ALZHEIMER''''S DISEASE: ITS DIAGNOSIS AND PATHOGENESIS - PART 2 doc
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (2.14 MB, 10 trang )
ALZHEIMER'S DISEASE 177
as compared with subjects with higher education levels. The level of in-
creased risk varies between studies (Katzman, 1993; The Canadian Study of
Health and Aging Study Center, 1994; Stern
et al.,
1994; Letenneur
et al.,
1999; Hall
et al.,
2000) but is generally found to be between 1.5 and 2 times
that of the higher-educated reference groups. Nevertheless, the finding of
an association between education and AD is by no means universal because
a number of studies have found no relationship (Beard
et al.,
1992; Cobb
et al.,
1995). In particular, no association between autopsy-confirmed AD
and either education or occupation was found in a study of 115 patients
with AD, although the authors suggest this may reflect different attitudes to
consent to autopsy among groups with different education levels (Munoz
et al.,
2000).
The mechanism that links low educational attainment and AD is unclear.
Some authors suggested education provides increased functional capacity or
"brain reserve," which requires the brain to undergo a greater period of de-
generation before the critical threshold for dementia is reached. Conversely,
low education may reflect other factors such as lower socioeconomic status,
increased likelihood of exposure to adverse events, or childhood deprivation
(Hall
et al.,
2000). This latter hypothesis, referred to as "brain battering,"
proposes that subjects with higher education have higher socioeconomic
status and enjoy healthier lives with fewer coexisting brain diseases (Del Ser
et al.,
1999). This hypothesis is supported by the work of Del Ser and col-
leagues (1999), who, in an autopsy study, found patients with low education
had more cerebrovascular disease than those with a high level of education.
Gaining a better understanding of this association between low education
and AD is of great importance because education is a modifiable factor and,
unlike increasing age or genotype, amenable to intervention and possible
correction.
A. BRAIN ATROPHY
It is well established that the brains of older individuals are, on average,
smaller than their younger counterparts (Dekaban, 1978). Although this
may be interpreted as a loss of brain tissue with age, it may also represent
cohort differences in body size as a result of improvements in nutrition and
health standards (Miller and Corsellis, 1977). Cross-sectional
in viva
studies
have demonstrated atrophy of all brain compartments (Murphy
et al.,
1996;
Yue
et aL,
1997), prefrontal grey matter (Raz
et al.,
1997), and hippocampus
(Convit
et aL,
1995). In several
in vivo
studies, age-associated atrophy was
found to be greater in men than in women (Matsumae
et al.,
1996; Murphy
et al.,
1996; Yue
et al.,
1997; Coffey
et al.,
1998). However, postmortem analy-
sis of normal subjects ages 46 to 92 years find no decrease in cortical volume,
178 JILLIAN J. KRIL AND GLENDA M. HALLIDAY
but significant white matter atrophy (Double
et al.,
1996), suggesting that
marked loss of cortical neurons is not a feature of normal aging. This is sup-
ported by studies in older primates (Peters
et al.,
1996) and by longitudinal
MRI studies (Mueller
et al.,
1998; Fox
et al.,
2000). Because brain atrophy oc-
curs in a number of conditions other than neurodegenerative disease (Kril
and Halliday, 1999) and factors such as hypertension, smoking, and high
alcohol consumption contribute to atrophy (Akiyama
et al.,
1997), rigorous
exclusion criteria are necessary in cross-sectional samples investigating true
age-related changes. A loss of cerebral white matter may underlie the slow-
ing of mental processing identified in many elderly subjects (Howieson
et al.,
1993; Ylikoski
et al.,
1993). Overall, the data are consistent with the clinical
finding that, at least in a proportion of the elderly, there is no substantial
deficit over time.
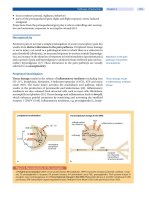
In contrast to normal aging, cross-sectional studies show that there is
marked cortical atrophy in AD (Fig. 2) and that the degree of atrophy cor-
relates with the severity of dementia (Double
et al.,
1996; Mouton
et al.,
1998; Regeur, 2000). Atrophy in AD is most severe in the temporal lobe,
particularly in the medial temporal lobe (Double
et al.,
1996; Convit
et al.,
1997; Detoledo-Morrell
et al.,
1997; Jack
et al.,
1998; Frisoni
et al.,
1999;
Visser
et al.,
1999). More important, longitudinal analyses of brain volume
confirmed that marked temporal lobe atrophy distinguishes AD (Fox
et al.,
1996, 2000; Smith and Jobst, 1996; Kaye
et al.,
1997; Yamada
et al.,
1998)
from the relatively constant brain volumes during healthy aging (Shear
et al.,
1995; Mueller
et al.,
1998). The greatly accelerated atrophy of the
temporal neocortex, not the hippocampus, in AD patients is associated with
the symptomatic onset of dementia (Fox
et al.,
1996, 2000; Smith andJobst,
1996; Convit
et al.,
1997, 2000; Detoledo-Morrell
et al.,
1997; Kaye
et al.,
1997;
Juottonen
et al.,
1998a, 1998b; Yamada
et al.,
1998), whereas atrophy of the
hippocampus occurs 1 to 2 years before dementia onset (Fox
et al.,
1996;
Convit
et al.,
1997). These data show that significant cortical atrophy occurs
in AD and distinguishes it from normal aging. The degeneration begins in
the hippocampus and spreads to involve first the temporal lobe and then
other cortical association areas (Fig. 2).
B. NEURONAL LOSS
Controversy exists over whether neuronal loss is a normal consequence
of aging or is only related to disease processes. Many earlier studies using
measures of neuronal density found widespread degeneration in older sub-
jects (Brody, 1955; Henderson
et al.,
1980; Anderson
et al.,
1983; Terry
et al.,
1987), although this finding was not universal (Haug and Eggers, 1991).
ALZHEIMER'S DISEASE 179
Hippocampal
atro )hy
AD diagnosis
~
Hippocampal and
temporal atrophy
, Global atrophy
l
(end-stage)
~t
i
/
FIG. 2. At autopsy, AD is characterized macroscopically by generalized atrophy of the cere-
bral hemispheres (left panel), which results in widening of the sucli (upper), ventricular dilata-
tion (V, lower), and atrophy of the hippocampal formation, causing dilatation of the temporal
horn (TH, lower) of the lateral ventricles. Atrophy of the hippocampal formation can be de-
tected in susceptible patients prior to the diagnosis of dementia (right upper). The atrophy
progresses to involve the adjacent temporal lobe (right center) and then, uhimately, spreads
to involve most regions of the brain (right lower).
The introduction of unbiased quantitative techniques has revolutionized
quantitative neuropathology; however, in many instances, there is still un-
certainty as to whether neuronal loss with aging occurs. Pakkenberg and
Gundersen (1997) found a 10% decline in total estimated neuron number
between 20 and 90 years of age. This study was performed on samples from
the entire neocortex, regardless of anatomical or functional location, but
has yet to be confirmed by others. Interestingly, they also demonstrated a
large (16%) difference in neuron number with gender, which is not as a
result of differences in body height.
Studies in which specific functional regions of the brain have been exam-
ined using unbiased techniques have reported variable results with regard
180 JILLIAN J. KRIL AND GLENDA M. HALLIDAY
to an age-associated loss of neurons. No loss of neurons was found in the su-
perior temporal (Gomez-Isla
et al.,
1997) or entorhinal cortices (Gomez-Isla
et al.,
1996) of nondemented controls between the sixth and ninth decades,
or from the locus coeruleus (Ohm
et al.,
1997). In the hippocampal for-
mation, a loss of CA1 (West and Gundersen, 1990; Simic
et al.,
1997), CA4
(West
et al.,
1994), and subicular (West, 1993; West
et al.,
1994) neurons was
reported. However, this is in contrast to the finding that the apparent reduc-
tion in CA1 neuron number with age can be accounted for by differences in
cerebrum volume between younger and older adults (Harding
et al.,
1998).
This relationship between premorbid brain size and hippocampal neuron
number highlights some of the difficulties with cross-sectional cohort stud-
ies and suggests multiple factors need to be analyzed to determine potential
cause and effect.
The most consistent finding in AD is substantial neuronal loss from
the entorhinal cortex and hippocampus (Fig. 3). This reflects the pattern
of neurofibrillary pathology, which is a cardinal feature of AD and ap-
pears to occur very early in the disease process (Braak and Braak, 1997).
A 32% loss of neurons from the entorhinal cortex was found in AD patients
Control AD
CA1
Ch4
FIG. 3. Marked neuronal loss and NFT formation is seen in AD (right panels) compared
with controls (left panels) in both the CA1 region of the hippocampus (upper panels) and
cholinergic basal forebrain (Ch4, lower panels). Eventually, neuronal loss exceeds NFT forma-
tion in the hippocampus but is equivalent in the basal forebrain. Nickel peroxidase with cresyl
violet counterstain.
ALZHEIMER'S DISEASE 181
with a CDR score of 0.5, whereas a 48% loss was found in all AD patients
(Gomez-Isla
et al.,
1996). When specific laminae were examined, the loss
was more dramatic with a 60% loss of layer II neurons in mild AD and a 90%
loss in severe AD (CDR = 3; Gomez-Isla
et al.,
1996). Marked neuronal loss
from the hippocampus has also been described. Simic and colleagues (1997)
found a 23% loss of neurons from the dentate gyrus and subiculum, whereas
West and colleagues (1994) found a 25% loss from the CA4, 47% from the
subiculum, and 68% from the CA1. The dramatic loss of neurons from the
CA1 and subiculum has been confirmed in other studies (Bobinski
et al.,
1995) and has been found to occur early in the disease process. Thus, the
early atrophy noted clinically in medial temporal lobe structures (see above)
is a result of marked neuronal loss in this region (Bobinski
et al.,
2000).
Other consistently affected regions in AD are the cholinergic nucleus
basalis (Vogels
et al.,
1990; Cullen
et al.,
1997; Fig. 3), the serotoninergic
raphe nuclei (Aletrino
et al.,
1992; Halliday
et al.,
1992), and the noradren-
ergic locus coeruleus (Busch
et al.,
1997). These subcortical nuclei innervate
cortical pyramidal neurons, capillaries, and arterioles, and play an impor-
tant role in cortical synaptic neurotransmission and the neurogenic control
of blood flow through the capillary bed. The early loss of cortical choliner-
gic transmission is believed to lead to hyperactivity of acetylcholinesterase
and a loss of cholinergic neurogenic control, thus significantly contributing
to the cognitive deterioration seen in AD (Bartus
et al.,
1982; Francis
et al.,
1999; Tong and Hamel, 1999). Hyperactivity of acetylcholinesterase under-
lies the currently recommended treatments for AD, which use cholinesterase
inhibitors such as tacrine, donepezil, or rivastigmine (Francis
et al.,
1999;
Ladner and Lee, 1998). Despite mixed success with such treatments, there
is a great deal of evidence supporting the cholinergic hypothesis of AD.
Choline acetyltransferase levels were found to correlate with cognitive im-
pairment in AD (Baskin
et al.,
1999), whereas degeneration in cholinergic
basal forebrain neurons correlates with MMSE score (Iraizoz
et al.,
1999),
cortical atrophy (Cullen
et al.,
1997), the stage of cortical pathology (Cullen
and Halliday, 1998; Iraizoz
et al.,
1999; Beach
et al.,
2000), and the ear-
liest depositions of A/~ (Beach
et al.,
2000). A/3 potently inhibits various
cholinergic neurotransmitter functions (Auld
et al.,
1998) by killing corti-
cally projecting cholinergic neurons (Harkany
et al.,
2000). Furthermore,
cortical cholinergic denervation elicits vascular Aft deposition (Roher
et al.,
2000), suggesting a link between Aft deposition, small vessel disease, and
cholinergic cell loss in AD. In addition, it has been shown that the action of
tacrine is through improving cerebral blood flow rather than due its effects
on neuronal cholinergic neurotransmission (Peruzzi
et al.,
2000).
Although cortical atrophy is a consistent feature of AD (see above),
whether this atrophy represents neuronal loss is not universally agreed upon.
182 JILLIAN j. KRIL AND GLENDA M. HALLIDAY
Earlier studies of neuron density found a widespread and marked loss of neu-
rons in AD (Colon, 1973; Shefer, 1973; Ball, 1977). However, using unbiased
techniques, Reguer and colleagues (1994) found no overall loss of cortical
neurons in AD. This study, which was conducted on entire lobes of the brain,
generated much debate (see commentaries in
Neurobiology of Aging
(1994)
15(3) :353-380), the consensus of which was that regional and population
differences do exist in AD and that they were masked by the quantitative
technique used. Using unbiased techniques, total neuron number was found
to decrease by 53% in the superior temporal gyrus (Gomez-Isla
et al.,
1997)
and 30% in visual areas 17 and 18 (Leuba and Kraftsik, 1994). In addi-
tion, a study described the loss of microcolumnar ensemble organization
in AD (Buldyrev
et al.,
2000), although the relationship between neuronal
patterning and cell loss remains to be determined. A considerable amount
of research is still required to evaluate the specificity of the disease process
for cortical regions and neuron type, and to correlate these findings with
atrophy, clinical indices, and the temporal sequence of events. As long as
research remains concentrated on individual brain regions affected by AD,
the entire disease process will not be fully understood.
C Aft DEPOSITION
Aft is a hydrophobic peptide, 39-43 residues long, which tends to form
insoluble aggregates. There has been considerable debate about the toxicity
of this peptide, with its neurotoxic activity believed to depend on its abil-
ity to form fibrils (Haas, 1996; Neve and Robakis, 1998; Storey and Cappai,
1999; Wilson
et al.,
1999; Coughlan and Breen, 2000; Gandy and Petanceska,
2000). The peptide is derived by the proteolytic processing of its high molec-
ular weight precursor, the amyloid precursor protein (APP). APP is a trans-
membrane protein with a small C-terminal cytoplasmic domain, one trans-
membrane domain, and a large N-terminal extracellular domain (Haas,
1996; Neve and Robakis, 1998; Storey and Cappai, 1999; Wilson
et al.,
1999;
Coughlan and Breen, 2000; Gandy and Petanceska, 2000). The Aft domain
is partially embedded within the phospholipid bilayer.
APP is cleaved via two proteolytic pathways, with only one pathway gener-
ating Aft peptide (Haas, 1996; Neve and Robakis, 1998; Storey and Cappai,
1999; Wilson
et al.,
1999; Coughlan and Breen, 2000; Gandy and Petanceska,
2000). During transport to the cell surface, APP is cleaved at the membrane
by an unknown protease called 0t-secretase into its soluble extracellular
domain (sAPP) and a membrane-bound 10-kD C-terminal fragment. The
membrane-bound fragment is further processed by, the as yet unidentified,
y-secretase at the C-terminal end of the Aft domain into a small rapidly
ALZHEIMER'S DISEASE 183
released peptide called p3. This pathway is the major processing pathway
for APP and does not involve the production of Aft. p3 is found in abun-
dance in the plaques associated with aging (Dickson, 1997). Uncleaved APP
that is reinternalized is processed in the endosome/lysosome system by two
hypothetical enzymes called/3- and y-secretases./3-secretase cleaves APP at
the N-terminus of the A/3 domain, creating a 12-kD intermediate peptide,
which recycles back to the cell surface, y-Secretase (s) cleave this intermedi-
ate peptide at the C-terminal end of the A/3 domain, releasing A/3 into the
extracellular space.
y-Secretase cleavage occurs at one of two main sites producing mainly
A/31-39/40 or sometimes A/31-42/43 (Haas, 1996; Neve and Robakis, 1998;
Storey and Cappai, 1999; Wilson
et al.,
1999; Coughlan and Breen, 2000;
Gandy and Petanceska, 2000). These peptides concentrate in the plaques
found in AD (Iwatsubo
et al.,
1996; Dickson, 1997), although there is a
general age-related increase in A/3 generation by neural cells (Turner
et al.,
1996), with the longer A/3 peptide being more amyloidogenic. The develop-
ment of specific antisera for A/31-40 and A/31-42/43 has enabled the evo-
lution and composition of plaques to be systematically studied (Iwatsubo
et al.,
1996; Dickson, 1997). The results suggest that A/31-42/43 initially
forms the nucleus of a plaque, enabling the subsequent deposition of the
more soluble Afll-40 and other protein fragments. This is consistent with
the identification of mainly A/31-42/43 in plaque cores of both demented
and nondemented individuals (Fukumoto
et al.,
1996). Evidence suggests
that protofibrils of A/3 may also be toxic and that fibril formation is concen-
tration dependent (Hartley
et al.,
1999), with A/3 peptides changing from
soluble forms in control brain to insoluble forms in AD brain (Wang
et al.,
1999).
Although we know a lot about the production of A/3, we know much less
about its clearance from brain tissue. Evidence suggests that A/3 deposition is
regulated by a specific protease that degrades extracellular A/3 (Iwata
et al.,
2000). Infusions of the protease inhibitor thiorphan into rat brain cause
extracellular deposits of endogenous A/3 as diffuse plaques. The enzyme
responsible for the clearance of Aft peptides is neutral endoprotease or
neprilysin (enkephalinase; Iwata
et al.,
2000). Cross-sectional analysis of cases
at different stages of AD suggests the A/3 plaque deposition occurs only
early in AD with resorption surpassing deposition at end-stage disease (Thal
et al.,
1998). This suggests that A/3 clearance mechanisms are largely intact
throughout the disease process and that the disease starts with early excessive
A/3 production and deposition.
Cross-sectional studies suggest the progressive deposition of A/3 in the
brain and microvasculature appears to precede the onset of dementia by
many years. Examination of a large unselected autopsy series shows a small
184 JILLIAN J. KRIL AND GLENDA M. HALLIDAY
proportion of people in their 40s begin to deposit Aft plaques in the basal
cortex (Braak and Braak, 1997). Few people at these ages have dementia,
and the low frequency of pathology is believed to represent very early "pre-
clinical" disease. By the age of 74, 50 % of the population will have Aft plaque
deposits (Duyckaerts and Hauw, 1997), although few people will have overt
dementia at this age (Jorm, 1990). At these and older ages, a subset of cog-
nitively intact individuals have extensive neocortical Aft plaque deposition
(Price and Morris, 1999), reinforcing the concept of "preclinical" disease.
Furthermore, an accumulation of AD-type pathology was shown to nega-
tively correlate with the change in MMSE score in nondemented subjects,
indicating that burden of pathology does reflect functional performance
(Morris
et al.,
1996; Green
et al.,
2000) and thus may represent "preclinical"
AD. However, the concept that normal aging is synonymous with preclini-
cal AD, which then proceeds to clinical AD, requires close scrutiny prior to
being universally accepted.
Several sets of data are difficult to reconcile with this model ofa contiuum
between aging and AD. NFTs are present in all autopsy samples from people
ages 91-95 years, whereas approximately 20% of these subjects are free from
plaques (Braak and Braak, 1997). This suggests that Aft plaque accumulation
may not be an inevitable component of aging. Alternatively, as discussed
above, plaque-dominant AD has been proposed as a developmental stage
of the disease only (Berg
et al.,
1998; Thal
et al.,
1998), with longitudinal
data of cerebrospinal fluid showing changes in Aft levels are greatest within
the first 2 years of diagnosis (Tapiola
et al.,
2000). Although much research
has concentrated on determining the cellular biology of Aft production,
there is only limited information on the relationship between Aft deposition
and measures of degeneration. Large cross-sectional studies incorporating
volumetric, neuronal, Aft deposition, and functional indices are necessary
to determine the time sequence and relationship between these measures,
particularly the role that Aft may play in the neurodegeneration of AD.
D. NYI" FORMATION
NFTs were first identified by Alzheimer in 1907. They consist of paired
helical filaments of the microtubule-associated protein tau. In the normal
brain, tau is bound to axonal microtubules where it stabilizes the micro-
tubles, promotes their assembly, and allows fast axonal transport to occur
(Goedert
et al.,
1991). In AD, tau becomes hyperphosphorylated and no
longer binds to the microtubules, impairing their stability, and consequently
impairing much of the normal function of the neuron. The hyperphosphor-
ylated tau aggregates into paired helical filaments and ultimately NFTs. The
ALZHEIMER'S DISEASE 185
gene for tau is on chromosome 17 and contains 15 exons. Mternative splic-
ing of these leads to six isoforms of tau, ranging from 352 to 441 amino
acids and with either three or four tandem repeats at the C-terminus end
(Goedert
et aL,
1991; Tolnay and Probst, 1999). In normal brain, three and
four repeat tau is expressed in approximately equal amounts, and these
same isoforms are present, in a hyperphosphorylated form, in AD (Tolnay
and Probst, 1999).
NFTs progressively accumulate in the cell body and processes of neu-
rons until the cell dies (Bancher
et al.,
1989; Braak
et al.,
1994). The earli-
est feature of NFT formation is the accumulation of hyperphosphorylated
tau, which aggregates into insoluble granules (Bancher
et al.,
1989). This
is called the "pretangle" stage and precedes the formation of the classi-
cal fibrillar NFTs ("mature tangles"). Once the neuron dies, the largely
insoluble NET remains in the neuropil as a "ghost" or "tombstone" tan-
gle (Bondareff
et al.,
1994). The time taken for an NFT to form and ma-
ture is unknown. Several estimates have been made based on extrapolation
from relationships with disease duration. Bobinski and colleagues (1998)
calculated it takes 3.4 years in the CA1 and 5.4 years in the subiculum
for a mature NFT to become a ghost tangle. This, together with the find-
ing of Morsch and colleagues (1999) that CA1 neurons with NFTs can
survive for 15-25 years, suggests that NFTs are slow to develop and that
the onset of pathology is many decades before the onset of clinical dis-
ease. This hypothesis is supported by the findings that the calculated time
taken to progress from NFT stage I to 1V is nearly 50 years (Ohm
et al.,
1995) and that lower scores on neuropsychological testing can be found as
much as 10 years prior to onset of dementia (Elias
et al.,
2000; Small
et al.,
2OOO).
NFTs and other abnormalities of tau are not unique to AD. Several other
neurodegenerative diseases such as Down syndrome, progressive supranu-
clear palsy, corticobasal degeneration, and parkinsonism-dementia com-
plex of Guam and Pick disease also have tau-positive inclusions (Tolnay
and Probst, 1999). This has led to the collective name of tauopathies, and
much effort has been expended to understand the commonality of these
disorders. To date, a number of differences were found in the cellular pop-
ulations affected and the tau isoforms expressed (Brion, 1998). However,
similarities in types of tau deposited and clinical expression of the diseases
were also described.
Cross-sectional studies suggest that progressive NFT formation in the
brain precedes the onset of dementia by many years. Examination of a large
unselected autopsy series shows that a small proportion of people in their
20s begin to form NFT in the entorhinal cortex (Braak and Braak, 1997).
Few people at these ages have dementia and the low frequency of pathology
186 JILLIAN J. KRIL AND GLENDA M. HALLIDAY
is not believed to affect cognitive function. By the age of 47, 50% of the
population will have NFTs (Duyckaerts and Hauw, 1997), although few peo-
ple will have overt dementia at this age (Jorm, 1990). As mentioned above,
all subjects ages 91-95 years have NFT formation (Braak and Braak, 1997),
and although dementia is more prevalent at these ages, it is not inevitable
(Jorm, 1990). By 86 years of age, 50% of the population have sufficient accu-
mulation of NFTs to suspect a pathological diagnosis of AD, particularly in
the presence of Aft plaques (Duyckaerts and Hauw, 1997). At the age of 86
and older, approximately 20% of people meet NFT criteria for AD (Braak
and Braak, 1997). This is consistent with the prevalence of clinical AD at
these ages (Jorm, 1990).
In contrast to the Aft deposits, NFTs accumulate in regions of neuron
loss (Braak and Braak, 1997; Cullen and Halliday, 1998; Duyckaerts
et al.,
1998; Iraizoz
et al.,
1999), and their accumulation correlates with measures of
functional decline (McKee
et al.,
1991; Arriagada
et al.,
1992; Bancher
et al.,
1993; Grober
et al.,
1999) and the degree of hippocampal atrophy (Bobinski
et al.,
1995; Nagy
et al.,
1996, 1999; Smith andJobst, 1996). However, as de-
scribed above, NFTs take many years to evolve and, therefore, the temporal
relationship between the formation of NFTs and the rapid neuronal loss
and brain atrophy in AD is difficult to reconcile. In addition, as dementia
is present only when NFTs occur in the neocortex and the extent of neo-
cortical neuron loss is unclear in AD (see above), the association between
this cortical degeneration and NFT and Aft deposition needs to be further
examined.
E.
MECHANISMS OF DEGENERATION
Studying the mechanism(s) of neuronal death in AD is difficult because
of the extended interval between the onset of symptoms and associated cell
death, and investigation at autopsy. NFT formation is considered to be the
major cause of neuron death in AD (Fig. 4), and cells dying as a result of
NFT formation can be identified by the presence of ghost NFTs. However,
reports show NFTs are not responsible for all the neuron loss seen in AD.
Studies on the temporal (Gomez-Isla
et al.,
1997) and occipital (Leuba and
Kraftsik, 1994) cortices, and hippocampus (Kril
et al.,
2000) have shown
that neuronal loss exceeds the degree of NFT formation. This is in contrast
to studies of the cholinergic basal forebrain in AD (Cullen and Halliday,
1998) and the parkinsonism-dementia complex of Guam (Schwab
et al.,
1998, 1999), where NFT formation does account for all the neuron loss.
In the CA1 region of the hippocampus, NFTs were found to account for
less than 20% of the neuron loss (Kril
et al.,
2000) suggesting that another