ALZHEIMER''''S DISEASE: ITS DIAGNOSIS AND PATHOGENESIS - PART 1 doc
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (1.63 MB, 10 trang )
ALZHEIMER'S DISEASE: ITS DIAGNOSIS AND PATHOGENESIS
Jillian J. Kril I
Centre for Education and Research on Ageing, Concord Hospital
Department of Medicine, The University of Sydney, Concord, New South Wales,
Australia 2139, and Department of Pathology, The University of Sydney, Sydney,
New South Wales, Australia 2006
Glenda M. Halliday
Prince of Wales Medical Research Institute
Randwick, New South Wales, Australia 2031
I. Introduction
II. Diagnostic Issues
A. A/3 Plaques and NFTs for Pathological Diagnosis
B. Evaluation of Other Pathologies
C. Clinical Correlates of AD Pathology
D. Reproducibility of Current Clinical Diagnostic Protocols
E. Summary
III. Pathogenesis
A. Brain Atrophy
B. Neuronal Loss
C. A/3 Deposition
D. NFT Formation
E. Mechanisms of Degeneration
E Summary
IV. Genetic Influences
A. Dominant Inheritance
B. Genetic Risk Factors
C. Summary
V. Inflammation and Anti-inflammatory Drugs
VI. Estrogen Therapy
VII. Vascular Pathology in AD
A. Vascular Risk Factors
B. Summary
References
lAuthor to whom correspondence should be addressed.
INTERNATIONAL REVIEW OF 167 Copyright © 2001 by Academic Press.
NEUROBIOLOGY, VOL 48 All rights of reproduction in any form reserved.
0074-7742/01 $35.00
168 JILLIAN J. KRIL AND GLENDA M. HALLIDAY
I. Intr(xludion
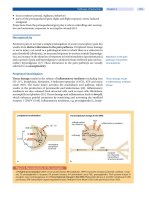
In the century since the neuronal inclusions [neurofibrillary tangles
(NFTs); Fig. 1] and extracellular protein aggregates (Aft plaques; Fig. 1)
that form the pathological hallmarks of Alzheimer's disease (AD) were de-
scribed, our knowledge of all aspects of AD has grown markedly. AD is
uniformly progressive and ultimately results in debilitating cognitive im-
pairment. In the early stages, the impairment may only be apparent on
Lesion type
"jAI~ plaques
0 e ; O 0 e
; • ; o •
"
D
Lewy bodies .
Clinical diagnosis
m° °
t ? other
dementi~
•''QI°
l~c,. 1. The major pathologies resulting in dementia are neurofibrillary tangles (NFTs; top
left), A¢I plaques (top center), and Lewy bodies (top right, arrow). Diagnosis of AD was pre-
viously based on age-corrected densities of A~ plaques; however, the finding that a significant
number of patients with dementia with Lewy bodies (DLB) also have A¢I plaques questions this
practice. Similarly, NFTs can be found in other forms of dementia and thus are not specific
for AD. Newer criteria proposed for the pathological diagnosis of AD use both A/~ plaques and
NFTs. This change in the way in which AD is diagnosed pathologically will have a significant
impact on the clinical criteria for the identification of AD. These clinical criteria have been val-
idated using A~ plaque-based pathology and now require re-evaluation in light of the advances
in our understanding of the pathogenesis of the disease.
ALZHEIMER'S DISEASE 169
neuropsychological testing; however, by end stage, few functions above the
automatic remain unaffected (Forstl and Kurz, 1999). The pattern and se-
quence of functional deficits and the relentlessness of the decline are rel-
atively reproducible, with the course of the disease in one patient similar
to that in others. AD remains the most prevalent form of late-life dementia
and is the most significant cause of morbidity in the elderly. Yet, we are still
unable to, in most instances, accurately predict who will succumb to AD or
to effectively treat it in those who do.
II. Diagnostic Issues
The definitive diagnosis of AD is made by pathological examination of
the brain; however, the accurate and reproducible diagnosis of AD during
life is of paramount importance. Not only is it essential to exclude possible
treatable causes of dementia, but it is also necessary for the identification
of homogeneous groups of patients for evaluation and study, and for the
recruitment of patients for drug and other therapeutic trials. Variability in
the clinical diagnosis of AD is well recognized and major diagnostic issues
continue to be addressed to develop accurate and reproducible criteria for
its identification. Yet, similar issues for the neuropathological diagnosis of
AD are only beginning to be evaluated in a systematic fashion. In most in-
stances, neuropathology is considered the "gold standard" for the diagnosis
of AD, but considerable variation exists between diagnostic protocols. This
has the potential to have a significant impact on our understanding of the
disease.
A.
A/3
PLAQUES AND NFTs FOR PATHOLOGICAL DIAGNOSIS
The majority of protocols for the pathological diagnosis of AD use only
one of the major pathological lesions first described by Alzheimer. The most
commonly used lesion for the diagnosis of AD is the neuritic plaque as
Aft deposition is more distinctive for AD than other neurodegenerative
diseases. The Consortium to Establish a Registry for Alzheimer's Disease
(CERAD; Mirra
et al.,
1991) developed the most widely used diagnostic pro-
tocol. It employs the earlier National Institutes of Health protocol based on
age-corrected plaque densities (Khachaturian, 1985) in a semiquantitative
fashion to arrive at diagnoses of varying certainties. The CERAD criteria
(Mirra
et al.,
1991; Table I) has gained wide acceptance because of its ac-
curacy and simplicity. In 142 cases with a clinical diagnosis of probable AD
170
JILLIAN J. KRIL AND GLENDA M. HALLIDAY
TABLE I
CONSORTIUM TO ESTABLISH A REGISTRY FOR ALZHE1MER'S DISEASE (CERAD) CRITERIA
FOR NEUROPATHOLOGICAL DIAGNOSIS OF AD a
Plaque Densities b
<50 Years 50-75 Years > 75 Years
N + No dementia N + No dementia N + No dementia, or
S + no dementia
S, M, or F + No dementia S + Dementia
Not defined M + Dementia
S, M, or F + Dementia
Normal brain
Possible AD S + No dementia
Probable AD S + Dementia, or
M + no dementia
Definite AD S, M, or F + Dementia
F + Dementia
aRegions examined middle frontal, superior and middle temporal, inferior parietal cor-
tices, hippocampus and entorhinal cortex, midbrain.
bN
= none, S = sparse, M = moderate, F = frequent plaques.
From Mirra
et aL
(1991).
(the National Institute of Neurological and Communicative Disorders and
Stroke (NINCDS)-Alzheimer's Disease and Related Disorders Association
(ADRDA) most certain clinical category, see Section II.C and Table II), 84%
were found to have definite AD 7% probable and 2% possible (Mirra
et al.,
1991). In 7% of cases, the age-related plaque score was negligible, suggesting
another cause for dementia in these cases. In an independent assessment of
the accuracy of the CERAD criteria, subjects with a clinical diagnosis of prob-
able AD had definite AD at autopsy in 27 out of 28 cases (96% Kosunen
et al.,
1996). Subsequent interlaboratory testing of the CERAD criteria revealed
75% agreement in the rank ordering of 10 cases between 24 neuropathol-
ogists at 18 centers (Mirra
et al.,
1994). The majority of variation was due
to staining differences between laboratories, but the data suggest there is
considerable variation between pathologists in the CERAD diagnosis of in-
dividual cases.
In contrast to the plaque-based protocols, Braak and Braak (1991) pro-
posed a staging scheme for the neuritic pathology ofAD (NFTs and neuropil
threads). This six-stage scale documents the temporal sequence and topo-
graphic spread of AD pathology. Although not proposed as a criteria for
the neuropathological diagnosis of AD, dementia is reliably associated with
stages V and VI and to a variable degree with stages III and IV (Braak and
Braak, 1991; Harding
et al.,
2000). NFTs first form in the pre-a layer of
the transentorhinal cortex (stage 1, Table III), then the pre-a layer of the
entorhinal cortex, the hippocampus, and finally the isocortex (Table III).
Compared with the CERAD criteria, inter-rater reliability for neuritic stag-
ing is high (weighted kappa 0.85 to 0.97; Nagy
et al.,
1997b). However, the
ALZHEIMER'S DISEASE 171
TABLE II
NINCDS-ADRDA CRITERIA FOR CLINICAL DIAGNOSIS OF An a
Possible AD
Probable AD
Key features
Probable AD
Supportive features
Probable AD
Suggestive features
Probable AD
Features not
consistent with AD
Definite AD
Clinical diagnosis of
• Dementia syndrome with variation in onset, presentation,
or course but in absence of neurological, psychiatric, or
systemic disorder sufficient to cause dementia
• Dementia in presence of second systemic or brain disorder
sufficient to produce dementia, but not considered to be
cause of dementia
• Single, severe, progressive deficit in single cognitive domain
Inclusion Criteria
• Onset between 40 and 90 years
• Dementia established on clinical examination and testing
• Deficits in two or more areas of cognition
• Progressive worsening of functions
Exclusion Criteria
• No disturbance of consciousness
• Absence of systemic or brain disease, which may account for
progressive deficits in memory and cognition
• Progressive deterioration in specific cognitive function,
such as language, motor skills, and perception
• Impaired activities of daily living and altered
behavior pattern
• Family history of similar disorder
• Normal CSF
• Normal or nonspecific changes on EEG
• Progressive cerebral atrophy on CT scan
After exclusion of other causes
• Plateaus in course of progression
• Associated symptoms of depression, insomnia,
incontinence, delusions, illusions, hallucinations,
catastrophic verbal, emotional or physical outbursts,
sexual disorders, weight loss
• Neurological abnormalities, especially in advanced
patients motor signs (increased muscle tone, myoclonus,
or gait disorder), epilepsy
• CT scan noixnal for age
• Sudden apoplectic onset
• Focal neurological signs hemiparesis, sensory loss,
visual field deficit, incoordination early in disease
• Seizures or gait disturbance at onset or early in disease
• Clinical criteria for probable AD
• Histopathologic evidence from biopsy or autopsy
aDiagnostic certainty is ranked as possible, probable, or definite based on the features
present.
From McKhann
et al.
(1984).
172 JILLIAN J. KRIL AND GLENDA M. HALLIDAY
TABLE III
BRAAK STAGING SCHEME OF
NFT
FORMATION DURING AGING AND AD
Stage Neurofibrillary tangles a
I
II
III
1V
V
VI
NFTs in pre-a layer of the transentorhinal cortex
Isolated NFTs in pre-a layer of entorhinal cortex proper
Numerous NFTs in transentorhinal cortex
Sparse NFTs in CA1 sector of hippocampal formation
Severe involvement of transentorhinal cortex, including eNFTs
Modest involvement of CA1
NFTs in subiculum
Numerous NFTs in CA1, some in CA4
Mild involvement of isocortices, sparing of primary cortices
All sectors of hippocampus involved
Moderate involvement of subcortical nuclei
Isocortex moderately involved
Severe involvement of isocortices
Severe involvement of subcortical nuclei
Mild involvement of primary cortices
aNFT = neurofibrillary tangle, eNFT = extracellular or "ghost" neurofib-
rillary tangle.
From Braak and Braak (1991 ).
strict hierarchical order of NFT formation is not observed in all cases. In a
study of 42 brains, Gertz and colleagues (1998) found that only six cases fully
fitted the expected pattern of NFT distribution. Most of these violations of
staging order were in the early stages, suggesting they would not alter the
effectiveness of the protocol for identifying AD.
The NINCDS and ADRDA working group proposed a number of criteria
for the neuropathological diagnosis ofAD, which evaluated both Aft plaques
and NFT and excluded cerebrovascular disease (Tierney
et al.,
1988). How-
ever, the protocols have not been widely adopted, in part, because of their
complexity and modest sensitivity. Comparison between NINCDS-ADRDA
clinical and pathological criteria showed agreement in 8 of 9 nondemented
controls, 18 of 38 cases with possible AD, and 18 of 19 cases with prob-
able AD (Nagy
et al.,
1998). An evaluation of the Khachaturian, CERAD,
NINCDS-ADRDA, and Braak methods for assessing AD in a group of 60 el-
derly subjects with known Mini-Mental State (MMS) score, revealed that all
criteria accurately identified individuals with severe dementia (MMS 0-10;
Jellinger
et al.,
1995). However, in moderately demented individuals (MMS
11-23)
reliance on plaque-based criteria resulted in significant underdiag-
nosis of AD. The majority of these subjects also had limbic NFTs (Braak
stages III and IV). In addition, the use of plaque densities without assessing
clinical state resulted in 5 of 9 nondemented subjects being classified as AD,
ALZHEIMER'S DISEASE 173
indicating the presence of plaques is a poor indicator of the presence of
dementia, at least in the very old.
Because of these difficulties, the neuritic staging scheme of Braak has
been combined with the CERAD protocol for assessment of plaques into the
National Institute on Aging (NIA)-Reagan Institute criteria for the diagnosis
of AD (Hyman and Trojanowski, 1997; National Institute on Aging and
Reagan Institute Working Group, 1997; Newell
et al.,
1999). Topographical
assessment of NFT type (intra- or extracellular) and A/3 plaque density is
used to classify cases into high, intermediate, or low likelihood of AD. These
criteria are the currently accepted "gold standard" for the diagnosis of AD,
even though their reliability, reproducibility, and overall accuracy are yet to
be determined.
B.
EVALUATION OF OTHER PATHOLOGIES
Further problems with assessing the accuracy of neuropathological di-
agnoses exist. In most studies, diagnostic validity is assessed by reporting
cases that meet criteria for AD, regardless of whether other pathologies are
present. In some instances, coexisting pathologies may contribute to the de-
mentia process and thus be of importance in the assessment of the clinical
criteria. In one study addressing this issue, diagnostic accuracy was 81% for
AD (using CERAD criteria) including coexisting disease, but only 44% for
pure cases (Bowler
et al.,
1998). The presence of infarction was the primary
reason for the differences encountered. Furthermore, the identification
of a number of previously unrecognized dementing disorders, with clini-
cal and/or pathological overlap with AD (e.g., dementia with Lewy bodies
(DLB) ; Kosaka
et al.,
1984; McKeith
et al.,
1996, 1999; Fig. 1; and small vessel
disease dementia (Pantoni
et al.,
1996)), calls into question the usefulness
of many of the existing criteria for the diagnosis of AD. Because the majority
of cases with DLB also have plaques (McKeith
et al.,
1996), the CERAD cri-
teria cannot differentiate these disorders and the evaluation of intracellular
pathology is required. In addition, the overlap between cerebrovascular
disease and AD is well known (see Di Iorio
et al.,
1999; Breteler, 2000;
de la Torre, 2000). However, the demonstration that small vessel disease
alone can cause a clinical syndrome indistinguishable from AD (Pantoni
et al.,
1996) suggests that reevaluation of the role of this pathology is also
necessary. Few studies have addressed these issues.
The NIA-Reagan Institute criteria for the diagnosis of AD (Hyman
and Trojanowski, 1997; National Institute on Aging and Reagan Institute
Working Group, 1997) states that all pathologies should be evaluated, but
does not suggest how overlapping diagnoses can be arrived at or how they
174
JILLIAN J. KRIL AND GLENDA M. HALLIDAY
should be incorporated into the diagnostic criteria. This represents a signif-
icant weakness in the current neuropathological criteria for the diagnosis
of AD and must be addressed before the effectiveness of any criteria can be
adequately evaluated.
C. CLINICAL CORRELATES OF AD PATHOLOGY
Reevaluation of the clinical diagnosis of AD in light of the changing con-
cepts of the pathology of the disease is necessary. It is no longer adequate
that clinical criteria for AD alone are developed. Better diagnostic crite-
ria for patients with similar core clinical features that differentiate the signs
and symptoms of other pathologies are required. The most widely used clin-
ical criteria are those established by the NINCDS-ADRDA (McKhann
et al.,
1984). Diagnoses of probable and possible AD (Table II) can be made using
these criteria. Possible AD is considered when dementia is apparent in the
presence of other systemic or brain disorders that, may themselves, result in
dementia. A diagnosis of probable AD is considered when the patient is free
from complicating diseases and when deficits in two or more areas of cogni-
tion are present. The CERAD group (Morris
et al.,
1989) proposed a battery
of clinical and neuropsychological tests to aid in the classification of cases
into the NINCDS-ADRDA possible and probable AD groups. A multicenter
study of the NINCDS-ADRDA criteria, which evaluated 60 cases (40 with
AD), showed an initial sensitivity of 0.81 and specificity of 0.73 (Blacker
et al.,
1994). These values were improved to 0.83 and 0.84, respectively, after
consensus rating (Blacker
et al.,
1994).
Validity studies that tested the NINCDS-ADRDA criteria against patho-
logically confirmed cases show variable results depending on the cases in-
cluded in the study. In "typical" cases, the agreement between probable and
definite AD has been shown to be 100% (Martin
et al.,
1987; Morris
et al.,
1988). However, in unselected cases, accuracies of 68-76% and 88% for
probable AD (Burns
et al.,
1990; Risse
et al.,
1990) and 78% for possible
AD (Burns
et al.,
1990) were found. Thus, the NINCDS-ADRDA protocol
for the diagnosis of AD has been well-validated within and across centers
as correlating with the CERAD plaque-based pathological criteria. However,
because these studies would have included cases with DLB and possibly other
pathologies, reevaluation of the accuracy of these criteria is required.
In addition to the NINCDS-ADRDA criteria for the clinical diagnosis of
AD, a number of other diagnostic protocols for use in clinical and popu-
lation settings have been developed. The
Diagnostic and Statistical Manual
of Mental Disorders, Fourth Edition (DSM-IV)
of the American Psychiatric
Association (APA; World Health Organization, 1992) criteria require a
ALZHEIMER'S DISEASE 175
deficit in memory, as well as one other cognitive domain of gradual onset
and progressive decline. These criteria result in similar classification of pa-
tients to the NINCDS-ADRDA criteria (see above). In addition, the Clinical
Dementia Rating (CDR; McCulla
et al.,
1989; Morris, 1993) and Mini-Mental
State Examination (MMSE; Folstein
et al.,
1975) are used to determine the
severity of dementia. These protocols are useful for screening for cognitive
impairment as they are easy to administer and have been validated across
a variety of social and ethnic populations. However, they lack specificity for
AD. Pathological validation of these protocols has been performed, but as
with other criteria, reevaluation is necessary in light of the changing na-
ture of dementia diagnosis. It would be of interest for those centers with
large clinical and pathological databases to reevaluate the clinical diagnosis
of AD and similar dementia syndromes using the currently recommended
neuropathological criteria for AD. This may help define better diagnostic
tools that can then be evaluated longitudinally.
D.
REPRODUCIBILITY OF CURRENT CLINICAL DIAGNOSTIC PROTOCOLS
Several studies to test the reliability of the clinical criteria for AD have
been performed. Both Lopez and colleagues (1990) and Kukall and col-
leagues (1990) used four raters to evaluate the NINCDS-ADRDA criteria.
Using cases with dementia (AD and non-AD) and nondemented controls,
percentage agreement ranged from 55% to 75% for pairs of raters (kappa
coefficients were 0.36-0.65) with the most experienced clinicians achiev-
ing the greatest agreement (Lopez
et al.,
1990). Interestingly, many of the
disagreements in diagnosis were found between possible and probable AD
categories. Although there is little disagreement on whether patients have
dementia, the underlying causes of the dementia syndrome are less reli-
ably agreed upon between clinicians. The inclusion of cases with multiple
pathologies using the current criteria probably contributes to this variability.
E. SUMMARY
Considerably more research is required on the diagnosis and definition
of AD. The current recommended "gold standard" has yet to be widely
validated, and protocols for overlapping pathologies need to be incorpor-
ated. This will, of course, have an impact on the clinical diagnosis of AD.
At present, clinical criteria for AD cannot differentiate patients with differ-
ent underlying disease mechanisms (e.g., DLB versus AD). In addition, it
will be important to determine the clinical profile of cases that have both
176
JILLIAN J. KRIL AND GLENDA M. HALLIDAY
NFTs and Aft. Difficulties with the definition of the disease have important
implications for any study of AD. From the point of view of researching the
pathogenesis of AD, pure groups are desired to eliminate potential con-
founding causes. The continual modification and improvement of criteria
for the diagnosis of AD is therefore necessary until we understand, and
either prevent or cure, this illness.
IIh Pathogenesis
To understand the pathogenesis of AD, it is necessary to determine the
sequence of events that occurs over the life of a patient. Because it is not
possible to perform longitudinal cellular analyses in humans, most of our
understanding of the pathogenesis of AD is inferred from patients sampled
cross-sectionally at different time points in the disease process. Information
concerning the initial events is the most patchy as it is extremely difficult to
determine, with accuracy, when the disease first begins. The single greatest
risk factor for the development of AD is age (Jorm, 1990). However, we do
not yet fully understand the normal aging process; thus, it is difficult to distin-
guish the pathological process (es) underlying AD. We do know that demen-
tia in old age isfar from a universal phenomenon and that other factors must
play a role in determining susceptibility to disease. These factors include ge-
netic, environmental, and lifestyle factors, as well as coexisting disease.
Although a decline in brain function with age is accepted as normal by
many authors, increasing evidence suggests cognitive decline is not an in-
evitable consequence of aging (Rubin
et al.,
1998; Morris, 1999; Unger
et al.,
1999) but rather a manifestation of underlying disease processes. Longitudi-
nal studies of community-dwelling elderly subjects do not find a decrease in
cognitive performance with advancing age (Rubin
et al.,
1998; Morris, 1999).
Interestingly, those who do develop dementia may have a long preclinical
period with stable deficits (usually memory), which precedes a precipitous
decrease in function (Rubin
et al.,
1998; Small
et al.,
2000). Such studies call
into question the idea of an age-related decline in brain function and more
likely represent cohort differences in health, education, and other factors.
Nevertheless, many of these studies are performed on highly selected groups
of elderly subjects who are free from neurological and systemic diseases, and
although adequately addressing the question of age-associated cognitive de-
cline, do not represent the majority of elderly subjects. Cognitive deficits
may be present in a proportion of elderly subjects, although these would be
expected to have greater brain pathology.
Numerous studies have shown an increased risk of AD in subjects with low
education levels (primary school level or around 6 or less years of schooling)