- Trang chủ >>
- Khoa Học Tự Nhiên >>
- Vật lý
Chemistry part 29, Julia Burdge,2e (2009) pdf
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (16.83 MB, 26 trang )
Figure 17.8
708
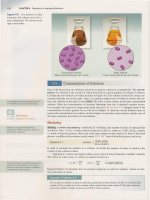
We
prepare a saturated solution
by
adding AgCl to water and stirring.
Neither
Agel
nor a saturated solution
of
AgCl is purple. The color has been used
in this illustration to clarify the process.
In the resulting saturated solution, the
concentrations
of
Ag
+ and
Cl
- are equal,
and the product
of
their concentrations
is equal
to
K sp
[Ag+
][Cl-]
= 1.6 X 10-
10
Therefore, the concentrations are
[Ag+]
=
l.3
X 10-
5
M
and [Cl- ] =
l.3
X 10-
5
M
2S.0C
/ After filtering
off
the solid AgCI, we
dissolve enough NaCI to make the
concentration
of
CI-
= 1.0
M.
Because the concentration
of
Cl
- is now larger, the product
of
Ag+ and
Cl-
concentrations is
no
longer equal to K sp
[Ag+][CI- ] = (1.3 X
10
-
5
M)(1.0
M)
> 1.6 X
10
-
10
In
any solution saturated with AgCl at
25
°
C,
the product
of
[Ag+] and
[Cl-]
must equal the Ksp of AgCl.
Therefore, AgCl will precipitate until the product
of
ion concentrations is again 1.6 X
10-
10
.
Note that this causes nearly all the
di
ssolved AgCl
-
to
precipitate. With a
Cl-
concentration
of
1.0 M, the
highest possible concentration
of
Ag+
is
1.6 X
10-
10
M.
i
[Ag+](1.0
M)
= 1.6 X
10
-
10
therefore, [Ag+] = 1.6 X
10
-
10
M
The amount
of
AgCI precipitated is
exaggerated for emphasis. The actual amount
of
AgCI would be extremely small.
What's the point?
When two salts contain the same ion, the ion they both contain is
called the
"common ion." The solubility
of
a slightly soluble salt
such
as
AgCl can be decreased by the addition
of
a soluble salt
with a common ion.
In
this example, AgCI is precipitated by
adding NaCl. AgCI could also be precipitated by adding a soluble
salt containing the Ag
+ ion, such
as
AgN0
3
.
7
09
710
CHAPTER
17 Acid-Base Equilibria
and
Solubility
Equilibria
Think
About
It When a salt
dissociates to give the conjugate
ba
se
of
a
weak
acid, H+ ions
in
an
acidic solution
consume a product
(base)
of
the dissolution. This
drives the equilibrium to the right
(more solid dissolves) according to
Le
Chatelier's principle.
Lewis
acid-base
reactions
in
which
a
metal
cation
combines
with a
Lewis
base
res
ult
in
the
format
io
n of
comp
l
ex
io
ns.
Sample Problem 17.11 demonstrates the effect
of
pH
on solubility.
. Sample Problem 17.11
Which
of
the following compounds will be
more
soluble in acidic solution than in water: (a) C
uS
,
(b)
AgC
l, (c)
PbS0
4
?
Strategy
For
each
salt, write the dissociation equilibrium equation and determine whether it
produces an anion that will react with
H+ Only an anion that is the conju
ga
te
ba
se
of
a
weak
acid
will react with H+. .
Setup
(a) CuS(s) , • Cu
2
+(aq) + S
2-(
aq)
S2
- is the conjugate
ba
se
of
the weak acid HS
S2- reacts with H+ as follows:
(
b)AgCl(s),
•
Ag
+(aq) + C
qaq)
Cl- is the conjugate base
of
the strong acid HCI.
Cl-
does n
ot
rea
ct
with H+.
(c)
PbS0
4
(s) , •
Pb
2+(
aq) +
SO
~
-
(
aq
)
SO
~-
is the conjugate
ba
se
of
the weak acid
HS0
4
.
It reacts with H+ as follows:
SO
~-(
aq
)
+ H+(aq)
-_.
HS0
4
(aq)
A salt that produces an anion that reacts with H+ will be
more
soluble in acid than
in
water.
Solution
CuS and
PbS0
4
are more soluble in acid than in wate
r.
(AgCl is no more
or
less soluble in
acid than in water.)
Practice
Problem
A Determine
if
the following compounds are more soluble in acidic solution than
in
pure
water: (a)
Ca
(
OH
)2, (b)
Mg
3
(P0
4
)2
, (c)
PbBr
2'
Practice
Problem
B Other than those in Sample Problem 17.11 and th
ose
in
Practice Problem A, list
three salts that are
more
soluble in acidic solution than
in
pure
water.
Complex Ion Formation
A complex ion is an ion containing a central metal cation bonded to one or more molecules or
• • • • • • • • • • • • • •
ions. Complex ions are crucial to many chemi
ca
l and biological processes. Here we will consider
the effect
of
complex ion formation on solubilit
y.
In Chapter 22 we will discuss the chemistry
of
complex ions in more detail.
Transition metals have a particular tendency to form complex ions. For example, a solution
of
cobalt(II) chloride (CoCI
2
)
is pink because
of
the presence
of
the
CO(H20)~
+
ions (Figure 17.9
).
When HCI is added, the solution turns blue because the complex ion
CoCl~
-
forms:
Copper(II) sulfate
(CUS04) dissolves in water to produce a blue solutio
n.
The
hydrated copper(II)
ions are responsible for this color; many other sulfates (e.g., Na2S04) are colorless. Adding a few
drops
of
concentrated ammonia solution to a CUS04 solution causes the formation
of
a light-blue
precipitate, copper(II) hydroxide:
Cu
?+
(aq) +
20H
- (aq) • Cu(OHl2(s)
The
OH-
ions are supplied by the ammonia solution.
If
more NH3 is added, the blue precipitate
redissolves to produce a beautiful dark-blue solution, this time due to the formation
of
the com-
plex ion
CU(NH
3
)~
+
(Figure 17.10):
Thus, the formation
of
the complex ion
Cu(NH3)~
+
increases the solubility
of
Cu(OHh.
A measure
of
the tendency
of
a metal ion to form a particular complex ion is given by the
formation constant (
Kr
) (also called the stability constant), which is the equilibrium constant for
the complex ion formation.
The
larger K
f
i
s,
the more stable the complex ion i
s.
Table 17.5 lists the
formation constants
of
a number
of
complex ion
s.
Complex
Ion
Ag(NH
3
);
Ag(CNh
Cu(CN)
~
Cu(NH
3)~
+
Cd(CN)~
CdI~-
HgCl
~
-
HgI~
Hg(CN)
~
Co(NH
3)~+
Zn(NH
3)~+
Equilibrium
Expression
Ag + + 2NH
3
• •
Ag(NH
3
);
Ag + +
2CN
• Ag(CN)?
Cu
2
+ +
4CN
-
+.=~'
Cu(CN)
~-
Cu2+
+ 4NH3 • •
Cu(NH
3
)~
+
Cd
2
+ + 4CN- . ·
Cd(CN)~
-
Cd
2
+ +
41
•
Cdl~-
Hg2
+ + 4Cl- . .
HgCl
~
-
Hg
2+
+
41
.
HgI
~
-
Hg
2+
+ 4CN- . •
Hg(CN)~
-
Co
3+
+
6NH
3
+.=~'
Co(NH3)~
+
Zn
2+
+
4NH
3 • •
Zn(NH
3)~
+
,
\
Formation
Constant
(K
f
)
1.5 x
10
7
l.0
X 10
21
l.0
X
10
25
5.0 X
1013
7.1 X 10
16
2.0 X 10
6
l.7
X
10
16
2.0 X 10
30
2.5 X 10
41
5.0 X 10
31
2.9 X 10
9
Figure 17.9
(Left)Anaque
ous
cobalt(II) chloride solution. The
pink color is due
to
the presence of
CO(H
2
0)
~
+
ions. (Right) After the
addition
of
HCI solution, the solution
turns blue because
of
the fonnation
of
the complex
CoCl~
-
ions.
Fig
u re 17.
10
(Left) An aqueous
solution
of
copper(II) sulfate. (Center)
After the addition
of
a few drops
of
concentrated aqueous ammonia
solution, a light-blue precipitate
of
CU(OH)2
is fonned. (Right)
When more concentrated aqueous
ammonia solution is added, the
Cu(OH)z is fonned. (Right) When
more concentrated aqueous ammonia
solution is added, the
Cu(OHh
precipitate dissolves to form the dar
k-
blue complex ion
CU
(
NH
3
)~+
'
•
7i '
712 CHAPTER 17 Acid-Base
Equilibria
and
Solubility
Equilibria
•
Formati
on
of a
complex
ion
consumes
the
metal
ion
produced
by
the
di
ssociation
of a
salt
,
increasing
the
salt's
solubility si
mpl
y d
ue
to
Le
Chiitel
ier
's
principle
[~
Section
15
.
5].
• - !
Multimedia
Solutions Precipitation- fractional
crystallization.
The
fOllnation
of
the
CU(NH
3)~
+
ion can be expressed as
The
corresponding fOlrnation constant is
The
large value
of
K
f
in this case indicates that the complex ion
is
very stable in solution and
accounts for the very low concentration
of
copper(II) ions at equilibrium .
Recall that
K for the s
um
of
two reactions is the product
of
the individual K values
[
~~
Section
15 .3] .
The
dissolution
of
silver chloride is represented by the equation
The
s
um
of
this equation and the one representing the fOlmation
of
Ag(NH
3
)! is
AgCl(s).
.
~)
+ Cl- (aq) K
sp
= 1.6 X 10-
10
+ aq) +
2NH
3(
aq).
•
Ag(NH
3
)!(
aq) K
f
= 1.5 X 10
7
AgCl(s) +
2NH
3
(
aq).
•
Ag(NH
3
)!
(aq) +
Cl
- (aq)
and the corresponding equilibrium constant is (1.6 X
10-
1
°)(1.5 X 10
7
)
= 2.4 X 10-
3
. This is
significantly larger than the
K
sp
value, indicating that much more AgCl will dissolve in the pres-
.
erlce
'
oLiqiieo
ui
ammo
ni
a '
thin
'
iii
pure
' water. Although the actual calculation
of
solubility in these
cases is somewhat complicated, the effect
of
complex ion formation generally
is
to increase the
solubility
of
a substance.
Finally, there is a class
of
hydroxide
s,
called amphoteric hydroxides, which can react with
both acids and
ba
ses. Examples are Al(OH)
3,
Pb(OHh,
Cr(OH)3, Zn(OH)1, and Cd(OH)2' Al(OH)3
reacts with acids and bases as follows:
Al
(
OH
Ms)
+ 3H+(aq) -
-+.
Al
3+
(aq) +
3H
2
0(l)
Al(OHMs)
+
OH-(aq
) . • Al(OH)4 (aq)
The increase in solubility
of
Al(OH)3 in a basic medium is the result
of
the formation
of
the com-
plex ion
Al(OH
)4
in which Al(OH)3 acts as the Lewis acid and OH- acts as the Lewis base. Other
amphoteric hydroxides react similarly with acids and bases.
Checkpoint 17.5
Factors Affecting Soubility
17
.5
.1
Calculate the molar solubility
of
AgCl
in 0.10
MCaCl
2
.
a) 1.6 X
10
-
10
M
b) 1.6 X
10-
9
M
c) 8.0 X 10-
10
M
d) 1.3 X
10-
5
M
e) 2.6 X
10-
8
M
17
.5.2 Which
of
the following substances will
be
more soluble
in
acidic solution than
in pure water? (Select all that apply.)
a)
PbC0
3
b)
AgS
c)
AgJ
d)
Fe
(OH)3
e)
CaF2
Separation
of
Ions Using Differences in Solubility
In
chemical analysi
s,
it
sometimes is necessary to remove one type
of
ion from solution by pre-
cipitation while leaving other ions in solution. For instance, the addition
of
sulfate ions to a solu-
tion containing both potassium and barium ions causes
BaS04
to precipitate out, thereby removing
most
of
the
Ba
2+
ions from the solution.
The
other "product," K
2
S0
4
,
is soluble and will remain in
solution.
The
BaS0
4 precipitate can be separated from the solution by filtration .
Fractional Precipitation
Even when both products are insoluble, we can still achieve some degree
of
separation by choosing
the proper reagent to bring about precipitation
.• Consider a solution that contains Cl
-,
Br
- , and 1-
SECTION 17.6 Separation
of
Ions Using Differences in Solubili
ty
713
ions. One way to separate these ions is to convert them to insoluble silver halides. As the K sp val-
ues in the margin show, the solubility
of
the silver halides decreases from AgCl to AgI. Thus, when
a soluble compound such as silver nitrate is slowly added to this solution, AgI begins to precipitate
first, followed by AgBr, and then AgCl. This practice
is
known as fractional precipitation.
Sample Problem
l7.12
describes the separation
of
only two ions (Cl- and
Br-),
but the pro-
cedure can be applied to a solution containing more than two different types
of
ions.
".
~
Sample Problem 17.12 '
,
Silver nitrate is
added
slowly to a solution that is 0.020 M in
Cl
- ions and
0.020
Min
Br
- ions.
Calculate the concentration
of
Ag
+ ions (in mol/L) required to initiate the precipitation
of
AgBr
without precipitating AgCL
Strategy
Silver nitrate dissociates in solution to give
Ag
+ and
NO
:; ions.
Adding
Ag
+ ions in
sufficient
amount
will cause the slightly soluble ionic
compounds
AgCl
and
AgBr
to precipitate
from
solution. Knowing the K
sp
values for AgCI and
AgBr
(and the concentrations
of
CI- and
Br
- already
in solution),
we
can
use
the equilibrium expressions to calculate
the
maximum
concentration
of
Ag
+
that
can
exist
in solution without exceeding K
sp
for
each
compound.
Setup
The
solubility equilibria, K sp values,
and
equilibrium expressions for AgCI and
AgBr
are
AgCI(s)
:;::.
=
::t"
Ag
+(aq) +
Cqaq
)
AgBr(s) • "
Ag
+(aq) +
Br-(aq)
Ksp = 1.6 X 10-
10
= [Ag+][Cl- ]
Ksp = 7.7 X 10-
13
= [Ag+
][Br
- ]
Because
the K sp for
AgBr
is smaller (
by
a factor
of
more
than 200),
AgBr
s
hould
prec
ipitate first; that
is,
it
will require a
lower
concentration
of
added
Ag
+ to begin precipitation. Therefore,
we
first solve
for
[Ag
+]
using the equilibrium expression for
AgBr
to determine the
minimum
Ag
+ concentration
necessary
to
initiate precipitation
of
AgBr. We then solve for [Ag +] again, using the equilibrium
expression for
AgCl
to determine the maximum
Ag
+ concentration that
can
exist in the solution
without
initiating the precipitation
of
AgC
l.
Solution Solving the
AgBr
equilibrium expres
sion
for
Ag
+ concentration,
we
have
+ K sp
[Ag ] = [Br ]
and
[
A
+] = 7.7 X 10-
13
=
39
X
10-
11
M
g
0.020 .
For
AgBr
to precipitate from solution, the silver ion concentration
mu
st
exceed
3.9 X
10-
11
M.
Solving
the
AgCl
equilibrium expression for the
Ag
+ concentration, we have
and
A + _ K
sp
[ g ] - [CI ]
[A +]
= 1.6 X 10-
10
= 8.0 X 10-
9
M
g
0.
020
For
AgCI not to precipitate from solution, the silver ion concentration
mu
st stay below 8.0 X 10-
9
M.
Therefore, in order to precipitate the
Br
- ions without precipitating the
Cl
-
from
this solution,
the
Ag
+ concentration
must
be
greater than 3.9 X 10-
11
M and less than 8.0 X 10-
9
M.
Practice Problem A Lead(II) nitrate is added slowly to a solution that is 0.020 M in CI- ions.
Calculate the concentration
of
Pb
z
+
ions (in
mol/L
) required to initiate the precipitation
of
PbCl
z
.
(K
sp for
PbCl
z
is 2.4 X 10-
4
.)
Practice Problem B Calculate the concentration
of
Ag
+ (in mol/L) necessary to initiate the
precipitation
of
(a) AgCI and (b)
Ag
3
P0
4
from a solution
in
which
[Cn
and
[PO
~
-
]
are
each
0.10
M.
(K
sp for
Ag
3
P0
4
is 1.8 X 10-
18
.)
Compound
K,p
Agel
1.6 x 10-
10
AgBr
7.7 x 10-
13
Agi
8.3 X 10-
17
Think
About
It
If
we
continue
adding
AgN0
3
until the
Ag
+
concentration is high
eno
u
gh
to
begin
the
precipitation
of
AgCI,
the concentration
of
Br
- remaining
in solution
can
also
be
determined
using
the
K sp expression:
7.7
X 10-
13
8.0 X 10-
9
= 9.6 X 10-
5
M
Thu
s,
by
the time AgCI begins
to precipitate, (9.6 X 10-
5
M)
.;-
(0.020 M) = 0.0048,
so
less than
0.5
percent
of
the original
bromide
ion
remains
in
the solution.
714
CHAPTER
17
Acid-Base
Equilibria
and
Solubility
Equilibria
Note
that
these
group
numbers
do
not
correspond
to
groups
in
the periodic
table.
.
•
Multimedia
•
Chemical
Analysis
- flame
tests
of
metals.
Fig
u
re
17.11 Flame tests
for
sodium
(ye
llow
flam
e)
and
pota
ss
ium
(violet
flame
).
Qualitative Analysis
of
Metal Ions
in
Solution
The
principle
of
selective precipitation can
be
used to identify the types
of
ions present in a solu-
tion.
This practice is called qualitative analysis. There are about 20 common cations that can be
analyzed readily in aqueous solution. These cations can
be
divided into five groups according to
the solubility products
of
their insoluble salts (Table 17.4). Because an unknown solution may
cO
·
rita-iii
from."
r to ·
iil1
·
20
'lons; '
any
an."<i1)
;sls ·iriiis
die
·carried 'oiit systemiltlciiily
fioill
'group 1 through
group 5.
The
general procedure for separating the
se
20
ions is as follows:
• Group 1 cations.
When
dilute
HCl
is added to the unknown solution, only the Ag
+,
Hg
~+,
and
Pb
2
+ ions precipitate as insoluble chlorides.
The
other ions, whose chlorides are soluble,
remain in solution.
• Group 2 cations. After the chloride precipitates have been removed by filtration, hydrogen
sulfide is added to the unknown solution, which is acidic due to the addition
of
HCI. Metal
ions from group 2 react to produce metal sulfides:
In the pre
se
nce
of
H+, this equilibrium shifts to the left. Therefore, only the metal sulfides
with the
smallest K sp values precipitate under acidic conditions. These are
Bi
z
S
3
, CdS, CuS,
and SnS (see Table 17.4).
The
solution is then filtered to remove the insoluble sulfides.
• Group 3 cations.
At
this stage, sodium hydroxide is added to the solution to
make
it basic. In
a basic solution, the metal sulfide equilibrium shifts to the right and the more soluble sulfides
(CoS , FeS, MnS, NiS, ZnS) now precipitate out
of
solution.
The
AI
3+
and
Cr
3+
ions actually
precipitate as the hydroxides Al(OH)3 and Cr(OH)
3,
rather than as the sulfides, because the
hydroxides are less soluble.
The
solution is filtered again to remove the insoluble sulfides
and hydroxides.
• Group 4 cations. After all the group 1, 2, and 3 cations have been removed from solu-
tion, sodium carbonate is added to the basic solution to precipitate
Ba
2
+,
Ca
2
+, and
Sr
2
+
ions as
BaC0
3
,
CaC0
3
,
and
SrC0
3
.
The
se precipitate
s,
too, are removed from solution
by
filtration.
• Group 5 cations. At this stage, the only cations possibly remaining in solution are
Na
+,
K+,
and
NH
t.
The
presence
of
NH
t ions can be determined by adding sodium hydroxide:
The
ammonia gas is detected either by its characteristic odor
or
by observing a wet piece
of
red lit-
mus paper turning blue when placed above (not in contact with) the solution. To confirm the pres-
ence
of
Na
+ and K+ ions, a flame test is usually used in which a piece
of
platinum wire (chosen
because platinum is inert) is dipped into the original solution and then held over a
Bun
sen burner
flame.
Na
+ ions emit a yellow flame when heated in this manner, whereas K+ ions
emit
a violet
flame (Figure
17
.11). Figure 17.12 summarizes this scheme for separating metal ions.
SECTION
17
.6 Separation
of
Ions Using
Di
ffe
rences in
So
l
ub
ili
ty
7
:>
Solution containing ions
of
all cation groups
+ HCl
Group 1 precipitates
Filtration
f
AgCl, Hg1
C1
1
, PbCl
1
Solution containing ions
of
all remaining groups
+
H1S
Group 2 precipitates
Solution containing ions
of
all remaining groups
T
Na
l
Group 3
pr
ecipitates
f
Filtration
CoS, FeS, MnS, NiS,
ZnS, Al(OH)
3'
Cr(
OH
)3
Solution containing ions
of
all remaining groups
IT .'!, 1-
Group 4 precipitates
f Filtration
BaC0
3
,
CaC0
3
,
SrS0
3
Solution contains
Na+ K+
NH
+ ions
, , 4
Checkpoint
17.6
Separation
of
Ions Using Differences
in
Solubility
17.6.1
A solution is
0.10 M in
Br
- ,
CO
~
-
,
CC
C and
SO
~
-
ions. Which
compound will precipitate first as silver
nitrate is added to the solution?
a)
AgBr
b)
Ag
1
C0
3
c)
AgCl
d)
AgI
e)
Ag
2
S0
4
17.6.2
Barium nitrate is added slowly to a
solution that is
0.
10
M in
SO
~-
ions
and
0.10 M in
F-
ions. Calculate the
concentration
of
Ba
2+
ions (in mol/L)
required to initiate the precipitation
of
BaS0
4 without precipitating BaF
1
.
a)
1.7
X 10-
6
M
b)
1.1
X 10-
9
M
c)
1.7
X
10
-
4
M
d)
1.7
X
10
-
5
M
e)
1.1
X
10
-
8
M
•
Figure 17.12 A flowchart for the
separation
of
cations in qualitative
analysis.
716
CHAPTER
17 Acid-Base E
quilibria
and
Solubility
Equilibria
•
Applying
What
You've Learned
Most toothpastes contain fluoride, which helps to reduce tooth decay.
The
F-
ions in
toothpaste replace
some
of
the
OH
- ions during the remineralization process:
SCa
2
+(aq) +
3PO
~
-
(aq)
+
F-(aq)
+-
• CaS(P04)3F(S)
Because
F-
is a weaker base than
OH
- , the modified enamel, called fluoroapatite, is
more resistant to the acid produced
by
bacteria .
Problems:
(a) Calculate the molar solubility
of
hydroxyapatite given that its K sp is 2 X
10
-
59
.
[
~~
Sample
Problem
17.
7]
(b) Calculate the K sp
of
fluoroapatite given that its molar solubility is 7 X
10-
8
M.
[
~~
Sample
Problem
17.7]
(c) Calculate the molar solubility
of
fluoroapatite in an aqueous solution in which the
concentration
of
fluoride ion is 0.10
M.
[
~~
Sample
Problem
17.
7]
Cd)
Calculate the
molar
solubility
of
hydroxyapatite in a buffered aqueous solution
with
pH
= 4.0.
[
~~
Sample
Problem
17.7]
-
CHAPTER SUMMARY
=~~
Section 17.1
o
The
presence
of
a
common
ion s
uppre
sses the ioni
zat
ion
of
a
weak
acid
or
weak
base.
This
is
known
as
the
common
ion effect.
o A
common
ion
is
added
to a solution in
the
form
of
a sa
lt.
Section 17.2
o A
solution
that
contains
significant
concentrations
of
both
memb
ers
of
a
conjugate
acid-base
pair
(weak
acid
-conjugate
base
or
weak
base
-
conjugate
acid)
is a buffer solution
or
simply
a buffer.
o
Buffer
solutions
resist
pH
change
upon
addition
of
sma
ll
amounts
of
strong
acid
or
s
trong
ba
se
.
Buffers
are
imp
o
rtant
to
biological
systems
.
o
The
pH
of
a buffer
can
be
calculated
us
ing
an
equilibrium
table
or
with
the
Henderson-Hasselbalch equation.
o
The
pKa
of
a
weak
acid is - l
og
Ka.
When
the
weak
ac
id
and
co
njugate
base
concentrations
in a
buffer
solution
are equal,
pH
= pK
a
.
o We
can
prepare
a buffer with a specific
pH
by
choosing
a
weak
acid
with
a
pK
a
clo
se to the
de
sired pH.
Section 17.3
o
The
titration
curve
of
a
strong
acid -
strong
base
titration
ha
s a
long
,
steep
region near
the
equivalence
point.
o
Titration
curves
for
weak
acid-strong
ba
se
or
weak
base-strong
acid
titrations
have
a
sig
nificantly s
horter
steep
region.
o
The
pH
at
the
equivalence
point
of
a
strong
acid-strong
ba
se
titration
is
7.00.
o
The
pH
at
the
equivalence
point
of
a
weak
acid-strong
ba
se
titration
is
above
7.00.
o
The
pH
at
the
equivalence
point
of
a
weak
base
- stro
ng
acid titration
is
below
7.00.
o
Acid-base
indicators
are
usually
weak
organic
acids that
exhibit
two
different
colors
depending
on
the
pH
of
the
so
lution.
The
end
point
of
KEyWORDS
Buffer, 683
Common
ion effect, 683
Complex
ion,
710
KEY EQUATIONS
End
point, 698
Formation
constant
(K
r
),
710
Fractional
pr
ec
ipitation, 713
KEY EQUATIONS 717
a titration is
the
point
at
which
the
color
of
the
indicator
changes.
It
is
used
to
estimate
the
equivalence point
of
a titration.
o
The
indicator
used
for
a
particular
titration
should
exhibit
a
color
change
in
the
pH
range
corresponding
to
the
steep
region
of
the
titration
curve.
Section 17.4
o
The
solubility
product
constant (
Ksp
) is
the
equilibrium
constant
that
indic
ates to
what
extent
a s
lightly
soluble
ionic
compound
dis
soc
iates
m water.
o Ksp
can
be
u
sed
to
determine
molar
solubility
or
solubility
in
gIL,
and
•
VIce
versa.
o Ksp
ca
n also
be
used
to
predict
whether
or
not
a
precipitate
will
form
when
two
so
lution
s are
mixed.
Section 17.5
o
Solubility
is affected by
common
ion
s,
pH,
and
complex
ion
formation.
Theformation
constant (Kr)
indicates
to
what
extent
complex
ions
form.
o A
sa
lt that di
ssoc
iates to give a
strong
conjugate
ba
se
s
uch
as fluoride
ion
will
be
more
so
luble
in
ac
idic
solution
than
in
pure
water.
o A
salt
that
dis
sociates
to
give
hydroxide
ion
will
be
more
soluble
at
lower
pH
and
le
ss so
luble
at
higher
pH.
o
The
solubility
of
an ionic
compound
increases
when
the
formation
of
a complex ion
consumes
one
of
the
products
of
dissociation.
Section 17.6
o
Ion
s
can
be
separated
using
fractional precipitation.
o
Fracti
ona
l
precipit
ation
sc
heme
s
can
be
designed
ba
sed
on
K,p values.
o
Group
s
of
cations
can
be
identified
through
the
u
se
of
selective
precipitation.
This
is
the
ba
sis
of
qualitative analysis.
Henderson-Hasselbalch
equation,
685
Molar
so
lubilit
y,
701
Qualitative
analysis, 714
Solubility, 701
Solubility
product
constant
(K
s
p)
,
70
1
17. I
[A
- ]
P
H
= pK + 10" = , :-
a b [HA]
17.2 pKa
=
-log
Ka
17.3
[conjugate
ba
se
]
pH
=
pK
a +
log
[weak
acid]
718 CHAPTER 17 Acid-Base Equilibria and Solubility Equilibria
QUESTIONS AND PROBLEMS
Section 17.1: The Common Ion Effect
Review Questions
17.1
Use
Le
Chatelier's principle to explain how the common ion
effect affects the
pH
of
a weak acid solution.
17.2 Describe the effect
on
pH
Gincrease, decrease,
or
no change)
that results from each
of
the following additions: (a) potassium
acetate to
an
acetic acid solution, (b) ammonium nitrate to an
ammonia
solution, (c) sodium formate (
HCOONa)
to a
fonnic
acid (HCOOH) solution, (d) potassium chloride to a hydrochloric
acid solution, (e) barium iodide to a hydroiodic acid solution.
17.3 Define
pKa
for a
weak
acid.
What
is the relationship between the
value
of
the
pK
a and the strength
of
the acid?
17.4
The
pK;s
of
two monoprotic acids
HA
and
HE
are 5.9 and 8.1,
respectively. Which
of
the two is the stronger acid?
Problems
17.5 Determine the
pH
of
(a) a 0.40 M
CH
3
COOH
solution and (b) a
solution that is
0.40 M
CH
3
COOH
and 0.20 M
CH
3
COONa.
17.6 Determine the
pH
of
(a) a 0.20 M
NH
3 solution, and (b) a
solution that is
0.20 M
NH
3 and 0.30 M
NH
4
Cl.
Section 17.2: Buffer Solutions
Review Questions
17.7
What
is a buffer solution?
What
must
a solution contain
in
order
to
be
a buffer?
17.8
Using only a
pH
meter, water, and a graduated cylinder, how
would you distinguish between an acid solution and a buffer
solution at the
same
pH?
Problems
17.9
Which
of
the following solutions can act as a buffer:
(a)
KCVHCI, (b)
KHS0
4
1H
2
S0
4
,
(c)
Na
2
HP0
4INaHz
P0
4,
(d)
KN0
2
IHN0
2
?
17.10 Which
of
the following solutions can act as a buffer:
(a)
KCN
/HCN, (b)
Na
2
S041NaHS0
4'
(c)
NH
i
NH
4
N0
3
,
(d) NaIIHI?
17.11 Calculate the
pH
of
the buffer system made up
of
0.15 M
NH
3/0.35 M
NH
4
Cl.
17.12 Calculate the
pH
of
the following two buffer solutions: (a) 2.0 M
CH
3COONa/2.0 M
CH
3
COOH,
(b) 0.20 M
CH
3
COONa/0.20
M
CH
3
COOH.
Which is the
more
effective buffer?
Why?
17.13
The
pH
of
a bicarbonate-carbonic acid buffer is 8.00. Calculate
the ratio
of
the concentration
of
carbonic acid (H
2
C0
3
)
to that
of
the bicarbonate
ion
(HC0
3"
).
17.14
What
is
the
pH
of
the buffer 0.10 M
Na
2
HPO
J
O.15
M
KH
Z
P0
4
?
17.15
The
pH
of
a sodium acetate-acetic acid buffer is 4.50. Calculate
the ratio
[CH
3
COO
- ]I[CH
3
COOH].
17.16
The
pH
of
blood
plasma
is
7.40. Assuming the principal buffer
system is
HC0
3"
1H
2
C0
3
,
calculate the ratio
[HC0
3"
]/[H
2
C0
3
].
Is
this buffer
more
effective against an added acid
or
an added base?
17.17 Calculate the
pH
of
the 0.20 M
NH
i
O.20
M NH4Cl buffer.
What
is the
pH
of
the buffer after the addition
of
10.0
mL
of
0.10 M
HCI to 65.0 mL
of
the buffer?
17.18 Calculate the pH
of
1.00 L
of
the buffer 1.00 M
CH
3
COONa/l.00
M
CH
3
COOH
before and after the addition
of
(a) 0.080 mol NaOH and
(b) 0.12 mol HC!. (Assume that there
is
no change in volume.)
17.19 A diprotic acid, H
2
A, has the following ionization constants:
Ka = 1.1 X 10-
3
and Ka = 2.5 X 10-
6
.
To make up a buffer
1 2
solution
of
pH
5.80, which combination would you choose:
NaHA!H
2
A or
Na
2A1NaHA?
17.20 A student is asked to prepare a buffer solution at
pH
8.60, using
one
of
the following
weak
acids:
HA
(Ka = 2.7 X 10-
3
),
HB
(Ka = 4.4 X 10-
6
),
HC
(Ka = 2.6 X
10-
9
).
Which acid should
the student choos
e?
Why?
17.21
The
following di
agram
s contain
one
or
more
of
the compounds:
17.22
HzA, NaHA, and NazA, where HzA is a
weak
diprotic acid.
(1
) Which
of
the solutions can act as buffer solutions? (2) \Vhich
solution is the most effective buffer solution? Water molecules
and
Na
+ ions have been omitted for clarity.
=
HA
-
(a)
(b) (c) (d)
The
following diagrams repres
ent
solutions containing a weak
acid
HA
(
pKa
= 5.0) and its sodium salt NaA.
(I)
. Which solution
has the lowest pH?
Which
ha
s the highest pH? (2)
How
many
different species are pres
ent
after the addition
of
two H+ ions to
solution (a)? (3)
How
many different species are present after the
addition
of
two
OH
- ions to solution (b)?
=HA
-
= A -
•
(a) (b) (c) (d)
Section 17.3: Acid-Base Titrations
Review Questions
17.23 Briefly describe what happens in an acid-base titration.
17.24 Sketch titration curves for the following acid-base titrations:
(a) HCI versus
NaOH, (b) HCI versus
CH
3
NH
2
, (c)
CH
3
COOH
versus
NaOH
. In each case, the
ba
se is added to the acid in an
Erlenmeyer flask. Your graphs should show the
pH
on the y axis
and the volume
of
ba
se added on the x axis.
17.25 Explain how an acid-base indicator works in a titration. What are the
criteria for choosing an indicator for a particular acid-base titration?
17.26 The amount
of
indicator used in an acid-base titration
must
be
•
smal
l.
Why?
Problems
17.27 A 0.2688-g sample
of
a monoprotic acid neutralizes 16.4
mL
of
0.08133 M KOH solution. Calculate the molar mass
of
the acid.
17.28 A
S.OO-g
quantity
of
a diprotic acid was dissolved in water and
made
up to exactly
2S0
mL. Calculate the molar mass
of
the acid
if
2S.0
mL
of
this solution required 11.1
mL
of
1.00 M KOH for
neutralization. Assume that both protons
of
the acid were titrated.
17.29 In a titration experiment,
12.S
mL
of
O.SOO
M H
2
S0
4
neutralizes
SO.O
mL
of
NaOH.
What
is the concentration
of
the NaOH
solution?
17.30
17.31
In a titration experiment, 20.4
mL
of
0.883 M
HCOOH
neutralizes 19.3
mL
of
Ba(OH)z.
What
is the concentration
of
the
Ba(OHh solution?
A 0.1276-g sample
of
an unknown.monoprotic acid was
dissolved in
2S.0
mL
of
water and titrated with a 0.0633 M
NaOH
solution. The volume
of
ba
se
required to bring the
solution to the equivalence point was 18.4 mL. (a) Calculate the
molar
ma
ss
of
the acid. (b) After 10.0 mL
of
base had been added
during the titration, the pH was determined to be
S.87.
What
is
the
Ka
of
the unknown acid?
17.32 A solution is made by mixing exactly
SOO
mL
of
0.167 M
NaOH
with exactly
SOO
mL
of
0.100 M
CH
3
COOH.
Calculate the
equilibrium concentrations
ofH
+,
CH
3
COOH, CH
3
COO- ,
OH-,
and
Na
+.
17.33 Calculate the
pH
at the equivalence point for the following
titration:
0.20 M HCI versus 0.20 M methylamine (CH
3
NH
2
).
17.34 Calculate the
pH
at the equivalence point for the following
titration:
0.10 M
HCOOH
versus 0.10 M NaOH.
17.35 A 2S.0-mL solution
of
0.100 M
CH
3
COOH
is titrated with a
0.200 M KOH solution. Calculate the pH after the following
additions
of
the KOH solution: (a) 0.0 mL, (b)
S.O
mL,
(c)
10.0 mL, (d)
12.S
mL, (e) IS.0 mL.
17.36 A
10.0-mL solution
of
0.300 M
NH3
is titrated with a 0.100 M
HCI solution. Calculate the
pH
after the following additions
of
the HCl solution: (a) 0.0 mL, (b) 10.0 mL, (c) 20.0 mL,
(d) 30.0
mL
, (e) 40.0 mL.
17.37 Referring to Table 17.3, specify which indicator or indicators you
would use for the following titrations: (a)
HCOOH
versus NaOH,
(b) HCl versus KOH, (c)
HN0
3
versus CH
3
NH
2
.
17.38 A student carried out an acid-base titration by adding NaOH
solution from a buret to an Erlenmeyer
fla
sk
containing an
HCl solution and using phenolphthalein as the indicator. At
the equivalence point, she observed a faint reddish-pink
COIOf.
However, after a few minutes, the solution gradually turned
colorless. What do you suppose happened?
17.39 The ionization constant Ka
of
an indicator HIn is 1.0 X 10-
6
The
color
of
the nonionized form is red and that
of
the ionized form is
yellow. What is the color
of
this indicator in a solution whose
pH
is 4.00?
17.40
The
Ka
of
a certain indicator is 2.0 X 10-
6
The
color
of
HIn
is green and that
of
In- is red. A few drops
of
the indicator are
added to
an
HCI solution, which is then titrated against an NaOH
solution. At what pH will the indicator change color?
17.41
The
following diagrams repre
se
nt
solutions at various stages in
the titration
of
a weak base B (such as NH
3
) with HC!. Identify
/
/
QUESTIONS
AND
PROBLEMS 719
the solution that corresponds to (1) the initial stage before the
addition
of
HCl, (2) halfway to the equivalence point, (3) the
equivalence point, (4) beyond the equivalence point. Is the
pH
greater than, less than, or equal to 7 at the equivalence point?
Water and Cl- ions have been omitted for clarity.
=B
17.42
"'"
(a) (b)
(c)
•
•
•
(d)
The following diagrams represent solutions at various stages in
the titration
of
a weak acid
HA
with NaOH. Identify the solution
that corresponds
to
(1) the initial stage before the addition
of
NaOH, (2) halfway to the equivalence point, (3) the equivalence
point, (4) beyond the equivalence point. Is the pH greater than,
less than, or equal to 7 at the equivalence point? Water and Na +
ions have been omitted for clarity.
=HA
=
OH
-
(a) (b) (c) (d)
Section 17.4: Solubility Equilibria
Review
Questions
17.43 Use
BaS04
to distinguish between the terms solubility and
solubility product.
17.44 Why do we usually not quote the K sp values for soluble ionic
compounds?
1
7.4S
Write balanced
eq
uations and solubilIty product expressions for
the solubility equilibria
of
the following compounds: (a) CuBr,
(b) ZnC
2
0
4
, (c) Ag2Cr04, (d) Hg
2
C1
2
, (e) AuCI
3
, (f)
Mn
3
(P04h.
17.46 Write the solubility product expression for the ionic compound AxBy.
17.47 How can we predict whether a precipitate will form when two
solutions are mixed?
17.48
Silver chloride has a larger K sp than silver carbonate (see Table
17.4). Does this mean that AgCI also has a larger molar solubility
than Ag
2
C0
3
?
Problems
17.49 Calculate the concentration
of
ions in the following
sa
turated
solutions: (a)
W]
inAgI
sol
~
tion
with [Ag+] = 9.1 X
10-
9
M,
(b) [A]
3+
] in Al(OH)3 solution with
[OH-]
= 2.9 X 10-
9
M.
17
.SO
From
the solubility data given, calculate the solubility products
for the following compounds: (a)
SrF
2
,
7.3 X 10-
2
gIL,
(b) Ag
3
P0
4
,
6.7 X 10-
3
gIL.
17.51 The molar solubility
of
MnC0
3
is 4.2 X
10-
6
M.
What
is K
sp
for
this compound?
17.S2
The solubility
of
an
ionic compound
MX
(molar mass = 346 g)
is
4.63 X 10-
3
gIL.
What
is K sp for this compound?
720
CHAPTER
17
Acid-Base Equilibria and Solubility Equilibria
17.53
The
solubility
of
an ionic compound M
2
X
3
(molar
ma
ss = 288 g)
is 3.6
X 10-
17
gIL.
What
is Ksp for this compound?
17.54
Using data from Table 17.4, calculate the molar solubility
ofCaF
l
.
17.55
What
is the pH
of
a saturated zinc hydroxide solution?
17.56
The
pH
of
a saturated solution
of
a metal hydroxide
MOH
is
9.68. Calculate the
Ksp for this compound.
17.57
If
20.0
mL
of
0.10
MBa(N0
3
)l
is added to 50.0
mL
ofO
.IOM
Na2
C0
3, will
BaC0
3
precl.pitate?
•
17.58 A
vo
lume
of75
mL
of
0.060
MNaF
is
mixed with 25
mL
of
0.15 M
Sr(N0
3
)2
' Calculate the concentrations in the final solution
of
NO
) ,
Na
+,
S?
+, and F- . (Ksp for SrF2 = 2.0 X 10-
10
.)
Section 17.5: Factors Affecting Solubility
Review Questions
17.59 How does the corrunon ion effect influence solubility equilibria?
Use
Le
Chiltelier's principle to explain the decrease in solubility
of
CaC0
3
in an
Na
l
C0
3
solution.
17.60
The
molar solubility
of
AgC
l
in
6.5 X 10-
3
M
AgN0
3
is 2.5 X
10
-
8
M.
In deriving K
sp
from these data, which
of
the following
assumptions are reasonable? (a)
Ksp is the same as solubilit
y.
(b) Ksp
of
~gt
l
is }he same in 6.5 X 10-
3
M
AgN0
3
as in pure
water. (c) Solubility
of
AgCl is independent
of
the concentration
of
AgN0
3
.
(d) [Ag +] in solution does not chan
ge
significantly
upon the addition
of
AgCl to 6
.5
X 10-
3
M
AgN0
3
.
(e) [Ag +] in
solution after the addition
of
AgCl to 6.5 X 10-
3
M
AgN0
3
is the
same as it would be in pure water.
17.61 Give an example to illustrate the general effect
of
complex ion
formation on solubilit
y.
Problems
17.62 How many grams
of
CaC0
3
will dissolve in 3.0 X 10
2
mL
of
0.050 M
Ca(N0
3
h?
17.63
The
solubility product
ofPbBr
2 is 8
.9
X
10-
6
.
Determine the
,
molar solubility in (a) pure water, (b) 0.20 M
KEr
solution, and
(c)
0.20 M
Pb(N0
3
)2
solution.
17.64 Calculate the molar solubility
of
AgCl in a 1.00-L solution
containing 10.0 g
of
dissolved CaCl
l
.
17.65 Calculate the molar solubility
of
BaS04
in (a) water and (b) a
solution containing
1.0 M
SO~
-
ion
s.
17.66 Which
of
the following ionic compounds will be more soluble in
acid solution than in water: (a)
BaS04, (b) PbCl
l
, (c)
Fe(OH
h
(d)
CaC0
3
?
17.67 Which
of
the following will be more soluble in acid solution than
in pure water: (a) CuI, (b)
Ag
l
S0
4
,
(c)
Zn
(
OH
)l, (d) BaC
2
0
4
,
(e)
Ca3(P04)2?
17.68
Compare
the molar solubility
of
Mg(OH)l
in
water and in a
solution buffered at a
pH
of
9.0.
17.69 Calculate the molar solubility
of
Fe
(OH)2 in a solution buffered
at (a) a
pH
of
8.00 and (b) a
pH
of
10.00.
17.70
The
solubility product
of
Mg(OH)
l is 1.2 X 10-
11
•
What
minimum
OH- concentration must be attained (for example, by
adding
NaOH) to decrease the
Mg
concentration in a solution
of
Mg(N0
3
)2
to less than 1.0 X
10-
10
M?
17.71 Calculate whether
or
not a precipitate will form
if
2.00
mL
of
0.60 M
NH
3 is added to 1.0 L
of
1.0 X 10-
3
M
FeS04'
17.72
If
2.50 g
of
CUS04 are dissolved in 9.0 X 10
2
mL
of
0.30 M
NH
3
,
what are the concentrations
of
Cu2+,
Cu(NH
3
)~
+
'
and
NH
3 at
equilibrium?
17.73 Calculate the concentrations
of
Cd
2
+,
Cd(CN)~
-
,
and
CN
- at
equilibrium when
0.50 g of
Cd(N0
3
)2
dissolves in 5.0 X 10
2
mL
of
0.50 M NaCN.
17.74
If
NaOH
is added to 0.010 M Al
H,
which will be the
predominant species at equilibrium: AI(OH)3
or
AI(OH)4 ?
The
pH
of
the solution is 14.00.
[K
f
for Al(OH)4 = 2.0 X 10
33
]
17.75 Calculate the molar solubility
of
AgI in a 1.0 M
NH
3 solution.
17.76 Both
Ag
+ and
Zn
2+
form complex ions with
NH
3
. Write balanced
equations for the reaction
s.
However,
Zn(OHh
is soluble in 6 M
NaOH, and AgOH is not. Explain.
17.77 Explain, with balanced ionic equations, why (a) CuI
2
dissolves
in
ammonia solution, (b) AgBl' dissolves in NaCN solution, and
(c)
HgCl
l
dissolves in KCl solution.
Section 17.6: Separation
of
Ions Using Differences
in
Solubility
Review Questions
17.78 Outline the general procedure
of
qualitative analysis.
17.79 Give two examples
of
metal ions in each group
(1
through 5) in
the qualitative analysis sc
hem
e.
Problems
17.80 Solid NaI is slowly added to a solution that is 0.010 M in Cu+ and
0.010 M in
Ag
+. (a) Which compound will begin to precipitate
first? (b) Calculate [Ag +] when CuI
just
begins to precipitate.
(c)
What
percent
of
Ag
+ remains in solution at this point?
17.81 Find the approximate
pH
range suitable for the separation
of
Fe
H
and Zn2+ ions by precipitation
of
Fe(OH)3 from a solution that is
initially
0.010 M in both
Fe
H
and Zn
H
17.82 In a group I analysis, a student obtained a precipitate containing
both AgCI and PbCI
2
. Suggest one reagent that would enable the
student to separate AgCl(s) from PbCI
2
(s).
17.83 In a group 1 analysis, a student adds HCI acid to the unknown
solution to make
[Cl
~
]
= 0.15
M.
Some
PbCl
2
precipitates.
Calculate the concentration
of
Pb
2
+ remaining in solution.
17.84 Both KCl and
NH
4
Cl are white solid
s.
Suggest
one
reagent that
would enable you to distinguish between these two compounds.
17.85 Describe a simple test that would allow you to distinguish
between
AgN0
3
(s) and Cu
(N0
3
lz(s).
Additional Problems
17.86
The
buffer range is defined by the equation
pH
=
pK
a +
1.
Calculate the range
of
the ratio [conjugate base]/[acid] that
corresponds to this equation.
17.87 The
pK
a
of
the indicator methyl orange is 3.46. Over what
pH
range
does this indicator change from
90 percent HIn to 90 percent In- ?
17.88 Sketch the titration curve
of
a weak acid with a strong base like the
one shown in Figure 17.4.
On yo
ur
graph, indicate the volume
of
ba
se used at the equivalence point and also at the half-equivalence
point, that is, the point at which half
of
the acid
ha
s been
neutralized. Show how you can measure the
pH
of
the solution at
the
ha
lf-equivalence point. Using Equation 17.3, explain how you
can determine the
pK
a
of
the acid by this procedure.
17.89 A 200-
mL
volume
of
NaOH
solution
wa
s added to
400
mL
of
a
2.00 M
HNO
z
solution.
The
pH
of
the
mi
xed solution was 1.50
units greater than that
of
the original acid solution. Calculate the
molarity
of
the N
aOH
solution.
17.90
The
pK
a
of
butyric acid (
HBut
) is 4.7. Calculate Kb for the
butyrate ion (But - ).
17.91 A solution is made by mixing exactly
500
mL
of 0.167 M
Na
OH
with exactly
500
mL
0.100 M
HCOOH
. Calculate the equilibrium
concentrations
ofH
+,
HCOOH
,
HCOO
-,
OH-
, and
Na
+.
17.92 Tris [tris(hydroxymethyl)aminomethane] is a c
ommon
buffer for
studying biological sys
tem
s: (a) Calculate the
pH
of
the tris buffer
after mixing
15.0
mL
of
0.10 M
HC
I solution with 25.0
mL
of
0.10 M tris. (b)
Thi
s buffer was used to study an enzyme-
ca
talyzed
reaction. As a result
of
the reaction, 0.00015 mole
of
H+ was
consumed.
What
is the pH
of
the buffer at the end
of
the reaction?
(c)
What
would be the
fin
al
pH
if
no buffer were
pr
esent?
HOCH
2
I +
HOCH
2
-C-
NH
3
I
HOCH
2
•
pK
a = 8
.1
•
HO
CH
2
I
HOCH
2
-C-
NH
? + H+
I -
HO
CH?
17.93 Cd(OH)2 is an
in
soluble c
omp
ound. It dissolves in excess N
aOH
in solution. Write a balanced ionic equation for this reactio
n.
What
type
of
reaction is this?
17.94 A student mixes
50.0
mL
of
1.00 M Ba(
OH
)2 with 86.4
mL
of
0.494 M H
2
S0
4
,
Calculate the
ma
ss of BaS0 4 fo
rm
ed and the pH
of
the mix
ed
solution.
17.95
For
which
of
the follow
ing
reactions is the equilibrium constant
called a solubility product?
(a)
Zn
(
OH
lz(s) + 2
0W
(a
q) . •
Z
n
(
OH
)~
-
(
aq
)
(b) 3Ca
2+
(aq) +
2
PO
~
-
(aq
)
.
• Ca
}(
P0
4lz(
S)
(c)
CaC0
3
(s)
+ 2H+(aq) . • Ca2+(aq) + H
2
0 (l) + CO
z(
g)
(d) PbI
2
(s) • •
Pb
1+(aq) +
2r-(aq
)
17
.96 A 2.0-L kettle contains 116 g
of
boiler scale (CaC0
3
). How many
times would the kettle have to be c
omple
tely
fi
lled with distilled
water to remove all the deposit at
')
SO
C?
17.97 Equal v
olum
es of 0.12 M
AgN
O} and 0.14 M ZnCl
2
solution are
mixed. Calculate the equilibrium con
ce
ntrations
of
Ag
+,
C
I-
,
Zn
2+,
and
NO
.1
.
17.98 Find the approx
imat
e pH ran
ge
suitable for separating
Mg
2+
and Zn
2
+ by the
pre
cipitation of Zn(OH)2 from a solution that is
initially
0.010
Min
Mg
2+
and Zn
2
+.
17.99 Calculate the solubility
(i
n
gIL)
of
Ag
1
CO
}.
17.100 A volume
of
25.0 mL
of
0.100 M HCI is titrated aga
in
st a
0.100 M
CH
3N
H2 solution added to it from a bure
t.
C
al
culate the
pH
values
of
the solution after (a) 10.0
mL
of CH}
NH
2 solution
ha
ve been added, (b) 25.0
mL
of
CH
}NH2 solution have been
added, (c)
35.0
mL
of
CH
3NH2 solution
ha
ve been adde
d.
17.101
The
molar solubility
of
Pb
(
I0
3
)2
in
a 0.10 M
Na
rO} solution is
2.4
X 10-
11
mollL.
Wh
at is K,p for Pb(
IO
})2?
17.102
When
a
KI
solution was added to a solution of mercury(II)
chloride, a precipitate [merc
ur
y(II) iodide] fo
rmed
. A student
QUESTIONS
AND
PROBLEMS
-:2~
plotted the
ma
ss
of
the precipitate versus the volume
of
the
KJ
solution added and obtained the following graph.
Exp
lain the
shape
of
the graph.
v
, ,
<Ll
§
<B
'"
~
:r:
<.; ;
o
'"
'"
cO
~
IL-
_______
_ _ J
Volume
of
KI
added
17.103 Barium is a toxic substance that can
se
riously impair heart
function.
For
an X ray
of
the
ga
strointestinal tract, a patient
drinks an aqueous suspension
of
20 g
BaS0
4'
If
this substan
ce
w
ere
to equilibrate with the 5.0 L
of
the blood
in
the patient's
bod
y,
what would
be
[Ba
2+
]?
For
a good estimate,
we
may
ass
um
e that the Ksp
of
BaS0
4 at body temperature is the
sam
e as
at 25°C.
Wb
y is
Ba
(N0
3
lz not chos
en
for this procedure?
17.104 The
pK
a
of
ph
enolphthalein is 9.10. O
ve
r what
pH
range does
this indi
ca
tor chan
ge
f
rom
95
per
cent
Hln
to 95 percent
In-
?
17.105
So
lid NaBr is slowly added
to
a solution that is 0.010 M in Cu T
and 0.010 M in
Ag
+ (a) Which compound will begin to precipitate
first?
(b) Calculate [
Ag
+] when
CuBr
ju
st begins to precipitate.
(c)
Wba
t
pe
rcent of
Ag
+ remains in solution at this point?
17.106 Cacodylic acid is (
CH
3)2A sOz
H.
Its ionization constant is 6.4 X
10-
7
(a) Calculate the
pH
of
50.0
mL
of
a 0.10 M solution
of
the
acid. (b)
Ca
lculate the
pH
of 25.0
mL
of
0.15 M (
CH
3
lzAs0
2
a
(c) Mix the solutions
in
parts (a) and (b). Calculate the pH
of
the
resulting solution.
17.107 Radioche
mi
ca
l techniques are useful in estimating the solubility
product of many
compound
s. In one experiment, 50.0
mL
of
a 0.010 M AgN0
3
solution containing a s
il
v
er
isotope with a
radioac
ti
vity
of
74,025 counts per min per
mL
was mixed with
100
mL
of
a 0.030 M NaIO} solutio
n.
Th
e
mi
xed solution was
diluted to
50
0
mL
and filtered to remove all the
AgI0
3
precipitate_
The remaining solution was found to
ha
ve a radioactivity
of
44.4
counts per min per mL.
Wb
at is t
he
Ksp
of
AgI0
3
?
17.108
The
molar
ma
ss of a certain metal carbonate,
MCO
" can be
determined by adding an excess
of
HCI acid to react with all
the c
ar
bonate a
nd
then "
ba
ck-titratin
g"
the remaining acid with
NaOH. (a) Write an equation for these reactions. (b) In a cert
ai
n
experiment,
20.00
mL
of 0.0800 M
HCl
was added to a O.I022-g
sa
mp
le
of M
C0
3
.
Th
e excess HCl required 5.64
mL
of
0.1000 M
Ta
OH
for
neu
tralizatio
n.
Calculate the molar mass
of
the
carbonate a
nd
ide
ntif
y M.
17.109
Ac
id-ba
se
reactions usually go to completion. Confirm this
statement by calculating the equilibrium constant for each
of
the
foll
ow
in
g cases: (a) A strong ac
id
reacting with a strong base.
(
b)
A strong acid reacting with a w
eak
ba
se (NH
3)
' (c) A weak
•
ac
id
(
CH
}
COOH
) reacting with a strong
ba
s
e.
(d) A
weak
acid
(
CH
}
COOH
) reacting with a we
ak
base (
NH
3
).
(Hint: Strong
ac
id
s exist as H + ions and strong bases exist as
OH
- ions in
solution. You need to look up
K
a
, K
b
, and Kw')
17.
11
0 Calculate
x,
the number
of
molecules of water in oxalic acid hy
dr
ate
(H
2C10 4 . xH
2
0
),
from the following data: 5.00 g
of
the compound
is made up to exactly
2
50
mL
solution, and 25.0 mL
of
this solution
requires 15.9 mL of
0.500 M NaOH solution for neutralization.
17.111 Describe how you would
prep
are a
l-L
0.20 M
CH
3
COONaJ
0.
20 M
CH
J
COOH
buffeI
sys
tem
by (a)
mJxjJ]g!l
solo/joJ]
of
722
CHAPTER
17 Acid-Base Equilibria and
Solubility
Equilibria
•
CH
3
COOH
with a solution
of
CH
3
COONa,
(b)
mixing
a solution
of
CH
3
COOH
with
a s
olution
of
NaOH,
and (c) mixing a
solution
of
CH
3
COONa
with
a solution
of
HCL
17.112
Phenolphthalein
is the
common
indicator
for
the titration
of
a
strong acid
with
a strong base. (a)
If
the
pK
a
of
phenolphthalein
is 9.10, what is the ratio
of
the
nonionized
form
of
the indicator
(colorless) to the ionized
form
(reddish
pink)
at
pH
8.00? (b)
If
2
drops
of
0.060
M
phenolphthalein
are used in a titration involving
a
50.0-mL
volume,
what
is
the
concentration
of
the
ionized
form
at
pH
8.00?
(Assume
that 1
drop
=
0.050
mL)
17.113
Glutamic
acid is
one
of
the
20
amino
acids in proteins:
+ 9.67
NH3
4.?5 I
?19
HOOC-CH
?
-CH
2
-CH-COOH
The
numbers
refer to the pKa value of
each
side
chain
of
glutamic
acid in solution.
These
values
can
change
appreciably
when
glutamic
acid is
part
of
a
protein
molecule
,
depending
on its
environment.
How
would its
pK
a be affected (increase, decrease,
no change)
when
(a) the terminal -
COO
-
group
of
the protein
is
brought
into
close
proximity, (b) the terminal
-NH
j
group
is
brought
into
close
proximity, (c) it is expos
ed
on
the surface
of
the
protein,
and
(d)
it
is buried
in
the
interior
of
the protein?
17.114
Oil
paintings containing lead(II)
compounds
as constituents
of
their
pigments
darken over the
year
s.
Suggest
a chemical reason
for the
color
change.
17.115
What
reagents
would
you
employ
to separate the following pairs
of
ions in solution: (a)
Na
+ and
Ba
2+,
(b) K+
and
Pb
z
+,
(c)
Zn
2+
and
Hgz+?
17.116
Look
up
the Ksp values for
BaS0
4 and
SrS04
in Table 17.4.
Calculate
the concentrations
of
Ba
2+
,
Sr
2
+,
and
SO
~-
in
a
solution that is saturated with
both
compound
s.
17.117
In
principle,
amphoteric
oxides,
such
as Al
z
0
3
and
BeO
can
be
used to prepare buffer solutions
because
they possess
both
acidic
and
basic properties (see Section 16.11).
Explain
why
these
compounds
are
of
little practical
use
as buffer
components.
17.118
CaS04
(K
sp =
2.4
X 10-
5
)
has
a larger K
sp
value than that
of
Ag
z
S0
4
(K
sp = 1.4 X 10-
5
).
Does
it
necessarily follow that
CaS0
4 also has
greater
solubility (gIL)?
17.119
When
l
emon
juice
is
added
to tea,
the
color
becomes lighter.
In
part, the
color
change
is
due
to dilution,
but
the
main
rea
s
on
for
the
change
is
an
acid-base reaction.
What
is the reaction? (Hint:
Tea contains
"po
lyphenols,"
which
are
weak
acids, and
lemon
juice
contains citric acid.)
17
.120
How
many
milliliters
of
1.0 M
NaOH
must
be
added
to
200
mL
of
0.10
M N
aH
2
P0
4
to
make
a buffer solution with a
pH
of
7.50?
17.121
The
maximum allowable concentration
ofPb
z
+
ions in drinking
water is
0.05
ppm
(that is, 0.05 g
of
Pb
2+
in 1 million grams
of
water). Is this guideline exceeded
if
an underground water supply is at
equilibrium with the mineral anglesite
(PbS0
4
)
(Ksp = 1.6 X 1O-
8
)?
17.122
One
of
the
most
common
antibiotics
is
penicillin G
(benzylpenicillinicacid
),
which
has
the following structure:
o
II
H
C-OH
'\
/
~O
H3
C
C N-C?'
H
\/
I I I f
H3~
's
-
T-T-N-~-CH2
H H 0
It is a
weak
monoprotic
acid:
HP • • H+ + P-
Ka
= 1.64 X 10-
3
where
HP denotes the
parent
acid
and
P-
the conjugate base.
Penicillin G is
produced
by growing
molds
in fermentation tanks
at
25°C and a
pH
range
of
4.5 to 5.0.
The
crude
form
of
this
antibiotic is obtained by extracting the fermentation
broth
with an
organic solvent
in
which the acid is soluble. (a) Identify the acidic
hydrogen atom. (b)
In
one
stage
of
purification, the organic extract
of
the
crude
penicillin G is treated with a buffer solution at
pH
=
6.50.
What
is the ratio
of
the conjugate base
of
penicillin G to the
acid at this
pH?
Would you expect the conjugate
base
to be more
soluble
in
water than the acid? (c) Penicillin G is
not
suitable
for oral administration, but the
sodium
salt (NaP) is
because
it
is
soluble. Calculate the
pH
of
a 0.12 M
NaP
solution formed when
a tablet containing the salt is dissolved
in
a glass
of
water.
17
.123
Which
of
the following solutions has the highest [H+]: (a) 0.10 M
HF, (b)
0.10
M
HF
in
0.10
M NaF, (c)
0.10
M
HF
in
0.10
M
SbFs? (Hint: SbFs reacts with F- to
form
the
comp
lex
ion
SbF
6')
17.124 Distribution curves show how the fract
ion
s
of
a nonionized
acid
and
its
conjugate
base
vary as a function
of
the
pH
of
the
medium.
Plot
distribution curves for
CH
3
COOH
and
its conjugate
base
CH
3
COO
-
in
solution. Your
graph
should show fraction as
the
y axis and
pH
as the x axis.
What
are
the fractions and
pH
at
the
point
where
these
two
curves intersect?
17.125
Calcium
oxalate is a
major
component
of
kidney stones. Predict
whether the formation
of
kidney stones
can
be
minimized
by
increasing
or
decreasing the
pH
of
the fluid
present
in the kidney.
The
pH
of
normal
kidney
fluid is
about
8.2. [The first and
second
acid ionization constants
of
oxalic acid
(H
Z
C
Z
0
4
) are 6.5 X
lO-
z
and 6.1 X
lO
-
s
,
respectively.
The
so
lubility
product
of
calCium
oxalate is
3.0
X 10-
9
.]
17.126 Water containing
Ca
2+
and
Mg
2+
ions is called hard water and is
unsuitable for
some
household
and
industrial use
because
these
ions
react
with
soap to
form
insoluble
salts,
or
curds.
One
way
to
remove
the
Ca
2
+ ions
from
hard water is
by
adding
wa
s
hing
soda
(Na
z
C0
3
.
lOH
z
O). (a)
The
molar
solubility
of
CaC0
3
is
9.3
X 10-
5
M.
What
is its
molar
solubility in a
0.050
M
Na
Z
C0
3
solution? (b)
Why
are
Mg
2+
ions
not
removed
by
this
procedure?
(c)
The
Mg
2+
ions are removed as
Mg(OH)
z by
adding
slaked
lime
[Ca(OHh]
to the
water
to
produce
a saturated solution.
Calculate the
pH
of
a saturated
Ca(OHh
solution. (d)
What
is the
concentration
of
Mgz+ ions
at
this
pH?
(e) In general,
which
ion
(
Ca
2+
or
Mg
2+
) would you remove first?
Why?
17.127
Amino acids are building blocks
of
proteins. These compounds
contain at least one amino group
(-NH
2
)
and one carboxyl group
(- COOH). Consider glycine (NH
Z
CH
2
COOH). Depending on the
pH
of
the solution, glycine can exist in one
of
three possible forms:
Fully protonated:
+
NH
3
-CH
z
-COOH
Dipolar
ion: +
NH
3
-CH
2
-COO
-
Fully ionized:
NHz-CH
z
-COO
-
Predict
the
predominant
form
of
glycine
at
pH
1.0, 7.0,
and
12.0.
The
pK
a
of
the
carboxyl
group
is 2.3
and
that
of
the
ammonium
group
(-
NH
j ) is 9.6.
17.128
Consider
the ionization
of
the following acid-base indicator:
HIn(aq) • • H+(aq) + In- (aq)
The
indicator
changes
color
according to the ratios
of
the
concentrations
of
the acid to its
conjugate
ba
se.
When
[Hln]/[In - ]
:>
10,
color
of
acid (HIn) predominates.
When
[Hln]/[In - ]
<:
0.1,
color
of
conjugate
base
(In
-)
predominates.
Show that the
pH
range over which the indicator changes
from
the acid color to the base color is
pH
= pKa +
1,
where
Ka
is the
ionization constant
of
the acid HIn.
17.129 One way to distinguish a buffer solution with an acid solution
is
by
dilution. (a) Consider a buffer solution
made
of
0.500 M
CH
3
COOH
and 0.500 M
CH
3
COONa.
Calculate its
pH
and the
pH
after it has
been
diluted lO-fold. (b) Compare the result in
part
(a) with the
pHs
of
a 0.500 M
CH
3
COOH
solution before
and after it has been diluted 10-fold.
l7
.l30
(a) Referring to Figure
17
A,
describe how you would determine
the
pKb
of
the base. (b) Derive an analogous Henderson-
Hasselbalch equation relating
pOH
to pKb
of
a
weak
base B
and its conjugate acid HB+.
Sketch a titration curve showing
the variation
of
the
pOH
of
the
ba
se
solution versus the volume
of
a strong acid added from a buret.
De
scribe how you would
determine the
pKb
from this curve.
•
17.131 A sample
of
0.96 L
of
HCl gas at 372
mmHg
and
22
°C is
bubbled into
0.034 L
of
0.57 M
NH
3
.
What
is the
pH
of
the
resulting solution? Assume the volume
of
solution remains
constant and that the HCI is totally dis sol ved in the solution.
17.132 Draw distribution curves for an aqueous carbonic acid solutio
n.
Your graph should show fraction
of
species present as the y axis
and
pH
as the x axis. Note that at any pH, only two
of
the three
ANSWERS TO IN-CHAPTER PROBLEMS 723
species (H
2
C0
3
,
HC0
3
, and
co
j-) are present in appreciable
concentrations.
Use
the
Ka
values in Table 16.8.
17.133 Histidine is
one
of
the
20
amino acids found in proteins. Shown
here is a fully protonated histidine molecule, where the numbers
denote the
pK
a values
of
the acidic groups:
9.1J
~
1.82
H3N
-CH-C-OH
I
CH
2
6.
00
~~
LNH
(a) Show stepwise ionization
of
histidine in solution. (
Hint:
The
H+ ion will first come
off
from the strongest acid group followed
by
the next stronge
st
acid group and so on.) (b) A dipolar
ion
is
one in which the species has an equal
number
of
positive and
negative charges. Identify the dipolar ion in part (a). (c)
The
pH
at which the dipolar
ion
predominates is called the isoelectric
point, denoted by
pl.
The
isoelectric point is the average
of
the
pK
a values leading to and following the formation
of
the dipolar
ion. Calculate the
pI
of
histidine. (d)
The
histidine group plays an
important role in buffering blood (the
pH
of
blood is about
704).
Which
conjugate acid-base pair shown in part (a) is responsible
for maintaining the
pH
of
blood?
PRE-PROFESSIONAL PRACTICE
EXAM
PROBLEMS:
PHYSICAL
AND
BIOLOGICAL SCIENCES
Aqueous acid reacts with carbonate ions to produce carbonic acid, which
produces carbon dioxide. A 1.0-L
sa
turated silver carbonate solution at
S
oC
is
treated with enough hydrochloric acid to consume all the carbonate
in solution.
The
carbon dioxide generated is collected in a 19-mL vial
and
exerts a pressure
of
114
mmHg
at 25°C.
1.
Which
of
the following reactions represents the overall process
of
acid and carbonate reacting to give carbon dioxide?
a)
CO~
-
+ 2H+ • H
2
C0
3
b) H
2
C0
3
• •
H
2
0 + CO
2
c)
coj-
+ 2H+ • H
2
0 +
CO
2
d)
Ag
2
C0
3
+
2H
+ • 2Ag + +
CO
2
+ H
2
0
2.
What
is the Ksp
of
silver carbonate at SOC?
a) 2.5 x
10
-
11
b)
SA
X 10-
8
c) 6.3 X
10-
12
d)
2.7 X
10
-
8
3. · At 25°C, the Ksp
of
silver carbonate is 8.1 X 10-
12
.
Based
on this
and the answer to Question
1,
what can
be
said about the dissolution
of
silver carbonate?
a) It is endothermic.
b) It is exothermic.
c)
It
is neither exothermic nor endothermic.
d) It produces hydrogen gas.
4. Which
of
the following,
if
added to a saturated solution
of
Ag
2
C0
3
,
would increase the solubility
of
Ag
2
C0
3
?
a)
Na
2
C0
3
b)
NaHC0
3
c)
AgN0
3
d)
HN0
3
ANSWERS TO
IN-CHAPTER
PROBLEMS
Answers
to
Practice Problems
17.1A 4.22. 17.1B 4.89. 17.2A 5.0. 17.2B 4.77. 17.3A Dissolve 0.6 mol
CH
3
COONa
and 1
mol
CH
3
COOH
in enough water to make 1 L
of
solution. 17.3B 2.35-4.35. 17.4A (a) 5.04, (b) 8.76, (c) 12.2. 17.4B
(a) 8.3 mL, (b) 12.5 mL, (c) 27.0 mL. 17.5A
5.9l.
17.5B 5.21. 17.6
(a) Bromophenol blue, methyl orange, methyl red, or chlorophenol,
(b) any but thymol blue, bromophenol blue,
or
methyl orange, (c) cresol
red
or
phenolphthalein. 17.7A
l.8
X
10
-
3
gIL.
17.7B 1.5 X
10-
11
gIL.
17.8A 1.9 X
10
-
14
gIL.
17.8B
1.7
X
10
-
6
gIL. 17.9A No. 17.9B Yes.
17.10A (a) 9.1 X
10
-
9
M, (b) 8.3 X 10-
14
M.
17.10B (a) 1.0 X
10
-
5
M,
(b)
1.1
X 10-
7
M.
17.11A (a) Yes, (b) yes, (c) no.
17.11BAny
salts
containing the
CO
~-
ion, the
OH-
ion, the
S2
- ion,
or
any anion
of
a
weak
acid such as the
SO~
-
ion
or
the F- ion. 17.12A 0.60
M.
17.12B
(a)
l.6
X
10-
9
M, (b) 2.6 X
10-
6
M.
Answers
to
Checkpoints
17.1.1 = a, c,
d.
17.1.2 =
b.
17.2.1 = b, c, d. 17.2.2 =
a.
17.2.3 =
c.
17.2.4 =
b.
17.3.1 = a,
c,
d, e. 17.3.2 =
c.
17.3.3. =
a.
17.3.4 =
a.
17.4.1 =
a.
17.4.2 =
c.
17.5.1 =
a.
17.5.2 =
a,
b, d,
e.
17.6.1 =
d.
17.6.2 =
b.
Answers
to
Applying
What
You've Learned
a) 9 X
10
-
8
M. b) 3 X 10-
60
M.
c) 1 X
10
-
8
M. d) 2 X 10-
7
M.
-Jntro
,
ree
_ner
,
• •
•
UII
rlum
•
18.1 Spontaneous Processes
18.2 Entropy
•
Probability
•
Microstates
•
Standard
Entropy
•
Entropy Changes
in
a
System
18.3
The
Second
and
Third
Laws
of
Thermodynamics
•
Entropy Changes in the
System
•
Entropy Changes
in
the
Surroundings
•
Third
Law
of
Thermodynamics
18.4 Gibbs Free Energy
•
Standard Free-Energy
Changes
•
Using
L1
C
and
L1
C o to Solve
Problems
18.5
Free Energy
and
Chemical
Equilibrium
•
Relationship Between
L1
C
andL1Co
•
Relationship Between
L1C
o
andK
18.6
Thermodynamics in
Living Systems
Living
Systems
and
Entropy
All things in nature obey certain laws. Among these are the laws
of
thermodynamics.
, The first law
of
thermodynamics states that energy is neither created nor destroyed in
chemical reactions. The second law
of
thermodynamics states that any process that
occurs spontaneously must result in an overall increase in entropy.
On the face
of
things,
living systems might appear to violate this
law.
Organisms, especially humans, seem to
be able
to
create order, thereby decreasing entropy. For us
to
cause a decrease in entropy
somewhere, however, we must cause an even larger increase in entropy somewhere else.
For example, although we may plant orange trees in straight rows, harvest the oranges,
pack them in crates, and stack the crates neatly onto a truck, to do so requires a great deal
of
energy. The energy required for these activities comes from the consumption
of
large
quantities
of
food, which consists mostly
of
carbon, oxygen, and hydrogen. In addition
to providing energy for human activity, the metabolism
of
food generates carbon diox-
ide gas and water vapor.
The?e gaseous products have far greater entropy than the solid
foods that went into their production.
For us
to
cause a reduction in entropy in one part
of
the universe, the principles of
entropy, free energy, and equilibrium require that we generate an even greater increase
in entropy somewhere else.
•
In
This Chapter, You Will Learn about the three laws
of
thermodynamics and how
to
use thermodynamic
quantities to determine whether or not a process is expected to be spontaneous.
Before you begin, you should review
• System and surroundings
[I
••
Section
5.1]
• Hess's law
[
~.
Section
5.5]
• Equilibrium expressions
[~
.
Section
15.3]
Overcoming
nature's
tendency
toward
disorder, as
we
do
when
we
harvest oranges from a
grove
and
arrange
them
in neatly
stacked
crates, requires
an
enormous
input
of
energy.
Media
Player/
MPEG
Content
Chapter in
Re
v
ie
w
,
725