- Trang chủ >>
- Khoa Học Tự Nhiên >>
- Vật lý
Chemistry part 28, Julia Burdge,2e (2009) pps
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (16.3 MB, 26 trang )
682
CHAPTER
17 Acid-Base Equilibria
and
Solubility Equilibria
The
percent
ioniza
t
ion
of
acetic
acid
is
13~~~~
3
M X 100% = 1.3%
By
adding
so
dium
acetate,
we
also
add
so
d
ium
ions
to
the
solutio
n,
However,
sodium
ions
do
not interact with water or with
any
of the other
species
present
[
~
Section
[6
.
10].
Remember
that prior to the ionization of a
wea
k
acid,
the concentrat
io
n of
hydrogen
ion
in
water
at
25°(
is
1.0 x 1
0-
7
M.
H
owever,
becau
se
this
concentration
is
insignificant
comp
ared
to the
conc
entration
re
su
lting
from the i
oniza
ti
on,
we
can
neglect it
in
our
equilibrium
table.
The Common Ion Effect
Up
until now, we have discussed the properties
of
solutions containing a single solute. In this sec-
tion, we will examine how the properties
of
a solution change when a second solute is introduced.
Recall that a system at equilibrium will shift in response to being stressed and that stress
can be applied in a variety
of
ways, including the addition
of
a reactant or a product [I
••
Section
15.5]. Consider a liter
of
solution containing 0.10 mole
of
acetic acid. Using the
Kafor
acetic acid
(1.8
X
10-
5
)
and an equilibrium table
[
~.
Section
16.5], the
pH
of
this solution at 25°C can be
deter mined:
CH
3
COOH(aq)
+:.
===z:.
H +(aq) +
CH
3
COO- (aq)
Initial concentration (M): 0.10 0 0
Change in concentration (M):
-x
+x +x
Equilibrium concentration (M):
0.10 - x
x x
Assuming that (0.10 - x) M = 0.10 M and solving for
x,
we get 1.34 X 10-
3
.
Therefore,
. . . . . . . . . . . . . . .
.
,
.,
3
[CH
3
COOH] = 0.09866 M, [H+] = [CH
3
COO
- ] = 1.34 X 10- M and
pH
= 2.87.
Now consider what happens when we add
0.050 mole
of
sodium acetate
(CH
3
COONa) to
the solution.
Sodium acetate dissociates completely in aqueous solution to give sodium ions and
acetate ions:
CH
3COONa(aq) H2
0
,
Na
\aq)
+
CH
3
COO- (aq)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
.,
Thus, by adding sodium acetate, we have increased the concentration
of
acetate ion. Because
acetate ion is a product in the ionization
of
acetic acid, the addition
of
acetate ion causes the equi-
librium to shift to the left.
The
net result is a reduction in the percent ionization
of
acetic acid.
Addition
/
H+(aq) + CH3COO- (aq)
Equilibrium is driven toward reactant.
Shifting the equilibrium to the left consumes not only some
of
the added acetate ion, but also some
ofthe
hydrogen ion. This causes the
pH
to change (in this case the
pH
increases). Sample Problem
17.1 shows how an equilibrium table can be used to calculate the
pH
of
a solution
of
acetic acid
after the addition
of
sodium acetate.
Sample
Problem 17.1
",
"
Determine the pH at 25°C
of
a solution prepared by adding 0.050 mole
of
sodium acetate to 1.0 L
of
0.10 M acetic acid. (Assume that the addition
of
sodium acetate does not change the volume
of
the
solution.)
Strategy
Construct a new eqUilibrium table to solve for the hydrogen ion concentration .
Setup
' We'
~se
'
the
'
s
'
tat~d
concei;t~~iion
ci
~~etic
'
a
c
i'd
:
o.io
M:
~nd
[H
+] = 0 M as the initial
concentrations in the table:
CH
3
COOH(aq)
:.;::.
=::t, H+(aq) +
CH
3
COO- (aq)
Initial concentration
(M):
0.10
o 0.050
Change in concentration (M): - x
+x
+x
Equilibrium concentration (M):
0.10 - x
x 0.050 + x
Solution
Substituting the equilibrium concentrations, in terms
of
the unknown
x,
into the
equilibrium expression gives
1.8
X 10-5 =
(x)~o.~o
+ x)
.1
- x
Because we expect x to be very small (even smaller than 1.34 X 10-
3
M- see above), because the
•
ionization
of
CH
3
COOH
is suppressed by the presence
of
CH
3
COO
-,
we assume '
(0.10
-
x)
M = 0.10 M and
(0.050 - x) M = 0.050 M
SECTION 17.2
Buffer
Solutions
683
Therefore, the equilibrium expression simplifies to
~5
(x)(0.050)
1.8
X 10 = 0.10
and x = 3.6 X
1O
~5
M.
According
to
the equilibrium table, [H+] =
x,
so pH = - log (3.6 X
1O
~5)
= 4.44 .
.
Think
About
It
The equilibrium concentrations
of
CH
3
COOH,
C
H
3
COO
~,
and H+ are the same
regardless
of
whether we add sodium acetate to a solution
of
acetic acid, add acetic acid to a
solution
of
sodium acetate,
or
dissolve both s
pe
cies at the same tim
e.
We
could have constructed
an
equilibrium table starting with
th
e equilibrium concentrations in the 0.10 M acetic acid solution:
• • • •
• • • • • • • • • • • • • • • • • • • • • • • • • • •
In
this
case,
the
percent
i
oniza
t
ion
of
ace
tic
acid
is
3.6
OX1~0~s
M x 1
00%
= 0.036%
Th
is
is
cons
i
derably
sma
lle
r than the
per
cent
ionization prior to
the
addition of
so
diu
m
acetate.
Initial concentration (M):
0.09866 1.34 X
1O
~3
5.134 X
1O
~2
This
is
the
sum
of the
equ
ilib
rium
concentra
tio-
Change in concentration (M):
t I
of
acetate
ion
in
a 0.10 M
so
lu
tion of
ace
tic
aoc
+
::.y +
y"
, t
:o.y___
(1.3
4 x 1
0
~3
M)
and
the
added
ace
t
ate
ion
Equilibrium concentration (M):
0.09866 + Y 1.34 X
1O
~3
- Y 5.134 X
1O~
2
- Y (0.050 M).
In this case, the reaction proceeds
to
,the left. (The acetic acid concentration increases, and the
concentrations
of
hydrogen and acetate ions decrease.) Solving for y gives 1.304 X
1O~
3
M.
[H+] = 1.34 X
1O~
3
- Y = 3.6 X
1O
~
5
M and pH = 4.44.
We
get the same pH either way .
.
• • • • •
••
•••
Practice Problem A Determine the pH at 25°C
of
a solution
pr
epared by dissolving 0.075 mole
of
sodium acetate in 1.0 L
of
0.25 M acetic acid. (Assume that the addition
of
sodium acetate does not
change the volume
of
the solution.)
Practice Problem B Determine
th
e pH at 25°C
of
a solution prepared by dissolving 0.35 mole
of
sodium acetate in 1.0 L
of
0.25 M acetic acid. (Assume that the addition
of
sodium acetate does not
change the volume
of
the solution
.)
An aqueous solution
of
a weak: electrolyte contains both the weak: electrolyte and its ioniza-
tion products, which are ion
s.
If
a soluble salt that contains one
of
those ions is added, the equi-
librium shifts to the left, thereby
suppressing the ionization
of
the
weak:
electrolyte.
In
general,
when a compound containing an ion in common with a dissolved substance is added to a solution
at equilibrium, the equilibrium shifts to the left. This
phenom
enon is known
as
the common ion
effect.
Checkpoint 17.1 The Common Ion Effect
17.1.1 Which
of
the following would cause
a decrease in the percent ionization
of
nitrous acid (
HN0
2
)
when added
to
a
solution
of
nitrous acid at equilibrium?
(Select all that apply.)
a)
NaN0
2
b) H
2
0
c)
Ca(N0
2
h
d)
HN0
3
e)
NaN0
3
Buffer Solutions
17
.1.2 What is the pH
of
a solution prepared
by adding
0.05 mole
of
NaF
to 1.0 L
of
0.1 M
HF
at
2YC?
(Assume that the
addition
of
NaF
does not change the
volume
of
the solution.)
(Ka
for
HF
=
7.1 X
1O
~4
.
)
a) 2.1
b) 2.8
c) 1.4
d) 4.6
e) 7.3
. . . . .
A solution that contains a weak acid and its conjugate
ba
se (or a weak: base and its conjugate
acid) is a
buffer solution' or 'slmpiy a '
buffer:
B'
uff
er
'soiutioiis;
by
'
vli-tiie
'
o(i
h
e1r
'comPOSItIon; '
r(dst
'
.
changes in
pH
upon addition
of
small amounts
of
either an acid or a base.
The
ability to
re
sist
pH
change is very important to chemical and biological systems, including the human body. The
pH
of
blood is about 7.4, whereas that
of
gastric juices is about 1.5. Each
of
these
pH
values is crucial
for proper enzyme function and the balance
of
osmotic pressure, and each is maintained within a
very narrow
pH
range by a buffer.
Trea
ti
ng
the
problem
as
though both
CH
3
COm-
a
nd
CH
3
COO-
are
added
at
the
same
time a
nd
•
the
reaction
proceeds
to the
ri
ght
simp
li
fies
the
solut
ion.
The
common
ion
can
also
be
H+ or
O
H
~
.
For
example,
addition of a strong
acid
to a
so
luti
on
of a weak
acid
supp
r
esses
io
niza
tion of the
~
acid.
Similarly,
addition
of
a
st
rong
base
to a
solu
t
ion
of
weak
base
suppre
sses
ionizati
on
of
the
weak
base.
Any
solution of a
weak
acid
co
nta
in
s
some
co
njugate
base.
In a buffer
so
luti
on,
t
houg
h,
;;-::
amounts of
weak
acid
and
conj
ug
ate
base
m=
be
comparable,
meaning
that the
con
ju
ga
te
hax
must
be
sup
plied
by
a
dissolved
salt.
684
CHAPTER
17 Acid-Base Equilibria and Solubility Equilibria
Remember
that
sod
ium
acetate
is
a
strong
electrolyte
[
~
~
Sect
ion
4 ,1].
so
it
dissociates
completely
in
water to
give
sodium
ions
and
acetate
ions:
. ,, ' .
Multimed
ia
Acids
and
Bases
effect of addition of a
strong
acid
and
a
strong
base
on
a buffer,
The
forward
reaction
is
suppressed
by
the
presence
of
the
common
ion,
CH
3
COO
-,
and
the
reverse
process
is
suppressed
by
the
presence
of
CH
3
COOH.
As
long
as
th
e amount of strong
acid
added
to
the
buffer
does
not
exceed
the amount of
conjugate
ba
se
originally
present,
all the
added
acid
will
be
consumed
and
converted
to
weak
acid,
Calculating the
pH
of
a Buffer
., C'onsider' a
;;o
lution th'at i's 'i:6 M
Ii;
aceti'c '
aClC!"
and 'i:6 M sodium acetate.
If
a small amount
of
acid
is added to this solution, it is consumed completely by the acetate ion,
thus converting a strong acid (H+) to a weak acid (CH
3
COOH). Addition
of
a strong acid lowers
the
pH
of
a solution. However, a buffer's ability to convert a strong acid to a weak acid minimizes
the effect
of
the addition on the pH.
Similarly,
if
a small amount
of
a base is added, it is consumed completely by the acetic
acid,
thus converting a strong base
(OH-)
to a weak base (CH
3
COO-).
Addition
of
a strong base
increases the pH
of
a solution. Again, however, a buffer's ability to convert a strong base to a weak
base minimizes the effect
of
the addition on pH.
To
illustrate the function
of
a buffer, suppose that we have 1 L
of
the acetic acid-sodium
acetate solution described previously. We can calculate the
pH
of
the buffer using the procedure
in
Section 17.1:
CH3COOH(aq) +:.
=::z:'
H+(aq) +
CH
3
COO-(aq)
Initial concentration (M):
1.0 0 1,0
Change in concentration (M):
-x
+x +x
Equilibrium concentration (M):
1.0 - x
x
1.0 + x
The equilibrium expression is
(x)(l.O + x)
K = -
, ::-
a 1.0 - x
. . . . . . . . . . . . . . . . . . . . . . . . .
.,
.
Because
it
is reasonable to assume that x will be very small,
(1.0 - x) M = 1.0 M and (1.0 + x) M = 1.0 M
Thus, the equilibrium expression simplifies to
1.8
X 10-
5
=
(x)(l
.O
) = x
1.0
At
equilibrium, therefore, [H+] = 1.8 X 10-
5
M and pH = 4.74.
Now consider what happens when we add
0.10 mole
of
HCI to the buffer. (We assume that
the addition
of
HCI causes no change in the volume
of
the solution.)
The
reaction that takes place
,
'
~
. "
when
we
add a strong acid is the conversion
of
H to
CH
3
COOH. The added acid is all consumed"
along with an equal amount
of
acetate ion. We keep track
of
the amounts
of
acetic acid and acetate
ion when a strong acid (or base) is added by writing the starting amounts above the equation and
the final amounts (after the added substance has been consumed) below the equation:
Upon addition
of
H+: 1.0 mol 0.1 mol 1.0 mol
CH
3C
OO
- (aq) + H
+(
aq) ,
CH
3
COOH(aq)
After H+ has been consumed:
0.9 mol
o mol
1.1
mol
We
can use the resulting amounts
of
acetic acid and acetate ion to construct a new equilibrium
table:
CH
3COOH(aq) +:.
=::z:'
H+(aq) +
CH
3
COO-(aq)
Initial concentration (M):
1.1
0 0.9
Change in concentration (M):
-x
+x
+x
Equilibrium concentration (M):
1.1
- x
x
0.9 + x
We
can solve for pH as we have done before, assuming that x is small enough
to
be
neglected,
SECTION 17.2
Buffer
Solutions 685
8
- 5 (x)(0.9 + x) (x)(0.9)
1.
XlO
=
=-
- -
1.1
- x
1.1
x = 2.2 X 10-
5
M
Thus, when equilibrium is reestablished, [H+] = 2.2 X 10-
5
M and
pH
= 4.66
~
.
~h~~g~
'
~f"~
'
~iy
"""
0.08
pH
unit.
In the determination
of
the
pH
of
a buffer such as the
one
just
described,
we
always neglect
the small amount
of
weak acid that ionizes (x) because ionization is suppressed by the presence
of
a
common
ion. Similarly, we ignore the hydrolysis
of
the acetate ion because
of
the presence
of
acetic acid. This enables us to derive an expression for determining the
pH
of
a buffer. We begin
with the equilibrium expression
Rearranging to solve for [H+] gives
Taking the negative logarithm
of
both sides,
we
obtain
or
Thus,
where
+ [HA]
- log [H ]
=
-log
Ka
- log [A ]
[A
- ]
- log [H
+]
=
-log
Ka + log [HA]
[A
- ]
pH
=
pKa
+ log [HA]
pKa
= - log Ka
Equation 17.1
Equation 17.2
Equation 17.1 is known as the
Henderson-Hasselbalch equation. Its more general for
III
is
[conjugate base]
pH
=
pKa
+ log [weak acid]
Equation 17.3
In the case
of
our acetic acid (1.0
M)
and sodium acetate (1.0
M)
buffer, the concentrations
of
weak
acid and conjugate base are equal. When this is true, the log term in the Henderson-Hasselbalch
equation is zero and the
pH
is numerically equal to the
pK
a
.
In
the case
of
an
acetic acid-acetate
ion buffer,
pKa
=
-log
1.8 X
10
-
5
= 4.74.
After the addition
of
0.10 mole
of
HCl,
we
determined that the concentrations
of
acetic acid
and acetate ion were 1.1
M and 0.9 M,
re
spectively. Using these concentrations in the Henderson-
Hasselbalch equation gives
. [CH
3
COO-]
pH
= 4.74 + log [CH
3
COOH]
= 4.74 + 1 0.9 M
og
1.1
M
= 4.74 + (- 0.087) = 4.65
The small difference between this
pH
and the 4.66 calculated using an equilibrium table is due to
differences in rounding. Figure 17.1 illustrates how a buffer solution resists drastic changes in
pH
.
•
Had
we
added
0.1
0
mole
of He I to 1 L of pU
lE
water,
the
pH
would
have
gone
from
7.
00 to
1
.00!
Media
Player
/MP
EG
Animation
:
Figure
17.1,
Buffer
Solutions,
pp.
686-68
7.
Figure 17.1
686
0.100
MCH
3
COOH
0.100
MCH
3
COO-
[CH
3
COO- ]
= 4.74 +
log
[CH
3
COOH] = 4.74
The
buffer solution is 0.100 M in acetic acid
and
0.100 M in
sodium
acetate. 100 mL
of
this
buffer contains (0.100 mollL)(O.lO L) = 0.010
mol
each
acetic acid
and
acetate ion.
Water
pH
= 7.00
aO
When
we
add 0.001 mol
of
strong acid, it
is
completely
consumed
by the acetate ion
in
the buffer.
Before reaction:
0.001
mol
0.010
mol
0.010 mol
•
H+(aq) +
CH
3
COO-(aq)
•
CH
3
COOH(aq)
After reaction: 0 mol
0.009
mol
2S.0
·c
When we add 0.001
mol
of
strong base, it is
completely consumed by the acetic acid
in the buffer.
0.011 mol
Before
reaction: 0.001
mol
0.010 mol 0.010 mol
OH-(aq)
+
CH
3
COOH(aq) •
H20
(0
+
CH
3
COO- (aq)
After reaction: 0
mol
0.009 mol
0.011 mol
N
7
•
-
We
can calculate th€ new
pH
using the
Henderson-Hasselbalch equation:
0.009
pH
= 4.74 + log 0.011 = 4.65
We
can calculate the new
pH
using the
Henderson-Hasselbalch equation:
0.011
pH=
4.74 + log 0.009
=4.83
-
~
There is nothing in pure water to consume strong
acid. Therefore, its
pH
drops drastically.
H
=
-1
0.001 mol =
200
P og O.lDL .
.
Ther€ is nothing
in
pure water to Gonsume strong
base. Therefore, its
pH
rises drastically.
0.001 mol
pOH
=
-log
0.10 L = 2.00, pH = 12.00
What's the point?
A buffer contains both a weak acid and its conjugate base. *
Small amounts
of
strong acid or strong base are consumed by
the buffer components, thereby preventing drastic
pH
changes.
Pure water does not contain species that can consume acid
or base. Even a very small addition
of
either causes a large
change in pH.
* A buffer could also be prepared using a weak base and its
conjugate acid.
687
688
CHAPTER
17 Acid-Base Equilibria
and
Solubility Equilibria
The
volume
of
the buffer
is
1 L in this ex
ampl
e,
so
the
number of
moles
of a
substance
is
equal
to the molar
con
centration. In c
ases
where the
buffer volume
is
something
other
than
1
L,
however,
we
can
still
use
molar amounts
in
the
Henderson-Hasselbalch
equati
on
becau
se
the
volume
would
cancel
in
the top
and
bottom of
the
log
term.
Think
About
It Always do a
"reality check" on a calculated pH.
Although a buffer does minimize
the effect
of
added base, the
pH
does increase.
If
you find that
you've calculated a lower pH after
the addition
of
a base, check for
errors like mixing up the weak acid
and conjugate base concentrations
or losing track
cif
a minus sign.
Weak Acid
Ka
pKa
HF
7.1
x
10-
4
3.15
HN0
2
4.5 X 10-
4
3.35
HCOOH
1.7 x 10-
4
3.77
C
6
H
s
COOH
6.5 x
lO
-
s
4.19
CH
3
C
OOH
1.8 X
lO
-
s
4.74
HCN
4.9 x
10-
10
9.31
C
6
H
s
OH
1.3 x 10-
10
9.
89
Sample Problem 17.2 shows how the Henderson-Hasselbalch equation is used to determine
the
pH
of
a buffer after the addition
of
a strong base.
Sample
Problem 17.2
Starting with 1.00 L
of
a buffer that is 1.00 M in acetic acid and 1.00 M in sodium acetate, calculate
the
pH
after the addition
of
0.100 mole
of
NaOH. (Assume that the addition does not change the
volume
of
the solution.)
Strategy
Added base will react with the acetic acid component
of
the buffer, converting
OH-
to
CH
3
COO- :
• • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • •
Write the starting amount
of
each species above the equation and the final amount
of
each species
below the equation.
Use the final amounts as concentrations in Equation 17.1.
Setup
Upon addition
of
OH
- :
1.00
mol 0.10 mol 1.00 mol
CH
3
COOH(aq) +
OW(aq)
H
2
0(/)
+
CH
3
COO- (aq)
After OH- has been consumed:
0.90 mol o mol 1.10 mol
Solution
H = 4.74 + I a 1.10 M
P 0
0
0.90M
=
474
+ I 1.10 M =
483
. og 0.90 M .
Thus, the pH
of
the buffer after addition
of
0.10 mole
of
NaOH is 4.83.
Practice
Problem
A Calculate the
pH
of
1 L
of
a buffer that
is
1.0 M in acetic acid and 1.0
Min
sodium acetate after the addition
of
0.25 mole
of
NaOH.
Practice
Problem
B Calculate the
pH
of
1 L
of
a buffer that is 1.0 M in acetic acid and 1.5
Min
sodium acetate after the addition
of
0.20 mole
of
HCI.
Preparing a Buffer Solution
with
a Specific
pH
A solution is only a buffer
if
it has the capacity to resist
pH
change when either an acid or a base
is added.
If
the concentrations
of
a weak acid and conjugate base differ by more than a factor
of
10, the solution does not have this capacity. Therefore, we consider a solution a buffer, and can use
Equation 17.1 to calculate its pH, only
if
the following condition is met:
[conjugate base]
10 >
>0.1
[weak acid]
Consequently, the log term in Equation 17.1 can only have values from
-1
to
1,
and the
pH
of
a
buffer cannot be more than one
pH
unit different from the
pKa
of
the
weak
acid it contains. This is
known as the
range
of
the buffer, where
pH
= pKa +
1.
This enables us to select the appropriate
conjugate pair to prepare a buffer with a specific, desired pH.
First, we choose a weak acid whose
pKa is close to the desired pH. Next,
we
substitute the
pH
and
pKa
values into Equation 17.1 to obtain the necessary ratio
of
[conjugate base ]/[ weak
acid]. This ratio can then be converted to molar quantities for the preparation
of
the buffer. Sample
Problem 17.3 demonstrates this procedure.
Sample Problem
17.3
.
Select an appropriate weak acid from the table in the margin, and describe how you would prepare a
buffer with a pH
of
9.50.
SECTION 17.2
Buffer
Solutions 689
Strategy
Select an acid with a
pKa
within one
pH
unit
of
9.50. Use the
pKa
of
the acid and Equation
17.1 to calculate the necessary ratio
of
[conjugate base]/[ weak acid]. Select concentrations
of
the
buffer components that yield the calculated ratio.
Setup Two
of
the acids listed in Table 16.6 have
pKa
values in the desired range: hydrocyanic acid
(HCN,
pK
a = 9.31) and phenol (C
6
H
s
OH,
pK
a = 9.89).
Solution Plugging the values for phenol into Equation 17.1 gives
Therefore, the ratio
of
[C
6
H
s
O- ] to [C
6
H
s
OH] must
be
0.41 to
1.
One way to achieve this would be
to dissolve
0.41 mole
of
C
6
H
s
ONa and 1.00 mole
of
C
6
H
s
OH in 1 L
of
water.
Practice Problem A Select an appropriate acid from Table 16.6, and describe how you would
prepare a buffer with
pH
= 4.5.
Practice Problem B What range
of
pH
values could
be
achieved with a buffer consisting
of
nitrous
acid
(HN0
2
)
and sodium nitrite
(N0
2
)7
Bringing Chemistry
to
Life
Maintaining the
pH
of
Blood
There are about 5 L
of
blood in the average adult. Circulating blood keeps cells alive by providing
them with oxygen and nutrients and by removing carbon dioxide and other waste materials. The
efficiency
of
this enolmously complex system relies on buffer
s.
The two primary components
of
blood are blood plasma and red blood cells, or erythrocytes.
Blood plasma contains many compounds, including proteins, metal ions, and inorganic phosphates.
The erythrocytes contain hemoglobin molecules,
as
well
as
the enzyme carbonic anhydrase, which
catalyzes both the formation and the decomposition of carbonic acid (H
2
C0
3
) :
The substances inside the erythrocytes are protected from extracellular fluid (blood plasma) by a
semipermeable cell membrane that allows only certain molecules
to
diffuse through it.
The
pH
of
blood plas
ma
is maintained at about 7.40 by several buffer systems, the most
important
of
which is the HCO
;-
IH
2
C0
3
system. In the erythrocyte, where the pH is 7.25, the prin-
cipal buffer systems are
HCO
;-
IH
2
C0
3
and hemoglobin. The hemoglobin molecule is a complex
protein molecule (molar mass
of
65,000 g) that contains a number
of
ionizable protons. As a very
rough approximation, we can treat it
as
a monoprotic acid in the form HHb:
where HHb represents the hemoglobin molecule and Hb - is the conjugate base
of
HHb. Oxyhe-
moglobin
(HHb0
2
),
fOlmed by the combination
of
oxygen with hemoglobin,
is
a stronger acid
than HHb:
Carbon dioxide produced by metabolic processes diffuses into the erythrocyte, where it is rapidly
converted to
H
2
C0
3
by carbonic anhydrase:
The ionization
of
the carbonic acid,
Think
About
It
There is an
infinite number
of
combinations
of
[conjugate base] and [weak acid]
that will give the necessary ratio.
Note that this
pH
could also be
achieved using
HCN
and a cyanide
salt. For most purpose
s,
it is best
to use the least toxic compounds
available.
690
CHAPTER
17
Acid-Base Equi libria and Solubility Equilibria
ha
s two important consequences. First, the bicarbonate ion diffuses out
of
the erythrocyte and
is carried by the blood plasma
to
the lungs. This is the major mechanism for removing carbon
dioxide. Second, the H+ ions shift the equilibrium in favor
of
the un-ionized oxyhemoglobin
molecules:
Because
HHb0
2
releases oxygen more readily than does its conjugate base
(Hb0
2
),
the formation
of
the acid promotes the following reaction from left to right:
HHbO
zeaq)
+:.
==:!:'
HHb(aq) + 0
2(a
q)
The O
2
molecules diffuse out
of
the erythrocyte and are taken up by other cells to carry out
metabolism.
When the venous blood returns
to
the lungs, the preceding processes are reversed. The bicar-
bonate ions now diffuse into the erythrocyte, where they react with hemoglobin to form carbonic
acid:
HHb
(aq) +
HC0
3
(a
q)
+:.
==:!:
' Hb- (aq) + H
2
C0
3
(aq)
Most
of
the acid is then converted
to
CO
2
by carbonic anhydrase:
The carbon dioxide diffuses to the lungs and is eventually exhaled. The formation
of
the Hb - ions
(due to the reaction between HHb and
HC0
3")
also favors the uptake
of
oxygen at the lungs,
Hb
-(aq)
+ O?(aq)
:;::::.
===z:'
Hb0
2
(aq)
because
Hb-
has a greater affinity for oxygen than does HHb. '
When the arterial blood flows back to the body tissues, the entire cycle
is
repeated.
Checkpoint
17.2
Buffer Solutions
17.2.1
Which
of
the following combinations
ca
n
be
used to prepare a buffer?
a)
HCIICI-
b)
HFIF-
c)
CH
3
COOHlOH
-
d)
HN0
2
IN0
2
e)
HN0
3
IN0
3
17.2.2
What
is the
pH
of
a buffer that is
0.76 M in
HF
and 0.98
Min
NaF?
a)
3.26
b)
3.04
c)
3.15
d) 10.85
e)
10.74
Acid-Base Titrations
17.2.3
17.2.4
Consider 1 L
of
a buffer that is 0.85 M
in formic acid (HCOOH) and 1.4 M in
sodium formate
(HCOONa). Calculate
the
pH
after the addition
of
0.15 mol
HCI. (Assume the addition causes no
volume change.)
a) 4.
11
b)
3.99
c)
3.87
d)
10.13
e)
10.01
Consider 1 L
of
a buffer that is 1.5 M
in hydrocyanic acid (HCN) and 1.2 M
in sodium cyanide (NaCN). Calculate
the pH after the addition
of
0.25 mol
NaOH. (Assume the addition causes no
volume change.)
a) 9.21
b) 9.37
c)
9.04
d) 4.63
e) 4.96
In Section 4.6 we introduced acid-base titrations as a form
of
chemical analysis. Having discussed
buffer solutions, we can now look in more detail at the quantitative aspects
of
acid-base titrations.
We will consider three types
of
reactions: (1) titrations involving a strong acid and a strong base,
SECTION 17.3 Acid-Base Titrations 6
9'
. u
"
~
I
!
(2) titrations involving a weak acid 'and a strong base, and (3) titrations involving a strong acid and
a weak base. Titrations involving a weak acid and a weak base are complicated
by
the hydrolysis
of
both the cation and the anion
of
the salt formed. These titrations will not
be
discussed here.
Figure 17.2 shows the experimental setup for monitoring the pH over the course
of
an acid-base
,
titration.
Strong Acid- Strong
Base
Titrations
The reaction between the strong acid. HCl and the strong
ba
se NaOH can be represented by
NaOH(aq) + HCI(aq)
NaCI(aq) + H
2
0(l)
or by the net ionic equation,
,
Consider the addition
of
a 0.100 M NaOH solution (from a buret) to a vessel containing 25.0
mL
of
0.100 M HCI. For convenience, we will use only three significant figures for volume and con-
Fig
u
re
17.2 A
pH
meter
is us
ed
[0
monitor
an
acid-base titration.
I
Multimedia
Acids and
Bases
- titration
of
He
l
wit
h
NaOH
.
. . . . . . . . . . . . . . . . . . , . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
centration and two significant figures for pH. Figure 17.3 shows the titration curve the plot
of
pH
.
.
x
.
. .
.
.
.
.
as
a function
of
titrant volume added. :
Before the addition
of
NaOH begins, the
pH
of
the acid is given by
-log(0,100),
or 1.00. :
•
Re
call
that
only
the di
gits
to the
right
of
t
he
de
cim
al
point
are
significant
in
a
pH
va
lu
e.
When NaOH is added, the
pH
of
the solution increases slowly at first. Near the equivalence point,
T
he
ti
trant
is
th
e
so
l
ution
that is added
fro
m
the buret.
.
~
">"
.
the
pH
begins to rise steeply, and at the equivalence point, when equimolar amounts
of
acid and :
•
base have reacted, the curve rises almost vertically. In a strong
acid-strong
base titration, both the .
·
hydrogen ion and hydroxide ion concentrations are very small at the equivalence point (roughly ·····
Re
c
all
that f
or
an
acid
and
base
that com
bine
1 X
10-
7
M); consequently; the addition
of
a single drop
of
the
ba
se causes a large increase in
[OH- ] and a steep rise in the
pH
of
the solution. Beyond the equivalence point, the
pH
again
increases slowly with the continued addition
of
NaOH.
14
13
12
11
10
9
8
-
:r:
7
0,
6
5
-
4
3
2
1
0
20
-
F-
Equivalence
point
J
__
I
I _
I
- I -
I
~
30
Volume
of
NaOH added
(mL)
-~
40
50
in a 1: 1 ra
tio,
the
equi
valence point
is
wh
ere
equal
mol
ar
am
o
unts
of
ac
id and
base
ha
ve
beer
comb
i
ned
[
~~
Sec
tion
4.5
].
Figure 17.3
Titrationcurve(pH
as a function
of
volume
titrant added)
of
a
strong
acid-strong
base
titration.
A 0.100 M
NaOH
solution, the titrant,
is
added
from
a
buret
to 25.0
mL
of
a
0.100 M
Hel
solution in an
Erlenm
ey
er
flask.
692 CHAPTER
17
Acid-Base Equilibria and Solubility
Equ
ilibria
These
calculations
could
also
be
done
using
moles,
but
using
m
ill
imoles
sim
plifies
the
ca
l
culations.
Remember
that
millimoles
= M x
mL
[
~
Section
4.5]
.
Volume
OH-
OH-
It
is possible to calculate the pH
of
the solution at every stage
of
titration. Here are three
sample calculations.
1.
Consider the addition
of
10.0
mL
of
0.100 M NaOH to 25.0
mL
of
0.100 M HCI: The total
vcii
ume
' of"ihe ·
scii
utl
ori.
·
ls
·
3S".()
·
fiC
.·
The
· number· of"millirnoles
of
NaOH
in 10.0
mL
is
10.0
mL
X 0.100
mIllOI
NaOH = 1.00 mIllol
1
mL
The
number
of
millimoles
of
HCI originally present in 25.0
mL
of
solution is
25.0
mL
X 0.100
~l
HCI = 2.50 mIllol
Thu
s,
the amount
of
HClleft
after partial neutralization is 2.50 - 1.00, or 1.50 mIllol. Next,
we determine the resulting concentration
of
H+. We have 1.50 mIllol in 35.0 mL:
1.50 mIllol HCI = 0.0429 M
35.0
mL
Thus [H+] = 0.0429
M,
and the pH
of
the solution is
pH
= - log (0.04289) = 1.37
2. Consider the addition
of
25.0
mL
of
0.100 M
NaOH
to 25.0 mL
of
0.100 M HCI: This is a
straightforward calculation, because it involves a complete neutralization reaction and nei-
ther ion in the salt (NaCl) undergoes hydrolysis
[
~~
Section
16.10]. At the equivalence
point, [H+]
=
[OH-]
= 1.00 X
10-
7
M and the
pH
of
the solution is 7.000.
3. Consider the addition
of
35.0 mL
of
0.100 M NaOH to 25.0 mL
of
0.l00
M HCI:
The
total
volume
of
the solution is now 60.0 mL.
The
number
of
rnillimoles
of
NaOH added is
35.0
mL
X 0.100
~:
NaOH = 3.50 mIllol
Ther
e are 2.50 mIllol
of
HCl in 25.0 mL
of
solution. After complete neutralization
of
HCI,
2.50 mIllol
of
NaOH
have been consumed, and the number
of
rnillimoles
of
NaOH
remain-
ing is 3.5 - 2.5 or
1.00 mrnol.
The
concentration
of
NaOH in
60
.0
mL
of
solution is
1.00 mIllol NaOH =
00167
M
60.0
mL
.
Thu
s [OH
-]
= 0.0167 M and pOH = - log (0.0167) = 1.78. The
pH
of
the solution is
14.00 - 1.78
or
12.22.
Table 17.1 lists the data at nine different points during a strong acid - strong base titration along
with the calculated
pH
at each point.
H+
Total
[H+]
added
(mL)
added
(mmol)
remaining
(mmol)
volume
(mL)
(moI
/
L)
pH
0
0
2.5 25.0
0.100
LOOO
5.0
0.50
2.0 30.0 0.0667
1.176
10.0
1.0
1.5
35.0
0.0429
1.364
15.0
1.5 1.0 40.0 0.0250
1.602
20.0 2.0
0.5 45.0
0.0111
1.955
25.0 2.5
0 50.0
1.00
X
10-
7
7.000
Volume
OH-
OH-
Total
[OH - ]
added
(mL)
added
(mmol)
Excess
OH-
(mmol)
volume
(mL)
(moI
/
L)
pOH pH
30.0 3.0
0.5 55.0 0.0091 2.04
11.96
35.0 3.5
1.0 60.0
0.0167
1.78
12.223
SECTION 17.3 Acid-Base
Tit
r
ati
o
ns
69
3
Weak Acid-Strong
Base
Titrations
Consider the neutralization reaction between acetic acid (a weak acid) and sodium hydroxide (a
strong base):
CH3COOH(aq) + NaOH(aq) -
-+.
CH
3COONa(aq) + H
2
0(l)
This equation can be simplified to
The acetate ion that results from this neutralization undergoes hydrolysis
[
~~
Section
16.10] as
follows:
At the equivalence point, therefore, when we only have sodium acetate in solution, the pH will be
greater than 7
as
a result
of
the OH- formed by hydrolysis
of
the acetate ion.
The curve for titration
of
25.0
mL
of
0.1 M acetic acid with 0.10 M sodium hydroxide is
shown in Figure 17.4. Note how the shape
of
the curve differs from the one in Figure 17.3. Com-
pared to the curve for titration
of
a strong acid with a strong base, the curve for titration
of
a weak
acid with a strong base has a higher initial pH, a more gradual change in pH
as
base is added, and
a shorter vertical region near the equivalence point.
Again, it is possible to calculate the pH at every stage
of
the titration. Here are four sample
calculations.
1.
Prior to the addition
of
any base, the
pH
is determined by the ionization
of
acetic acid.
We
use its concentration (0.10
M)
and its Ka (1.8 X 10-
5
)
to
calculate the H+ concentration
using an equilibrium table:
CH
3COOH(aq)
:;:::
,
~
.
H+(aq) +
CH
3
COO- (aq)
Initial concentration (M): 0.10 o o
Change in concentration (M):
-x
+x +x
Equilibrium concentration (M):
0.10 - x
x x
2
X = 1.8 X 10-
5
0.10 - x
We
can neglect x on the bottom
of
the equation
[
~~
Section
16.5]. Solving for
x,
2
0~1O
= 1.8 X 10-
5
x
2
= (
1.8
X 10-
5
)(0.10) = 1.8 X 10-
6
x =
~
1.8
X
10-
6
= 1.34 X 10-
3
M
gives
[H
+] = 1.34 X 10-
3
M and pH = 2.87.
:a
14-
13
-
12 -
11
-
10
-
9-
- - - - - - - - - - - - - - - - - Equivalence
8 - point
7-
6-
.?
:
5-
I
4-
I
I
-'
3
I
I
2-
I
I
I -
I
I
0
,
I
,
0
10
20
30
Vo
l
ume
of
NaOH
added
(mL)
I
40
1
50
Figure 17.4 Titration curve
of
a weak
acid-stro
ng base titration.
A
0.100 M NaOH solution is added
from a buret to
25.0 mL
of
a 0.100 M
CH
3
COOH solution in an Erlenmeyer
fla
s
k.
Because
of
the hydrolysis
of
the
salt formed, the pH at the equivalence
point is greater than 7.
694
CHAPTER
17
Acid-Base Equilibria and Solubil
ity
Equili bria
Remember
that
we
can
use
mol
or
mmol
amounts
in
place
of concentrations
in
the
Henderson-Hasse
l
balch
equation.
K
a
XKb=Kw
[
~.
Section
16.7].
Prior
to the additi
on
of
any
base,
and
at
th
e
eq
ui
val
e
nce
point,
this
is
an
equilibrium
problem
that
is
solved
using
a
concentration,
an
ionization
constant,
and
an
equilibrium
table
.
Prior
to t
he
equivalence
point,
pH
is
determined
using
the
Hender
son-Hasselbalch
equation:
.
I [conjugate
base]
pH
= pKa +
og
~ =
o
-,-;: '
[weak acid]
After the
equivalen
ce
point,
the
titration
curve
of a
weak
acid
is
identical
to that of a
strong
acid.
2.
After the first addition
of
ba
se, some
of
the acetic acid
ha
s been converted to acetate ion via
the reaction
CH3COOH(aq) +
OH-(aq
) - -+.
CH
3
COO- (aq)
+ H
2
0 (I)
With significant amounts
of
both acetic acid and acetate ion in solution, we now treat the
solution as a buffer and use the Henderson-Hasselbalch equation to calculate the pH.
After the addition
of
10.0
mL
of
base, the solution contains 1.5 mmol
of
acetic acid
. . . . . . . . . . . . . . . . . . . .
and 1.0 mmol
of
acetate ion (see Table 17.2).
H
=
474
+ I a 1.0 mmol =
456
p .
0",
1.5 mmol .
Each
of
the points between the beginning
of
the titration and the equivalence point can
be
calculated in this way.
3.
At
the equivalence point, all the acetic acid
ha
s been neutralized and we are left with
acetate ion in solution. (
There
is also sodium ion, which does not undergo hydrolysis and
therefore does not impact the
pH
of
the solution.)
At
this point,
pH
is determined by the
concentration and the
Kb
of
acetate ion,
The
equivalence point occurs when 25.0
mL
of
ba
se
ha
s
be
en added, making the total volume 50.0 mL. The 2.5
mmol
of
acetic acid (see
Table 17.2)
ha
s all been conv
er
ted to acetate ion. Therefore, the concentration
of
acetate
. ,
lOn
IS
[CH
COO-]
= 2,5 mmol =
0050
M
3 50.0
mL
.
As we did at the beginning
of
the titration, we construct an equilibrium table:
CH
3
COO-(aq)
+ H
2
O(l)
•
•
OH-(aq)
+
CH
3COOH(aq)
Initial concentration
(M): 0.050
0 0
Change in concentration
(M)
:
-x
+x
+x
Equilibrium concentration (M):
0.050 - x
x x
'
The
Kb
for acetate ion is 5.6 X 10-
10
.
Volume
OH -
added
(mL)
K - [OH- ][CH
3
COOH] _ x
2
_
X - 10
b - [CH
3
COO]
- 0.050 _ x - 5.6 10
OH-
added
(mmol)
• •
remaining
pH
· . . .
'"
. . . . . . . .
. . . . . . . .
• • • • • • • • • • •
.
. . . . . . . . . . . . . . . . . . . -
· .
'
o
10.0
15.0
20.0
25,0
o
. . . . .
.
0.50
1.0
1.5
2.0
2.5
2,5
0.0 2,87*
. . . . . . . . .
.,.
. . . . .
.
. . . . . . . . . . . . . . . . . . .
2.0 0.50 4.14
1.5
1.0
0.5
0,0
1.0
1.5
2.0
2.5
Total
4,56
4,92
5.34
8.
72t
Volume
OH-
OH-
added
Excess
volume
[OH-]
added
(mL)
(mmol)
OH-
(mmol)
(mL)
(moIlL)
pOH
pH
30,0 3.0 0.5 55.0
0.0091 2.04
11.96
• • • • • • •
••
•
••
• • • • • • • • • • • • • • • • • • • • • • • • • • • • •
••
••••••••••••••••••••••
•••••••••••
••••••••••
•
•••••
• • • • • • • • • •
••
•••
•••••
35,0 3.5 1.0 60.0
*[CH
3
COOH] = 0.10
M,
Ka = 1.8 x 10-
'.
t[CH
3
COO- ] = 0.050 M, Kb = 5.6 X 10-
10
•
!
0.Ql7
1.78 12.22
As
before,
we
can
neglect
x
in
the
denominator
of
the
equation.
Solving
for
x,
2
x =
56
X 10-
10
0.050 .
x
2
= (5.6 X
10-
10
)(0.050) = 2.8 X 10-
11
x =
~2.78
X 10
II
= 5.3 X
10-
6
M
gives
[OH
- ] = 5.3 X
10-
6
M,
pOH
= 5.28,
and
pH
= 8.72.
SECTION 17.3 Acid-Base
Titrations
695
4.
After
the
equivalence
point,
the
curve
for
titration
of
a
weak
acid
with
a
strong
base
is
identi-
cal
to
the
curve
for
titration
of
a
strong
acid
with
a
strong
base.
Because
all
the
acetic
acid
has
been
consumed,
there
is
nothing
in
solution
to
consume
the
additional
added
OH
- ,
and
the
pH
levels
off
between
12
and
13.
Table
17.2
lists
the
data
for
the
titration
of
25.0
mL
of
0.10 M
acetic
acid
with
0.10 M
NaOH.
Sample
Problem
17.4
shows
how
to
calculate
the
pH
for
the
titration
of
a
weak
acid
with
a
strong
base.
I Sample Problem 17.4
Calculate the
pH
in
the titration
of
50.0 rnL
of
0.120 M acetic acid by 0.240 M sodium hydroxide
after the addition
of
(a) 10.0 rnL
of
base, (b) 25.0 rnL
of
base, and (c) 35.0 rnL
of
base.
Strategy The reaction between acetic acid and sodium hydroxide is
Prior to the equivalence point [part (a)], the solution contains both acetic acid and acetate ion,
making the solution a buffer. We can solve part (a) using Equation 17.1, the Henderson-Hasselbalch
equation. At the equivalence point [part (b)],
all the acetic acid has been neutralized and we have
only acetate ion in solution.
We
must determine the concentration
of
acetate ion and solve part (b)
as an equilibrium problem, using the
Kb
for acetate ion. After the equivalence point [part (c)], all
the acetic acid has been neutralized and there is nothing to consume the additional added base.
We
must determine the concentration
of
excess hydroxide ion in the solution and solve for
pH
using
Equations 16.4 and 16.6.
Setup Remember that M can be defined as either molfL or mmoVrnL
[
~
Section
4.5].
For
this
type
of
problem, it simplifies the calculations to use millimoles rather than moles. Ka for acetic acid
is 1.8
X
10-
5
,
so
pKa
= 4.74.
Kb
for acetate ion is 5.6 X 10-
10
(a)
The
solution originally contains (0.120 mmoVrnL)(50.0 rnL) = 6.00 mmol
of
acetic acid. A
1O.0-rnL amount
of
base contains (0.240 mmoVrnL)(1O.0 rnL) = 2.40 mmol
of
base. After the
addition
of
10.0 rnL
of
base, 2.40 mmol
of
OH-
has neutralized 2.40 mmol
of
acetic acid, leaving
3.60 mmol
of
acetic acid and 2.40 mmol acetate ion in solution:
Upon addition
of
OH
- :
6.00
mmol 2.40 mmol 0 mmol
CH
3
COOH(aq) +
OH-
(aq)
:;::.
==::::!:'
H
2
0 (l) +
CH
3
COO- (aq)
After
OH
- has been consumed:
3.60 mmol Ommol
2.40
mmol
(b) After the addition
of
25.0 rnL
of
base, the titration is at the equivalence point. We calculate the
pH
using the concentration and the
Kb
of
acetate ion.
(c) After the addition
of
35.0 rnL
of
base, the titration is past the equivalence point and we solve for
pH
by determining the concentration
of
excess hydroxide ion.
Solution (a)
pH
=
pKa
+ log 2.40 = 4.74 - 0.18 = 4.56
3.60
(b)
At
the equivalence point, we have 6.0 mmol
of
acetate ion in the total volume. We determine the
total volume by calculating what volume
of
0.24 M
ba
se contains 6.0 mmol:
(volume)(0.240 mmoVrnL)
= 6.00 mmol
6.00 mmol
volume
= 0.240 mmoVrnL = 25.0 rnL
(Continued)
696
CHAPTER
17 Acid-Base Equilibria
and
Solubility Equilibria
Think
About
It
For each point
in a titration, decide first what
species are in solution and what
type
of
problem it is.
If
the
solution contains only a weak
acid (or weak base),
as
is the case
before any titrant
is
added, or
if
it contains only a conjugate base
(or conjugate acid),
as
is the case
at the equivalence point, when pH
is determined by salt hydrolysis,
it is an
equilibrium problem
that requires a concentration,
an ionization constant, and
an
equilibrium table.
If
the solution
contains comparable concentrations
of
both members
of
a conjugate
pair, which is the case at points
prior to the equivalence point, it
is a
buffer problem and is solved
using the Henderson-Hasselbalch
equation.
If
the solution contains
excess titrant, either a strong
base or strong acid, it
is
simply
a pH problem requiring only a
concentration.
Therefore, the equivalence point occurs when
25.0 mL
of
base has been added, making the total
volume
50.0
mL
+ 25.0 mL = 75.0 mL. The concentration
of
acetate ion at the equivalence point is
therefore
6.00 mmol CH
3
COO- = 0.0800 M
75.0mL
We
can construct
an
equilibrium table using this concentration and solve for pH using the ionization
constant for
CH
3
COO-
(Kb
= 5.6 X
10-
10
):
CH
3
COO-(qq) + H
2
0(I)
:;::.
=::!:"
OW(aq)
+
CH
3
COOH(aq)
Initial concentration (M): 0.0800 o o
Change in concentration (M):
-x
+x +x
Equilibrium concentration (M): 0.0800 - x x x
Using the equilibrium expression and assuming that x
is
small enough to be neglected,
[CH
3
COOH]
[OW]
(x)
(x)
x
2
Kb
= [CH
3
COO-]
= 0.0800 _ x = 0.0800 = 5.6 X 10-
10
x =
~4.48
X
10-
11
= 6.7 X
10-
6
M
According
to
the equilibrium table, x = [OH-], so [OH- ] = 6.7 X
10
-
6
M.
At equilibrium, therefore,
pOH =
-log
(6.7 X
10-
6
)
= 5.17 and pH = 14.00 - 5.17 = 8.83.
(c) After the equivalence point, we must determine the concentration
of
excess base and
calculate
pOH and pH using Equations 16.4 and 16.6. A 35.0-mL amount
of
the base contains
(0.240 mmol/mL)(35.0 mL) = 8.40 mmol
of
OH
After neutralizing the 6.00 mmol
of
acetic acid
originally present in the solution, this leaves
8.40 - 6.00 = 2.40 mmol
of
excess
OH
The total
volume is
50.0 + 35.0 = 85.0 mL. Therefore, [OH- ] = 2.40 mmo1/85.0
mL
= 0.0280
M,
pOH =
-log
(0.0280) = 1.553, and pH = 14.000 - 1.553 = 12.447.
In
summary, (a) pH = 4.56, (b) pH = 8.83, and (c) pH = 12.447.
Practice
Problem
A For the titration
of
10.0 mL
of
0.15 M acetic acid with 0.10 M sodium
hydroxide, determine the pH when (a)
10.0 mL
of
base has been added, (b) 15.0
mL
of
base has been
added, and (c)
20.0 mL
of
base has been added.
Practice
Problem
B For the titration
of
25.0
mT
,
of
0,20 M hydrofluoric acid with 0.20 M sodium
hydroxide, determine the volume
of
base added when pH is (a) 2,85, (b) 3,15, and (c) 11.89,
Strong Acid-Weak
Base
Titrations
Consider the titration
of
HCl, a strong acid, with
NH
3
, a
weak:
base:
or simply
The
pH
at the equivalence point is less than 7 because the ammonium ion acts
as
a
weak:
Bn'lnsted
acid:
or simply
Because
of
the volatility
of
an aqueous ammonia solution, it is more convenient to use hydro-
chloric acid as the titrant
(i.e"
to add HCl solution from the buret), Figure 17.5 shows the titration
curve for this experiment.
Analogous to the titration
of
a
weak:
acid with a strong base, the initial pH is determined by
the concentration and the
Kb
of
ammonia:
SECTION 17.3 Acid-Base Titrations
69-
14
13
12
Volume
HCI
11
added
(mL)
pH
10
0.0 11.13
5.0 9.86
9
10
.0
9.44
8
15.0 9.08
:r:
7
0-
20.0 8.66
22.0 8.39
6
24.0 7.88
5
25.0 5.28
26.0 2.
70
- - - - - - - -
- - -
Equi
valence - - - - - - - - -
point
4
I
28.0 2.22
3
30.0 2.00
35.0
1.70
I
I
I
2
I
40
.0 1.52
1
0
45.0
1.40
50.0 1.30
I
I
•
I
I
0 10
20
30
40
50
Volume
of
HCI added (
mL
)
Consider the titration
of
25
.0 mL
of
0.10 M
NH
3 with 0.10 M HC!. We calculate the initial
pH
by
constructing an equilibrium table and solving for
x:
NH
3(aq) + H
2
0(l
) +=.
===
'
NH
1(
aq) +
OH-(aq)
Initial concentration
(M)
: 0.10 0 0
Change in concentration (M):
-x
+x +x
Equilibrium concentration (M):
0.10 - x
-_
x x
Using the equilibrium expression and assuming that x is small enough to be neglected,
[NH1]
[OH-]
Kb
= [NH
3
]
2
= X = 1.8 X 10-
5
0.10 - x 0.10
(x) (x)
x
2
=
l.8
X
10-
6
x =
~
l.8
X 10-
6
= 1.3 X
10
-
3
pH
= 11.11
The pH at the equivalence point is calculated using the concentration and
Ka
of
the conjugate
ba
se
of
NH
3
, the
NH1 ion, and an equilibrium table. Sample Problem 17.5 shows how this is done.
Sample Problem 17.5
Calculate the
pH
at the equivalence point when 25.0
mL
of
0.100 M
NH
3 is titrated with 0.100 M HC!.
Strategy
The
reaction between
NH
3 and
HCl
is
NH
3
(aq) + H+(aq)
-_
.
NH
t (aq)
At
the equivalence point, all the
NH
3 has been converted to
NH
t . Therefore, we must determine
the concentration
of
NH
t at the equivalence point and use the
Ka
for
NH
t to solve for
pH
using an
equilibrium table.
Setup
The
solution originally
co
ntains (0.100 mmollrnL)(25.0 rnL) = 2.50
mmol
NH
t .
At
the
equivalence point, 2.50
mm
ol
of
HCI h
as
been added.
The
vol
um
e
of
0.100 M
HCl
that contains
2.50
mmol
is
(volume)(0.100 mmolirnL)
= 2.50
mmol
I
2.50
mm
ol 25 0 rnL
vo
ume
= = .
0.100
mmol
/
mL
(Continued)
Figure 17.5 Titration curve
of
a strong
acid-weak
base titration. A
0.100 M HCI solution is added fr
om
a buret to 25.0 rnL
of
a 0.100 M
NH
3
solution
in
an Erlenmeyer flask. As a
result
of
salt hydrolysis, the
pH
at the
equivalence point is lower than 7.
698
CHAPTER
17 Acid-Base Equilibria and
Solubility
Equilibria
Think
About
It
In the titration
of
a
weak
base with a strong
acid, the species in solution at the
equivalence point is the conjugate
acid. Therefore, we should expect
an
acidic pH. Once all the NH3 has
been converted to
NH
1, there is no
longer anything in the solution to
consume added acid. Thus, the pH
after the equivalence point depends
on
the number
of
rnillimoles
of
H+
added and not cons
umed
divided by
the new total volume.
The
end point is whe
re
the
col
or
change
s. T
he
equi
val
e
nce
po
int is w
her
e neutral
izat
ion
is
co
mplete.
Experimentall
y, we
use
t
he
end
point
to
es
timate the
equi
v
alen
ce
point.
It takes 25.0
mL
of
titrant to reach the equivalence point, so the total solution volume is 25.0 +
25.0 = 50.0 mL. At the equivalence point, all the
NH
3 originally present has been converted to
NH1.
The
concentration
of
NH
1 is (2.50 mmol)/(50.0
mL
) = 0.0500 M. We must use this concentration as
the starting concentration
of
ammonium ion in our equilibrium table.
Solution
NH
1(
aq) + H
2
0(l)
+.
=="
NH
3
(aq) + H3
0(aq)
Initial concentration (M): 0.0500 o o
Change in concentration (
M):
- x
+x
+x
Equilibrium concentration (M):
0.0500 - x
x x
The equilibrium expression is
K = [
NH
3][H+] = (x) (x) _ x = x
2
= 5.6 X 10-
10
a [NH1] 0.0500 0.0500
x
2
=2.8
X
IO
-
11
x =
~
2.8
X 10
II
= 5.3 X 10-
6
M
[H+] = x = 5.3 X 10-
6
M. At equilibrium, therefore, pH =
-log
(5.3 X 10-
6
)
= 5.28.
Practice Problem A Calculate the
pH
at the equivalence point in the titration
of
50.0
mL
of
0.10 M
methylamine
(s
ee Table 16.7) with 0.20 M HCI.
Practice Problem B Calculate the
pH
at the equivalence point in the titration
of
35
mL
of
0.12 M
NH
3 with 0.16 M
RN0
3
.
Acid-Base Indicators
The
equivalence
point
is the
point
at
which
the acid has
been
neutralized
completely
by
the added
base.
The
equiValence
point
in
a titration
can
be
determined
by
monitoring the
pH
over
the course
of
the titration,
or
it
can
be
determined using
an
acid-base indicator.
An
acid-base
indicator
is usu-
ally a
weak
organic acid
or
base
for
which the ionized
and
un-ionized forms are different colors.
Consider
a
weak
organic acid that
we
will
refer
to as HIn. To
be
an
effective acid-base indi-
cator,
HIn
and its
conjugate
base, In
-,
must
have
distinctly different colors. In solution, the acid
ionizes to a small extent:
In a sufficiently acidic
medium,
the
ionization
of
HIn
is suppressed according to
Le
Chatelier's
principle,
and
the
preceding
equilibrium
shifts to the left.
In
this case, the
color
of
the solution will
be
that
of
HIn.
In
a
basic
medium,
on the
other
hand, the
equilibrium
shifts to the
right
and the
color
of
the solution will
be
that
of
the conjugate base,
In
- .
'the
' end point
of
a titration is the
point
at
which the
color
of
the indicator changes.
Not
all
indicators
change
color
at the
same
pH, however, so the
choice
of
indicator
for
a particular titra-
tion
depends
on
the
strength
of
the
acid (and the
ba
se) us
ed
in
the
titration. To
use
the
end
point
to
determine the equivalence
point
of
a titration,
we
must
select
an
appropriate indicator.
The
end point
of
an indicator does not occur at a specific pH; rather, there is a range
of
pH
over
which the color change occurs.
In
practice, we select an indicator whose color change occurs over a
pH
range that coincides with the steepest part
of
the titration curve. Consider the information in Figure 17.6,
which shows the titration curves for hydrochloric acid and acetic acid each being titrated with sodium
hydroxide. Either
of
the indicators shown can
be
used for the titration
of
a strong acid with a strong
base because both end points coincide with the steepest part
of
the HCl-NaOH titration curve. However,
methyl red changes from red to yellow over the
pH
range
of
4.2 to 6.3. This end point occurs signifi-
cantly
before the equivalence point in the titration
of
acetic acid, which occurs at about
pH
8.7. There-
fore, methyl red is
not a suitable indicator for use in the titration
of
acetic acid with sodium hydroxide.
Phenolphthalein, on the other hand,
is
a suitable indicator for the
CH
3
COOH-NaOH
titration.
Many
acid-base indicators
are
plant
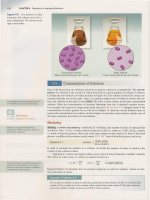
pigments.
For
example, boiling red
cabbage
in
water
extracts
pigments
that
exhibit a variety
of
colors at different
pH
values (Figure 17.7).
Table 17.3 lists a
number
of
indicators
commonly
used
in
acid-base titrations.
The
choice
of
indicator for a particular titration depends
on
the strength
of
the acid and
base
to
be
titrated.
Indicator
Thymol
blue
14
13
12
11
10
9
8
:a.
7
6
5
4
3
2
1
o
Bromophenol
blue
Methyl
orange
Methyl
red
Chlorophenol blue
Bromothymol
blue
Cresol red
Phenolphthalein
-
-
-
-
-
-
-
-
-
/'
-
0
,
I
!
/
I
Phenolphthalein (8.3 - 10)
,
-
Methyl red (4.2 - 6.3)
-
•
.
10
20
3
'0
<:
io
'.
,~
::
;'0
Volume
of
0,
10
M
NaOH
added (mL)
Color
In Acid
In Base
Red
Yellow
Yellow
Bluish purple
Orange Yellow
Red
Yellow
Yellow
Red
Yellow
Blue
Yellow
Red
Co
lorless
Reddish pink
r,
~,
I
r
SECTION
17
.3 Acid-Base
Titrations
699
pH
Range
1.2
-2
.8
3.0- 4.6
3.1-4.4
4.2-6.3
4.8-6.4
6.0-7.6
7.2-8.8
8.3-10.0
Figure 17.6 Titration curve
of
a strong acid with a strong base
(blue) and titration curve
of
a weak
acid with a strong base (red). The
indicator phenolphthalein can be used
to determine the equivalence point
of
either titration. Methyl red can be used
for the strong acid- strong base titration
but cannot be used for the weak
acid- strong base titration because i
ts
color change does not coincide with the
steepest part
of
the curve.
Figure 17.7 Solutions containing
extracts
of
red cabbage (obtained by
boiling the cabbage in water) produce
different colors when treated with an
acid and a base. The pH
of
the solutions
increases from left to right.
700 CHAPTER 17
Ac
id-Base Equilibria and Solubility Equilibria
Think
About
It
If
we don't select
an appropriate indicator, the end
point (color change) will not
coincide with the equivalence point.
Th
e co
mp
ounds
de
s
cr
i
bed
as
"i
n
soluble"
in
Chap
ter 4
[
~~
Sect
ion 4.2, Table 4.3] a
re
actuall
y
very
slig
ht
ly
soluble
each
to a
diffe
rent
de
gr
ee.
Sample Problem 17.6 illustrates this point.
Sample Problem 17.6
Which indicator (or indicator
s)
listed in Table 17.3 would you use for the acid-base titrations shown
in (a) Figure 17.3, (b) Figure 17.4, and (c) Figure 17.5?
Strategy
Determine the
pH
range that corresponds to the steepest part
of
each titration curve and
select an indicator (or indicators) that changes color within that range.
Setup (a)
The
titration curve in Figure 17.3 is for the titration
of
a strong acid with a strong base.
The
steep part
of
the curve spans a
pH
range
of
about 4 to 10.
(b) Figure 17.4 shows the curve for the titration of a weak acid with a strong base.
The
steep part
of
the curve spans a
pH
range
of
about 7 to 10.
( c) Figure
17
.5 shows the titration
of
a weak
ba
se with a strong acid.
The
steep part
of
the curve
spans a pH range
of
about 7 to 3.
Solution (a)
Most
of
the indicators listed in Table 17.3, with the exceptions
of
thymol blue,
bromophenol blue, and methyl orange would work for the titration
of
a strong acid with a strong
base.
(b) Cresol red and phenolphthalein are suitable indicators.
(c)
Br
omophenol blue, methyl orange, methyl red, and chlorophenol blue are all suitable indicators.
Practice Problem Referring to Table 17.3, specify at least
one
indicator that would
be
suitable for
the following titrations: (a)
CH
3
NH
2 with HEr, (b)
RN0
3
with NaOH, (c)
RN0
2
with KOH.
Checkpoint 17.3
Acid-Base Titrations
17.3.1
For which
of
the following titrations
will the
pH
at the equivalence point not
be
neutral? (Select all that apply.)
a)
HCN
with NaOH
b)
HEr
with NaOH
c)
NH
3 with
RN0
3
d)
RN0
2
with KOH
e)
HFwith
KOH
17.3.2
Calculate the
pH
at the equivalence
point in the titration
of
30
mL
of
0.25
M
CH
3
COOH
with 0.25 M KOH.
a)
7.00
b) 5.08
c) 8.92
d) 2.82
e) 11.18
Solubility Equilibria
17.3.3
Calculate the
pH
after the addition
of
25
mL
of
0.10 M NaOH to
50
mL
of
O.lOMHF.
a) 3.15
b) 9.31
c) 12.52
d) 1.48
e) 11.87
17.3.4
Calculate the
pH
after the addition
of
35
mL
of
0.10 M NaOH to 30
mL
of
0.10MHCN.
a)
11.89
b)
2.11
c)
12.22
d)
1.78
e)
13
.
00
The solubility
of
ionic compounds is important in industry, medicine, and everyday life. For exam-
ple, barium sulfate
(BaS0
4), an insoluble compound that is opaque to X rays, is used to diagnose
ailments
of
the digestive tract. Tooth decay begins when tooth enamel, which is mainly made
of
hydroxyapatite [CaS(P04)3
0H]
, is made more soluble in saliva by the presence
of
acid.
. . .
.
. .
.,
.
.
The
general rules for predicting the solubility
of
ionic compounds in water were introduced
in
Section 4.
2.
While these rules are useful, they do not allow us to make quantitative predictions
about how much
of
a given ionic compound will dissolve in water.
To
develop a quantitative
SECTION
17.4 Solubility Equilibria 7
01
approach,
we
must start with the principles
of
chemical equilibrium. Unless otherwise stated, the
solvent is water in the following discussion and the temperature is
25°C.
Solubility Product Expression and Ksp
Consider a saturated solution
of
silver chloride that is in contact with undissolved solid silver chlo-
ride.
The
equilibrium can
be
represented as
AgCI(s)
:;:::,
=::to
Ag
+(aq) +
CI
- (aq)
Although AgCl is
not
very soluble, all the
AgCl
that does dissolve in water dissociates completely
into
Ag
+ and Cl- ions. We can write the equilibrium expression for the dissociation
of
AgCl
as
Ksp = [Ag+
][Cn
where
Ksp
is called the solubility product constant. (Ksp is
just
another specially subscripted K
c,
. . . . . . . . . . . . . . . .
where "sp" stands for "solubility product.")
Because each
AgCl
unit contains only one Ag + and one
CI-
ion, its solubility product
expression is particularly simple to write. Many ionic compounds dissociate into
more
than two
ions. Table 17.4 lists a number
of
slightly soluble ionic compounds along with equations repre-
senting their dissolution equilibria and their solubility product constants. (Compounds deemed
"soluble" by the solubility rules in Chapter 4 are not listed for the same reason we did not list Ka
values for the strong acids in Table 16.6.)
In
general, the magnitude
of
K
sp
indicates the solubility
of
an ionic compound the smaller the Ksp value, the less soluble the compound. To make a direct
comparison
of
Ksp values, however,
we
must compare salts with similar formulas, such as AgCl
with ZnS (one cation,
one
anion) or
CaF
2
with Fe(OH)2 (one cation, two anions).
Calculations Involving Ksp and Solubility
There are two ways to express the solubility
of
a substance: molar solubility, which is the number
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. .
of
moles
of
solute in 1 L
of
a saturated solution (mol/L), and solubility, which is the number
of
grams
of
solute in 1 L
of
a saturated solution (gIL). Both
of
these expressions refer to concentra-
tions
of
saturated solutions at a particular temperature (usually 25°C).
Often
we
know the value
of
Ksp for a compound and are asked to calculate the compound
's
molar solubility.
The
procedure for solving such a problem is essentially identical to the procedure
for solving weak acid
or
weak base equilibrium problems:
1.
Construct an equilibrium table.
2. Fill in what
we
know.
3.
Figure out what
we
don't
know.
For example, the
Ksp
of
silver bromide (AgBr) is 7.7 x 10-
13
. We can construct an equilibrium
table and fill in the starting concentrations
of
Ag
+ and
Br-
ions:
AgBr(s)
:;:::,
=::to
Ag
+(aq) +
Br-
(
aq
)
Initial concentration
(M):
•
o 0
Change in concentration (M):
Equilibrium concentration (M):
Let s
be
the molar solubility (in mol/L)
of
AgBr. Because one unit
of
AgBr
yields one
Ag
+ cation
and one
Br
- anion, both [Ag +] and [Br - ] are equal to s at equilibrium:
Initial concentration (M):
•
0 0
Change in concentration (M):
+s +s
Equilibrium concentration (M):
s s
The equilibrium expression is
Therefore,
7.7
X 10-
13
= (s)(s)
A
solu
bility product
eq
uili
br
iu
m
ex
pr
ess
ion
is
l
ik
e any oth
er
eq
ui
libr
ium
ex
pr
ess
ion: K
is
eq
ua
l
to t
he
con
centrations of
produ
cts
over
the
conc
entrat
io
ns of
reactan
ts,
eac
h
rai
s
ed
to i
ts
coeffic
ient from the
ba
l
an
ced
chemi
c
al
equ
ation
.
Th
us
, for the p
roc
e
ss
MX
n(
s)
, ° Mn+(aq) + nX
-(
a
q)
the
Ksp
e
xp
r
essi
on
is
Ksp
= [M
n+][
X-r
MX
n
does
not
appe
ar
in t
he
ex
pr
ession
be
cause,
as
for
any
h
eteroge
neous e
qu
ili
bri
um,
the
e
qui
l
ibr
ium
ex
pre
ss
i
on
d
oes
not i
ncl
u
de
pur
e
liq
u
ids
or
soli
ds
[
~~
Secti on 15.
3]
.
Be
c
ar
eful
no
t to
confuse
t
he
ter
ms so
lu
b
ility
a
nd
Ksp.
Solu
bility is
the
co
n
cent
rat
io
n of a
sat
ur
at
ed
so
lution.
Ksp
is an e
quil
ib
ri
um
con
st
ant.
702
Compound
Aluminum hydroxide
Barium
carbonate
Barium
fluoride
Barium
sulfate
Bismuth sulfide
Cadmium sulfide
Calcium carbonate
Calcium fluoride
Calcium hydroxide
Calcium phosphate
Chromium(III) hydroxide
Cobalt(lI) sulfide
Copper(I) bromide
Copper(I) iodide
Copper(lI) hydroxide
Copper(lI) sulfide
Iron(lI) hydroxide
Iron(III) hydroxide
Iron(1I) sulfide
Lead(lI) carbonate
Lead(II) chloride
Lead(II) chromate
Lead(lI) fluoride
Lead(lI) iodide
Lead(II) sulfide
Magnesium carbonate
Magnesium hydroxide
Manganese(II) sulfide
Mercury(I) chloride
Mercury(II) sulfide
Nickel(II) sulfide
Silver bromide
Silver carbonate
Silver chloride
Silver iodide
Silver sulfide
Strontium carbonate
Strontium sulfate
Tin(II) sulfide
Zinc hydroxide
Zinc sulfide
Dissolution Equilibrium
Al(OHMs).
• Al3+(aq) +
30H
- (aq)
BaC0
3
(s).
• Ba2+(aq) +
CO~(aq)
BaFis).
• Ba2+(aq) + 2F- (aq)
BaS04(s)
:;::.
::::::='
Ba2+ (aq) +
SO~-
(aq)
Bi
2
S
3
(s).
• 2Bi3+(aq) +
3S
2
- (aq)
CdS(s).
• Cd2+(aq) + S2-(aq)
CaC0
3
(s).
• Ca2+(aq) +
CO~
-
(aq)
CaF
2
(s),
•
Ca
2
+(aq)
+ 2F- (aq)
Ca(OH)z(s).
• Ca2+(aq) +
20H-(aq)
Ca
3
(P04)z(S).
• 3Ca2+(aq) +
2POl-(aq)
Cr(OHMs).
•
Cr
3
+(aq) +
30H
- (aq)
,
CoS(s)
+,
=::!:'
Co
2
+ (aq) + S2-(aq)
CuBr(s).
• Cu+(aq) +
Br-(aq)
CuI(s).
• Cu+(aq) + I- (aq)
Cu(OH)z(s),
• Cu
2
+(aq) +
20H
- (aq)
CuS(s),
• Cu
2
+(aq) + S2-(aq)
Fe(OHMs).
~
Fe
2
+(aq)
+
20H-(aq)
Fe(OH)3
(S)'
•
Fe
3
+(aq) +
30H
- (aq)
FeS(s).
• Fe2+(aq) +
S2
- (aq)
PbC0
3
(
s),
• Pb2+(aq) +
CO~
-
(aq)
PbC1
2
(s).
• Pb
2+
(aq) +
2Cl-(aq)
PbCrOis).
•
Pb
2
+(aq)
+
CrO~
-
(aq)
PbF
2
(
s).
•
Pb
2
+(aq)
+
2F-(aq)
PbI
2
(s).
•
Pb
2
+(aq)
+
2I-(aq)
PbS(s).
•
Pb2+
(aq) + S2-(aq)
MgC0
3
(s),
• Mg2+(aq) +
CO~-(aq)
Mg(OH)z(s),
• Mg2+(aq) +
20H
- (aq)
MnS(s),
• Mn2+(aq) + S2-(aq)
Hg
2
Clis).
•
Hg~
+
(aq)
+ 2Cl- (aq)
HgS(s).
• Hg2+(aq) + S2-(aq)
NiS(s).
•
Nr(aq)
+ S2-(aq)
AgBr(s) • • Ag + (aq) +
Br-(aq)
Ag
2
C0
3
(S).
• 2Ag+(aq) +
CO~-(aq)
AgCl(s).
•
Ag
+(aq) +
C]-(aq)
AgI(s),
• Ag
\aq)
+
I-(aq)
Ag
2
S(s),
• 2Ag +(aq) +
S2
- (aq)
SrC0
3
(s).
• Sr2+(aq) +
CO~
-
(aq)
SrS04(s).
• Sr2+(aq) +
SO~-(aq)
SnS(s).
•
Sn2+
(aq) + S2-(aq)
Zn(OH)z(s).
•
Zn2
+(aq) +
20H
- (aq)
ZnS(s).
• Zn2+(aq) + S2-(aq)
Ksp
l.8
X
10
-
33
8.1
X
10-
9
l.7
X
10
-
6
1.1
X
10-
10
l.6
X
10
-
72
8.0 X
10
-
28
8.7 X
10
-
9
4.0 X
10-
11
8.0 X
10-
6
l.2
X
10
-
26
3.0 X
10-
29
4.0 X
10
-
21
4.2 X
10
-
8
5.1 X
10
-
12
2.2 X
10-
20
6.0 X
10
-
37
l.6
X
10
-
14
1.1
X
10-
36
6.0 X
10
-
19
3.3 X
10-
14
2.4 X
10-
4
2.0 X
10
-
14
4.0 X
10-
8
1.4 X
10
-
8
3.4 X
10
-
28
4.0 X
10
-
5
l.2
X
10
-
JI
3.0 X
10
-
14
3.5 X
10
-
18
4.0 X
10
-
54
1.4 X
10
-
24
7.7 X
10-
13
8.1
X
10-
12
l.6
X
10-
10
8.3 X
10-
17
6.0 X
10-
51
l.6
X
10-
9
3.8 X
10-
7
l.0
X 10-
26
l.8 X
10-
14
3.0 X
10
-
23