the concentrations
of
both species
if
we
decrease the volume
of
the cylinder
by
half.
Both
concentrations are initially doubled: [N
2
0
4
J =
1.286 M and [NOzJ 0.1094
M.
If
we plug the new concentrations into the reaction quotient
expression,
we
get
(0.1094
)2
3
=-1 =-:28=-=6-
= 9.3 1 X 10-
which is not equal to
Ke, so the system is no longer at equilibrium. Because Qe is greater than K
e
, the
equilibrium will have to shift to the left in order for equilibrium to be reestablished (Figure 15.7).
In general, a decrease in volume
of
a reaction vessel will cause a shift
in
the equilibrium in
the direction that minimizes the total
number
of
moles
of
gas. Conversely, an increase in volume
will
cause
a shift in the direction that maximizes the total
number
of
moles
of
gas.
SECTION 1S.5 Factors That
Affect
Chemical Equil i
brium
615
Sample
Problem
15
.12 shows how to predict the equilibrium shift that will
be
caused by a
volume change.
~
Sample Problem 15.12
For
each
reaction, predict in
what
direction the equilibrium will shift when the
volume
of
the reaction
vessel is decreased.
(a) PCIs(g)
• ' PCI
3
(g) + Clz(g)
(b) 2PbS(s)
+
30
z
(g).
'
2PbO(s)
2S0
z
(g)
(c) H
2
(g) +
Iz(g).
' 2HI(g)
Strategy
Determine
which
direction minimized the
number
of
mole
s
of
gas
in the reaction.
Count
only
moles
of
gas.
.
" . . . . . . . . .
.
" . . .
. .
Setup We have (a) 1
mole
of
gas
on
the reactant side and 2
mole
s
of
gas on the
product
side,
(b) 3
moles
of
gas
on
the reactant side
and
2 moles
of
gas
on the
product
side, and (c) 2 moles
of
gas
on each side.
Solution
(a) Shift to the left
(b) Shift
to
the right
(c)
No
shift
Practice Problem A
For
each reaction, predict the direction
of
shift caused by increasing the volume
of
the reaction vessel.
(a)
2NOCI(g)
+=.
==='
2NO(g)+Cl
z
(g)
(b)
CaC0
3
(s) . '
CaO(s)+C0
2
(g)
Practice Problem B
For
the following equilibrium, give an
example
of
a stress that will
cause
a
shift
to the right, a shift to the left, and
one
that will
cause
no shift:
.
It
is possible to change the total pressure
of
a system without changing its volume by add-
ing an inert gas such as helium to the reaction vessel. Because the total volume remains the same,
the concentrations
of
reactant and product gases do not change. Therefore, the equilibrium is not
disturbed and no shift will occur.
Changes
in
Temperature
A change in concentration or volume may alter the position
of
an equilibrium (i.e., the relative
amounts
of
reactants and products), but it does not change the value
of
the equilibrium constant.
Only a change in temperature can alter the value
of
the equilibrium constant. To understand why,
consider the following reaction:
The
forward reaction is endothermic (absorbs heat,
(t: H
> 0):
t: H
O = 58.0 kJ/mol
If
we treat heat as though it were a reactant, we can use
Le
Chil.teber's principle to predict what will
happen
if
we add or remove heat. Increasing the temperature (adding heat) will shift the reaction in
the forward direction because heat appears on the reactant side. Lowering the temperature (removing
heat) will shift the reaction in the reverse direction. Consequently, the equilibrium constant, given by
?
[N0
2
]eq
K =
-:
:
c
[N
2
0
4
]eq
increases when the system is heated and decreases when the system is cooled (Figure 15.8). A
similar argument can
be
made in the case
of
an exothermic reaction, where heat can
be
considered
It
is a common error to count
all
the
species
in
a readion to determine which
side
has
fewer
moles.
To
determine what diredion shift a
volume
change
w
ill
cause,
it
is
only the number
of
moles
of
gas
that
matters.
Think
About
It
When
there is no
difference in the
number
of
moles
of
gas,
changing
the
volume
of
the reaction vessel will
change
the concentrations
of
reactant(s)
and product(
s)-
but the system
will
remain
at equilibrium. (Q will
remain
equal to K.)
616
CHAPTER 15 Chemical Equilibrium
Figure 15.8
(a)
N
Z
0
4
-NO
z
equilibrium.
(b)
Because the reaction is
endothermic, at higher temperature, the
N
Z
0
4
(g).
• 2NO
z
(g)
equilibrium
shifts toward product, making the
reaction mixture darker.
Media
P
layer/MPEG
Animation
:
Figures
15
.
10
and
15
.
11,
Le
Chiltelier's
Principle,
pp
.
618~62
1.
\
(a)
(a)
mL
5,.
_
.
.
_
.
(b)
.
to
be
a product. Increasing the temperature
of
an
exothermic reaction
causes
the
equilibrium
con-
stant to decrease, shifting the equilibrium
toward
reactants.
Another
example
of
this
phenomenon
is the
equilibrium
between
the
following ions:
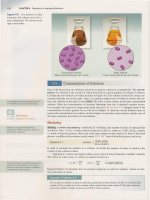
CoCl
~
~
+ 6H
2
0.
•
CO(H20)~
+
+
4CI~
+
Heat
Blue
Pink
The
reaction
as
written
, the
formation
of
CO(H
2
0)~
+
,
is
exothermic.
Thus,
the
reverse
reaction,
the
formation
of
CoCl
~~,
is
endothermic.
On
heating, the
equilibrium
shifts to
the
left
and
the
solution
turns blue.
Cooling
favors the
exothermic
reaction
[the
formation
of
CO(H20)~+]
and
the
solution
turns
pink
(Figure
15.9).
In summary, a temperature increase favors
an
endothermic
reaction, and a
temperature
decrease favors
an
exothermic
process. Temperature affects the
position
of
an
equilibrium
by
changing
the value
of
the
equilibrium
constant. Figures 15.10 and 15.11 illustrate the effects
of
various stresses
on
systems at equilibrium.
Catalysis
A catalyst speeds up a reaction
by
lowering
the
reaction's
activation energy
[
~~
Section
14.6]
.
However, a catalyst lowers the activation energy
of
the forward and reverse reactions
to
the
same
extent
(see
Figure
14.14).
The
presence
of
a catalyst, therefore, does
not
alter the
equilibrium
con-
stant,
nor
does
it
shift
the
position
of
an equilibrium system.
Adding
a catalyst to a reaction
mix-
(b)
(c)
Figure 15.9
(a)
An
equilibrium mixture of
CoCl
l~
ions and
Co(H
2
0)
~
+
ions appears violet.
(b)
Heating favors the formation of
CoCll~,
making the
solution look more blue.
(c)
Cooling favors the formation of
Co(H
z
O)~
+
,
making the solution look more pink.
SECTION
15.5 Factors That
Affect
Chemica l
Equilibrium
617
ture that is
not
at
equilibrium
will
simp
ly cause the mixture to
reach
equilibrium
sooner.
The
same
equilibrium mixture
could
be
obtained without the
cata
lyst, but it
might
take a much
longer
time.
Bringing Chemistry
to
Life
Hemoglobin Production at High Altitude
.
As
we
learned
at the
end
of
Chapter
11
, rapid
ascent
to a high altitude
can
cause
altitude sick-
ne
ss.
The
symptoms
of
altitu
de
sickness, including dizziness, headache,
and
nausea, are caused
by hypoxia,
an
insufficient
oxygen
supply to
body
tissues. In seve
re
cases, without
prompt
treat-
ment, a
victim
may
slip into a
coma
and die.
And
yet
a
per
son staying at a
high
altitude for weeks
or
months
can
recover gradually
from
altitude sickness, adjust to the low
oxygen
content
in
the
atmosphere, and live and function
nOllnally.
The
combination
of
oxygen
with the
hemoglobin
(
Hb
) molecule, which carries
oxygen
through
the
blood, is a complex reaction, but for
our
purpo
ses it
can
be repre
se
nt
ed
by
the follow-
ing simplified equation:
*
Hb(aq) + 02(aq)
+=
,
~
.
Hb0
2
(aq)
where
Hb0
2
is oxyhemoglobin, the
hemoglobin-oxygen
complex
that actually transports
oxygen
to tissues.
The
equilibri
um
expression for this process is
[Hb0
2
]
Kc = -[Hb- ] [-
O-
?]
At
an altitude
of
3
km,
the partial pressure
of
oxygen is only about 0.14 atm, compared with 0.20 atrn at
sea level. Accorcling to
Le Chfttelier's principle, a decrease in oxygen concentration will shift the hemo-
globin-oxyhemoglobin equiliblium from right to left. This change depletes the supply
of
oxyhemo-
globin,
ca
using hypoxia. Over time, the body copes with this problem by producing more hemoglobin
molecules. As the concentration
of
Hb
increases, the equilibrium gradually shifts back toward the right
(toward the formation
of
oxyhemoglobin). It can take several weeks for the increase in hemoglobin
production to meet the body's oxygen needs adequately. A return to full capacity
ma
y require several
years to occur. Studies show that long-time residents
of
high-altitude areas ha
ve
high hemoglobin levels
in their
blood-
sometimes as much as 50 percent more than individuals living at sea level.
The
produc-
tion
of
more hemoglobin and the resulting increased capacity
of
the blood to deliver oxygen to the body
have
made
high-altitude training and hypoxic tents popular among so
me
athletes.
*Biological processes such
as
this are not true equilibria, but rather they are steady-state situation
s.
In
a steady state, the
constant concentrations
of
reactants and products are not
th
e result
of
forward and re
ve
rse reactions occurring at the same
rate.
In
stead, reactant concentration
is
replenished by a previous reaction and product concentration
is
maintained by a
subsequent reaction. Nevertheless, many
of
the principles
of
equilibrium,
in
cluding Le Chiitelier's principle, still apply.
Checkpoint 15.5 Factors
That
Affect Chemical Equilibrium
15.5.1
Which
of
the following equilibria will shift to the right when
H2 is added? (Select all that apply.)
a)
2H
2 + O
2
• •
2H
2
0
15.5.3 Which
of
the following equilibria will shift to the left when
the temperature is increased?
[L'.H
(
kJ
/mol) values are given
in
parentheses
.]
(Select all that apply.)
b)
2HI
• • H2 +
12
c)
H2
+
CO
2
•
• H
2
0
+
CO
d)
2NaHC0
3
•
•
Na
2C
0 3 + H
2
0 +
CO
2
e)
2CO
+ O
2
•
•
2C0
2
15.5.2
Which
of
the following will cause the equilibrium
C(s)
+
CO
2
(g)
+=.
~
.
2CO(g)
to shift to the right? (Select all that apply.)
a) Decreasing the volume
b) Increasing the volume
c) Adding
more
C(s)
d) Adding
more
CO
2
(g)
e)
Remo
vi
ng
CO(g)
as it forms
a) S + H2 • • H
2
S
L'.H
(-20)
b) C + H
2
0 •
•
CO
+ H2
L'.H
(131)
c)
H2
+
CO
2
•
• H
2
0
+
CO
Lill(41)
d)
MgO
(s) +
CO
2
•
•
MgC0
3
L'.H
(-ll7)
e)
2CO
+ O
2
•
•
2C0
2
L'.H
(-566)
15.5.4 For which
of
the following reactions will a change in volume
not affect the position
of
the equilibrium? (Select all that apply.)
a)
MgO
(s) +
CO
2
(g) , •
MgC0
3
(s)
b)
H
2
(g) +
CI
2
(g)
• •
2HCl
(g)
c)
BaC0
3
(s)
• •
BaO
(s) +
CO
2
(g)
d)
Br(l
) + H
ig)
, •
2HBr
(g)
e) C(s) + CO
2
(g) • • 2CO(g)
Figure 15.10
Le
Chiitelier's
principle.
618
[Hz] = 0.112 M
[Iz] =
0.112M
[HI] = 0.825 M
Q = [HI]2 = (0.825)2 = 54.3
c [H
2
1[I
z
l (0. 112)(0.112)
Add a
reactant-
Iz(g)
•
,
[H
2
]
= 0.112 M
LI
z]
= 0.499 M
[HI] = 0.825 M
_ (0.825
)2
_
Qc
- (0.11 2)(0.499) -
12
Qc
eft
Kc
Equilibrium sh
if
ts toward produ
ct
[Hz] =
0.0404
M
LI
2
]
=
0.4?7
M
[HI] = 0.968 M
Q = (0.968)2 = 54.3
c
(0.0404)(0.427)
Q
c
=
Kc
[H
z
]=0.112M
LIz]
=
0.112M
[HI] = 0.825 M
Q = [HI]z = (0.825)z = 54.3
c LH
2
][Iz] (0.112)(0.112)
Qc
=
Kc
Add a
product-HI(g)
- -
[Hz] =
O.I!?
M
[I
z
] = 0.112 M
[HI]=3.17M
(3.17)2
Qc =
(0.
11
2)(0. 112) = 801
Qc
eft
Kc
Equilibrium shifts toward reactant
[Hz] =
0.362
M
[I
z
] =
0.362
M
[HI] = 2.67 M
(2.67)2
Q
c
=
"00
(0
:-::.
3=-"67:2
)=::(0~.3:-;6=2)
= 54.4
Qc
=
Kc
•
"/
LH
2
]
=
0.112M
[I
2]
= 0.112 M
[HI] = 0.825 M
Q = [HIl
z
= (0.825)2 = 54.3
c [H
2
]
[1
2]
(0.112)(0.112)
Qc
=
Kc
Add a species not involved
in
the equilibrium - He(g)
•
<
[H
2
] = 0.112 M
[I
2]
= 0
.11
2 M
[HI] = 0.825 M
[He] = 0.565 M
(0.825
)2
Qc = (0.112)(0.
1l2)
= 54.3
Qc =
Kc
Equilibrium does not shift in either direction
[H
2
]=0.112M
[I
z
] = 0
.1
12
M
[HI] = 0.825 M
[He] = 0.565 M
What's the point?
Adding
a
reactant
to an
equilibrium
mixture
shifts the
equilibrium
toward
the
product
side
of
the
equation.
Adding
a
product
shifts
the
equi
librium
toward
the
reactant
side.
Adding
a
species
that
is
neither
a
reactant
nor
a
product
does
not
cause
a
shift
in the
equilibrium.
• ""it
itA
•
Temperature decrease drives an
endothermic equilibrium toward reactants.
Temperature increase drives
an
exothermic equilibrium toward reactants.
Temperature increase drives an
endothermic equilibrium toward products.
Temperature decrease drives an
exothermic equilibrium toward product
s.
,
What's the point?
Increasing the temperature
of
an equilibrium mixture
causes a shift toward the product side for an endothermic
reaction, and a shift toward the reactant side for an
exothermic reaction.
619
Figure 15.11 Le Chatelier's principle .
•
)
[N
2
0
4
l = 0.540 M
[N0
2
l = 0.0354 M
,
_ (0.0354
)-
_
-3
Q
c
-
0.540 - 2.3 x 10
Q
c
* Kc
)
•
[N204l = 1.08 M
[N0
2
l = 0.0707 M
[NO,l2 (0.0707)2
Q - - = 4.63 X 10-
3
c -
[N2
0
4l
- 1.08
Q
c
=K
c
What's the point?
Decreasing the volume
of
an
equilibrium mixture
causes a shift toward the side
of
the equation
~~
:::.:-~
-: :;;;;J
with the smaller number
of
moles
of
gas.
620
)
•
[N
2
0
4
l = 0.533 M
[N0
2
l = 0.0497 M
(0.0497)2 _
-3
0.533 - 4.6 x 10
Q
c
=
Kc
Increasing the volume causes a shift toward the
side with the
larger number
of
moles
of
gas.
c _
___
:::>
- -
•
[N
2
0
4
l =
2.
16M
[N
0
2
l = 0.141 M
,
_ (0.141
)-
_ - 3
Q
c-
2.16 - 9.2 x
lO
Q
c
* Kc
C
___
.Y
)
•
[N
2
0
4
l = 2.18 M
[N0
2
l = 0.100 M
_ (O.lOW _ - 3
Q
c
- 2.18 - 4.6 x 10
Q
c
=
Kc
+
•
•
[Hz
] = 0.
056
M
[I
z
]
= 0.056 M
[HI
] = 0.413 M
(0.413
)z
Q
c
= (0.056)(0.056) = 54.3
Q
c
= K c
•
•
[Hz] = 0.
056
M
[1
2
]
=
0.056
M
[HI] =
0.413M
(0.413)2
Q
c
= (0.056)(0.056) = 54.3
Q
c
=
Kc
•
+
•
•
[Hz] = 0.
112M
[1
2
]
= 0. l12 M
[HI]
= 0.825 M
[HI]2 (0.825
)2
Q
c
=
[H
2
][I2] - (0.112)(0.112) = 54.3
Q
c
= Kc
What's the point?
For an equilibrium with equal numbers
of
gaseous moles on both sides, a change
in
volume
does not cause the equilibrium to shift in either
direction.
t
+
c
•
•
[H
2
]
= 0.224 M
[Izl = 0.224 M
[HI]
= 1.65 M
(1.65
)2
Q
c
= (0.224)(0.224) =
54
.3
Q
c
= Kc
c __ _
->
•
•
[H21 = 0.224
[I
zl = 0.224 M
[HI] = 1.65 M
(1.65)z
Q
c
= (0.224)(0.224) = 54.3
Q
c
= Kc
621