LASIK Fundamentals, Surgical Techniques, and Complications - part 1 docx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (916.12 KB, 52 trang )
Marcel Dekker, Inc. New York
•
Basel
LASIK
Fundamentals, Surgical Techniques,
and Complications
edited by
Dimitri T. Azar
Massachusetts Eye and Ear Infirmary
Schepens Eye Research Institute
and Harvard Medical School
Boston, Massachusetts, U.S.A.
Douglas D. Koch
Cullen Eye Institute
Baylor College of Medicine
Houston, Texas, U.S.A.
Copyright © 2002 by Marcel Dekker, Inc. All Rights Reserved.
Library of Congress Cataloging-in-Publication Data
A catalog record for this book is available from the Library of Congress.
ISBN: 0-8247-0797-4
This book is printed on acid-free paper.
Headquarters
Marcel Dekker, Inc.
270 Madison Avenue, New York, NY 10016
tel: 212-696-9000; fax: 212-685-4540
Eastern Hemisphere Distribution
Marcel Dekker AG
Hutgasse 4, Postfach 812, CH-4001 Basel, Switzerland
tel: 41-61-260-6300; fax: 41-61-260-6333
World Wide Web
The publisher offers discounts on this book when ordered in bulk quantities. For more information,
write to Special Sales/Professional Marketing at the headquarters address above.
Copyright © 2003 by Marcel Dekker, Inc. All Rights Reserved.
Neither this book nor any part may be reproduced or transmitted in any form or by any means, elec-
tronic or mechanical, including photocopying, microfilming, and recording, or by any information
storage and retrieval system, without permission in writing from the publisher.
Current printing (last digit):
10987654321
PRINTED IN THE UNITED STATES OF AMERICA
To my wife, Marcia, who makes it all so much more meaningful—and fun.
DDK
To Nathalie, Alexander, Nicholas, and Lara—for all the joyful moments that we share.
DTA
Preface
Dov’é mio figlio? . . . più non lo vedo:
In te più Alfredo–trovar non so
For decades, the majority of ophthalmologists have been embarrassed by and highly
suspicious of refractive surgery, at times with good justification. They have been repelled
by its tactics in patient recruitment, uneasy about its seemingly cavalier use without long-
term data, eager to defend its unsuspecting victims, and deeply concerned that its short-
term benefits are outweighed by its burdensome long-term consequences. And so the words
of Germont, uttered pursuant to the most dramatic moment in Verdi’s La Traviata, echo the
extreme and oftentimes passionate contempt expressed by so many colleagues and close
friends towards academically oriented ophthalmologists who marched among the van-
guards of laser refractive surgeons. Their repudiation was not unlike Germont’s scorn of
his son Alfredo for offending Violetta and for making himself worthy of disdain:
Where is my son? . . . no more do I see him.
I am unable to see Alfredo in you.
The ophthalmological peer-reviewed publications and textbooks prior to the late
1980s reflected this disinterest in or hostility toward refractive surgery. Unfortunately, in
the pre-LASIK era, there were relatively few high-quality peer-reviewed reports on refrac-
tive surgery. Criticisms of the scientific rigor with which clinical studies were conducted
were often justified, which, in turn, discouraged academically bound graduates of ophthal-
mology training programs from dedicating their careers to this subspecialty. Comprehen-
sive textbooks of ophthalmology in the pre-LASIK era also kept refractive surgery at arm’s
length, relegating it to a minor chapter on the topic. Refractive surgery was viewed as an
outlier of great potential but little practical merit in mainstream ophthalmology. But this
has all changed with the advent of LASIK!
This book is the first in a series dedicated to Refractive Surgery by Marcel Dekker,
Inc. It will most certainly be judged by many as just another LASIK book. While there is
abundant coverage of the topic in other books, this volume has several unique features. Its
coverage of LASIK is relatively comprehensive, in that it is not limited to LASIK history,
surgical techniques, complications, and their management. New aspects of lasers, optics,
refraction, diagnostics, and instrumentation are combined with the science and general
principles of LASIK, and indications for its use. Although not meant to be encyclopedic,
key references abound. They are intended to serve as a guide to the literature on the topic.
Thus, this book is not so much a chronicle of LASIK, as an attempt to serve as a source of
information relevant to clinical practice.
We are indebted to the students, residents, and colleagues who have made valuable
contributions to this book. Several have included original work and analysis in their chap-
ters. It is evident that the authors have attended diligently to their assignments. We are
grateful for their effort in integrating the sometimes limited information in peer-reviewed
literature with the knowledge derived from their clinical experiences and interactions with
colleagues. We hope that this has resulted in a text that is both clinically relevant and as ev-
idence-based as possible.
We thank Dr. Geoffrey Greenwood and Elizabeth Curione of Marcel Dekker, Inc.,
for their commitment to this project and Drs. Tsubota, Boxer Wachler, Hoang-Xuan, Ang,
and Gatinel for their assistance in future books in this series. Special thanks go to Leona
Greenhill, for her editorial assistance, and to Rhonda Harris, who managed this project with
care and precision. Her attention to detail and her dedication have enabled us to work co-
herently in the face of adversity.
We take the opportunity to acknowledge the pioneering surgeons and researchers in
the field of refractive surgery. Their work and vision have provided the basis not only for
current refractive developments that we can offer to our patients, but also for future ad-
vances to be made by the next generation of thoughtful contributors to this important field.
Dimitri T. Azar
Douglas D. Koch
vi Preface
vii
Contents
Preface v
Contributors xi
1. Refractive Errors and Their Treatment 1
Liane Clamen Glazer and Dimitri T. Azar
2. History of LASIK 21
Ioannis Pallikaris and Thekla Papadaki
3. Lasers in LASIK: Basic Aspects 39
Rodrigo Torres, Robert T. Ang, and Dimitri T. Azar
4. Microkeratomes 57
Sandeep Kakaria, Thanh Hoang-Xuan, and Dimitri T. Azar
5. Adjunctive Instrumentation in LASIK 71
Robert T. Ang and Dimitri T. Azar
6. LASIK Indications, Contraindications, and Preoperative Evaluation 91
Richard E. Braunstein, Marc Winnick, and Kenneth A. Greenberg
7. Preoperative Optical Considerations in LASIK: Refractive Errors,
Monovision, and Contrast Sensitivity 101
Balamurali K. Ambati, Leon Strauss, and Dimitri T. Azar
8. Corneal Topography and LASIK Applications 111
Li Wang, Douglas D. Koch, Dimitri T. Azar, Robert T. Ang,
and Rengin Yildirim
9. Wavefront Technology and LASIK Applications 139
Naoyuki Maeda
10. Preoperative Considerations: Diagnosis, Classification,
and Avoidance of Keratoconus Complications 153
Paul Chung-Shien Lu and Dimitri T. Azar
11. Corneal Stability and Biomechanics After LASIK 163
Esen Karamursel Akpek, Rana Altan-Yaycioglu, and Walter J. Stark
12. LASIK Techniques 175
Dimitri T. Azar, Kathryn Colby, and Douglas D. Koch
13. Microkeratomes and Laser Settings 189
William J. Lahners and David R. Hardten
14. Centration of LASIK Procedures 199
Marsha C. Cheung, Chun Chen Chen, and Dimitri T. Azar
15. Surgical Caveats for Managing Difficult Intraoperative
Situations 229
Samir G. Farah and Dimitri T. Azar
16. Bilateral Simultaneous LASIK: Advantages, Disadvantages,
and Surgical Caveats 243
David R. Hardten, Elizabeth A. Davis, Richard L. Lindstrom,
and William J. Lahners
17. Postoperative Management Protocols for Uncomplicated
LASIK Procedures 255
Melanie A. R. Graham and Dimitri T. Azar
18. Visual Outcomes After Primary LASIK 265
Samir G. Farah and Dimitri T. Azar
19. Quality of Vision After LASIK 277
Patrick C. Yeh and Dimitri T. Azar
20. LASIK for Hyperopia, Hyperopic Astigmatism, and Presbyopia 285
Neal A. Sher
21. LASIK Retreatments 297
Ayman F. El-Shiaty and Brian S. Boxer Wachler
22. LASIK Following Radial Keratotomy and Photorefractive
Keratectomy 313
Natalie A. Afshari and Dimitri T. Azar
viii Contents
23. LASIK After Penetrating Keratoplasty 319
Glenn C. Cockerham and Natalie A. Afshari
24. Bioptics: Combined LASIK and Phakic Intraocular Lens Surgery 329
José L. Güell, Mercedes Vázquez, Fortino Velasco, and Felicidad Manero
25. LASIK and Intrastromal Corneal Ring Segments (ICRS) 335
Jonathan D. Primack, Samir G. Farah, and Dimitri T. Azar
26. Intraoperative Complications 351
Li Wang, Manjula Misra, and Douglas D. Koch
27. Postoperative Complications of LASIK 365
Samir G. Farah, Jae Bum Lee, and Dimitri T. Azar
28. Optical Aberrations After LASIK 387
Samir A. Melki, Cinthia E. Proano, and Dimitri T. Azar
29. Posterior Segment Complications of LASIK 397
Ron Afshari Adelman and Natalie A. Afshari
30. Management of Topographical Irregularities Following LASIK 403
Jeffrey Johnson, Roselyn Jeun, and Dimitri T. Azar
31. LASIK and TopoLink for Irregular Astigmatism 421
Michael C. Knorz
32. Management of Flap Complications in LASIK 431
Manolette R. Roque, Samir A. Melki, Dimitri T. Azar, and Emily Yeung
33. Management of Interlamellar Epithelium 463
Nan Wang and Douglas D. Koch
34. Management of Infections, Inflammation, and Lamellar
Keratitis After LASIK 477
Bilal F. Khan, Margaret Chang, Sandeep Jain, Kathryn Colby,
and Dimitri T. Azar
35. The Future of LASIK 491
Nan Wang and Douglas D. Koch
Index 495
Contents ix
Contributors
Ron Afshari Adelman, M.D. Massachusetts Eye and Ear Infirmary, Boston, Mas-
sachusetts, and Yale University Eye Center, New Haven, Connecticut, U.S.A.
Natalie A. Afshari Cornea Service, Duke University Eye Center, Durham, North Car-
olina, U.S.A.
Esen Karamursel Akpek, M.D. Cornea and External Disease Service, The Wilmer Eye
Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland, U.S.A.
Rana Altan-Yaycioglu, M.D. Cornea and External Disease Service, The Wilmer Eye In-
stitute, Johns Hopkins University School of Medicine, Baltimore, Maryland, U.S.A.
Balamurali K. Ambati, M.D. Massachusetts Eye and Ear Infirmary and Department of
Opthalmology, Harvard Medical School, Boston, Massachusetts, U.S.A.
Robert T. Ang, M.D. Cornea and Refractive Surgery Service, Massachusetts Eye and
Ear Infirmary and Harvard Medical School, Boston, Massachusetts, U.S.A., and Asian Eye
Institute, Makati, The Philippines
Dimitri T. Azar, M.D. Cornea and Refractive Surgery Service, Massachusetts Eye and
Ear Infirmary, Schepens Eye Research Institute, and Harvard Medical School, Boston,
Massachusetts, U.S.A.
Brian S. Boxer Wachler, M.D. Refractive Surgery Service, Jules Stein Eye Institute at
UCLA, Los Angeles, California, U.S.A.
Richard E. Braunstein, M.D. Department of Ophthalmology, Columbia University
College of Physicians and Surgeons, and Harkness Eye Institute, New York, New York,
U.S.A.
Margaret Chang Columbia University College of Physicians and Surgeons, New York,
New York, U.S.A.
Chun Chen Chen, M.D. Cornea and Refractive Surgery Service, Massachusetts Eye and
Ear Infirmary, Schepens Eye Research Institute, and Harvard Medical School, Boston,
Massachusetts, U.S.A.
Marsha C. Cheung Massachusetts Eye and Ear Infirmary and Harvard Medical School,
Boston, Massachusetts, U.S.A.
Glenn C. Cockerham, M.D. Department of Surgery, Allegheny Ophthalmology and Or-
bital Associates, Pittsburgh, Pennsylvania, U.S.A.
Kathryn Colby, M.D., Ph.D. Cornea and Refractive Surgery Service, Department of
Opthalmology, Massachusetts Eye and Ear Infirmary, Schepens Eye Research Institute,
and Harvard Medical School, Boston, Massachusetts, U.S.A.
Elizabeth A. Davis, M.D., Ph.D. Department of Ophthalmology, University of Min-
nesota, and Minnesota Eye Consultants, Minneapolis, Minnesota, U.S.A.
Ayman F. El-Shiaty, M.D. Department of Ophthalmology, Faculty of Medicine, Cairo
University, Cairo, Egypt, and Jules Stein Eye Institute at UCLA, Los Angeles, California,
U.S.A.
Samir G. Farah, M.D. Massachusetts Eye and Ear Infirmary, Boston, Massachusetts,
U.S.A.
Liane Clamen Glazer, M.D. Massachusetts Eye and Ear Infirmary and Department of
Ophthalmology, Harvard Medical School, Boston, Massachusetts, U.S.A.
Melanie A. R. Graham, M.D. Greater Baltimore Medical Center, Baltimore, Maryland,
U.S.A.
Kenneth A. Greenberg, M.D. Department of Ophthalmology, Columbia University
College of Physicians and Surgeons, New York, New York, U.S.A.
José L. Güell, M.D., Ph.D. Instituto de Microcirugía Ocular, Barcelona, Spain
David R. Hardten, M.D. Department of Ophthalmology, University of Minnesota, and
Department of Medicine, Minnesota Eye Consultants, Minneapolis, Minnesota, U.S.A.
Thanh Hoang-Xuan, M.D. Fondation Ophtalmologique Adolphe de Rothschild, and
Paris University, Paris, France
Sandeep Jain, M.D. Cornea and Refractive Surgery Service, Massachusetts Eye and Ear
Infirmary, Schepens Eye Research Institute, and Harvard Medical School, Boston, Mas-
sachusetts, U.S.A.
Roselyn Jeun, O.D. Massachusetts Eye and Ear Infirmary, Boston, Massachusetts,
U.S.A.
xii Contributors
Jeffrey Johnson, O.D. Massachusetts Eye and Ear Infirmary, and Department of Oph-
thalmology, Harvard Medical School, Boston, Massachusetts, U.S.A.
Sandeep Kakaria, M.D. Department of Ophthalmology, Cornell University Medical
Center, New York, New York, U.S.A.
Bilal F. Khan, M.D. Massachusetts Eye and Ear Infirmary and Department of Ophthal-
mology, Harvard Medical School, Boston, Massachusetts, U.S.A.
Michael C. Knorz, M.D. University of Heidelberg, Heidelberg, Germany, and Baylor
College of Medicine, Houston, Texas, U.S.A.
Douglas D. Koch, M.D. Department of Ophthalmology, Cullen Eye Institute, Baylor
College of Medicine, Houston, Texas, U.S.A.
William J. Lahners, M.D. University of South Florida, Tampa, and Center for Sight,
Sarasota, Florida, U.S.A.
Jae Bum Lee Cornea and Refractive Surgery Service, Massachusetts Eye and Ear Infir-
mary, Schepens Eye Research Institute, and Harvard Medical School, Boston, Mas-
sachusetts, U.S.A.
Richard L. Lindstrom, M.D. Department of Ophthalmology, University of Minnesota,
and Department of Medicine, Minnesota Eye Consultants, Minneapolis, Minnesota, U.S.A.
Paul Chung-Shien Lu, M.D. Department of Ophthalmology, Chang Gung Memorial
Hospital, Taipei, Taiwan, and Harvard Medical School, Boston, Massachusetts, U.S.A.
Naoyuki Maeda, M.D. Departments of Ophthalmology and Medical Robotics and Im-
age Sciences, Osaka University Medical School, Osaka, Japan
Felicidad Manero Instituto de Microcirugía Ocular, Barcelona, Spain
Samir A. Melki, M.D., Ph.D. Cornea and Refractive Surgery Service, Massachusetts
Eye and Ear Infirmary, and Boston Cornea Center, Harvard Medical School, Boston, Mas-
sachusetts, U.S.A.
Manjula Misra, M.D. Cullen Eye Institute, Baylor College of Medicine, Houston,
Texas, U.S.A.
Ioannis Pallikaris, M.D. Department of Ophthalmology, University of Crete Medical
School, and Ophthalmology Clinic, University Hospital of Heraklion, Heraklion, Crete,
Greece
Thekla Papadaki, M.D. Refractive Surgery Service, Vardinoyannion Eye Institute of
Crete, University of Crete Medical School, Heraklion, Crete, Greece
Jonathan D. Primack, M.D. Massachusetts Eye and Ear Infirmary, Boston, Mas-
sachusetts, U.S.A.
Contributors xiii
Cinthia E. Proano, M.D. Cornea and Refractive Surgery Service, Massachusetts Eye
and Ear Infirmary and Harvard Medical School, Boston, Massachusetts, U.S.A.
Manolette R. Roque, M.D. Massachusetts Eye and Ear Infirmary and Department of
Ophthalmology, Harvard Medical School, Boston, Massachusetts, U.S.A.
Neal A. Sher, M.D., F.A.C.S. Department of Ophthalmology, University of Minnesota
Medical School, and Department of Surgery, Phillips Eye Institute, Minneapolis, Min-
nesota, U.S.A.
Walter J. Stark, M.D. Cornea, Cataract and Refractive Services, The Wilmer Eye Insti-
tute, Johns Hopkins University School of Medicine, Baltimore, Maryland, U.S.A.
Leon Strauss, M.D., Ph.D. The Wilmer Eye Institute, Johns Hopkins University School
of Medicine, Baltimore, Maryland, U.S.A.
Rodrigo Torres Massachusetts Eye and Ear Infirmary and Harvard Medical School,
Boston, Massachusetts, U.S.A.
Mercedes Vázquez, M.D Instituto de Microcirugía Ocular, Barcelona, Spain
Fortino Velasco Instituto de Microcirugía Ocular, Barcelona, Spain
Li Wang, M.D. Cullen Eye Institute, Baylor College of Medicine, Houston, Texas,
U.S.A.
Nan Wang Cullen Eye Institute, Baylor College of Medicine, Houston, Texas, U.S.A.
Marc Winnick, M.D. Department of Ophthalmology, Columbia University College of
Physicians and Surgeons, and Harkness Eye Institute, New York, New York, U.S.A.
Patrick C. Yeh Cornea and Refractive Surgery Service, Massachusetts Eye and Ear In-
firmary, Schepens Eye Research Institute, and Harvard Medical School, Boston, Mas-
sachusetts, U.S.A.
Emily Yeung, M.D. Massachusetts Eye and Ear Infirmary, Schepens Eye Research In-
stitute, and Harvard Medical School, Boston, Massachusetts, U.S.A.
Rengin Yildirim, M.D. Refractive Surgery Department, Cerrahpasa Medical School,
University of Istanbul, Istanbul, Turkey
xiv Contributors
1
1
Refractive Errors and Their Treatment
LIANE CLAMEN GLAZER
Massachusetts Eye and Ear Infirmary and Harvard Medical School,
Boston, Massachusetts, U.S.A.
DIMITRI T. AZAR
Massachusetts Eye and Ear Infirmary, Schepens Eye Research Institute,
and Harvard Medical School, Boston, Massachusetts, U.S.A.
A. LASIK: DEFINITION AND OVERVIEW
Laser in-situ keratomileusis (LASIK) involves creating a corneal flap so that midstromal
tissue can be ablated directly and reshaped with an excimer laser beam (1,2). The proce-
dure allows the ophthalmologist to surgically reshape the cornea in an attempt to obviate
the need for corrective lenses (Fig. 1.1). LASIK is a modification of Colombian José Bar-
raquer’s ingenious innovations. In 1949, Barraquer first described his technique, and in
1964 he published clinical results of his attempts to achieve emmetropia by shaving and re-
shaping the cornea (3–5). With Barraquer’s technique of keratomileusis (i.e., carving the
cornea), a lamellar button (lenticule) of the patient’s cornea was excised with a manual mi-
crokeratome. Barraquer then reshaped the lenticule so that the central corneal curvature
was flattened and the refractive power of the cornea decreased. He then replaced the lentic-
ule in position, either with or without sutures. Barraquer’s specific attempts to correct my-
opia were called cryolathe keratomileusis, because they involved freezing and reshaping
the removed lenticule with a cryolathe.
Troutman and Swinger introduced cryolathe keratomileusis to the United States in
1977 (6). While keratomileusis produced good results when performed by experienced sur-
geons, the procedure was technically very difficult and the results were therefore variable
(7–11). Innovations to this procedure, however, eventually led to the creation of the more
highly refined procedure of LASIK.
The introduction of the “excited dimer” (excimer) 193 nm UV laser allowed for the
development of LASIK. The argon–fluoride excimer laser is capable of precise ablation of
corneal tissue with minimal disruption of adjacent tissue. The excimer laser’s effect on the
cornea was first studied in animal models in 1983 (12). In 1989, Peyman first used a laser
to remove corneal stroma from a lamellar bed in animals (13). Shortly thereafter, similar
attempts were made in human eyes (14,15). This early work supported the theory that in
situ keratomileusis was better than surface ablation because it induced less activation and
proliferation of stromal keratocytes, thereby avoiding both haze and regression. In addition,
the excimer laser allowed for more accurate tissue removal, thereby eliminating one of the
main deterrents to lamellar surgery (see Chap. 2).
The LASIK procedure, in its current refined state, involves increasing the eye’s in-
traocular pressure to at least 65 mmHg with a suction device, and then using a microker-
atome to create a corneal flap that is at least 6 mm in diameter and 150 microns thick. This
flap, which allows the Bowman’s-layer epithelial complex to remain intact, is then care-
fully lifted to expose the layers of the cornea that will be reshaped by the excimer laser. The
size of the optical zone and the depth and profile of the laser ablation will determine the
correction achieved (Fig. 1.1).
B. THE EPIDEMIOLOGY OF LASIK
Refractive surgery is a young and rapidly growing field. In 1998, 400,000 Americans un-
derwent refractive surgical procedures. This represents an increase over 1997 of approxi-
mately 100% (16). Despite the many refractive surgeries that have been performed, the tar-
get population for laser vision correction at the turn of the century is still an estimated 44
million people. Currently, approximately 3,000 ophthalmologists have been trained to per-
form refractive surgery. This number is increasing at a rate of 500 to 1,000 surgeons per
year. Industry observers predict that the number of trained surgeons will approach 6,000 to
8,000, or approximately 50% of the U.S based ophthalmologists (16).
LASIK produces good refractive results for low, moderate, and high myopia (see
Chap. 18). Recent reports for low myopia show postoperative uncorrected visual acuities
of 20/40 or better in 100% of patients, and 20/20 or better in 81% of patients (17).
Potential complications of LASIK include halos, interface inflammation, haze, and
regression. However, significant complications causing a loss of two or more lines of best
2 Glazer and Azar
Figure 1.1 Schematic diagram of emmetropia. In emmetropia, the far point is optical infinity, and
the secondary focal point (F
2
) is at the retina. Parallel rays of light focus on the retina. (From
Ref. 115.)
corrected visual acuity (BCVA) are rare (1,18,19). In addition, with improved instrumen-
tation and increasing surgeon experience, LASIK complication rates continue to fall.
C. REFRACTIVE ERRORS THAT ARE TREATABLE WITH LASIK
Three components of the eye work together to determine the refractive power of the eye:
the shape of the cornea, the power of the lens, and the length of the eye. The overall re-
fracting power of the eye is approximately 60 diopters: the cornea contributes about 45
diopters of power, while the lens provides approximately 15 diopters of refractive power to
the eye. When the cornea, lens, and length of the eye combine and produce no refractive er-
ror, emmetropia is achieved. In the instance of emmetropia, a ray of light parallel to the op-
tical axis and limited by the pupil focuses at a point on the retina. In other words, the sec-
ondary focal point is located on the retina. In addition, the “far point” (i.e., the furthest point
at which the eye can see clearly) is optical infinity (Fig. 1.2). Because the cornea accounts
for approximately two-thirds of the refractive power of the eye, it is logical that most re-
fractive surgeries attempt to change the shape of the cornea. Other methods of refractive
surgery include implanting intraocular lenses.
Traditionally, four major types of naturally occurring ametropias, or refractive errors,
have been described: myopia, hyperopia, astigmatism, and presbyopia. Wavefront analysis
of human eyes after correction of these optical abnormalities often show additional irregu-
larities that have been classified using Zernike polynomials into complex subgroups that
may be simplified into lower- and higher-order aberrations.
In myopia, the secondary focal point is anterior to the retina. In other words, the re-
fractive power of the eye is greater than that required for emmetropia; parallel rays of light
entering the eye are in focus at a location in the vitreous, rather than on the retina. Refrac-
tive myopia is due to steep corneal curvature or high lens power. Axial myopia is due to an
eye that is too long (i.e., longer than 22.6 mm). For every millimeter of axial elongation of
the globe, there are 3 diopters of myopia. When myopia is corrected with spectacles, con-
tact lenses, or refractive surgery, the parallel rays of light entering the eye come into focus
on the retina, which is consistent with a far point at infinity. The prevalence of physiologic
myopia in the general population is approximately 25% (20). LASIK corrects for myopia
by removing tissue in the center of the cornea, thereby flattening the cornea and decreasing
the refractive power of the eye.
A hyperopic eye’s secondary focal point is posterior to the retina. In other words, par-
allel rays of light entering the eye come into focus at a location posterior to the retina. Re-
fractive hyperopia is due to a flat cornea (less corneal refracting power), or a lens with
lower power. Axial hyperopia is due to a short axial length. While hyperopia affects ap-
proximately 40% of the adult population, it is clearly less visually significant than myopia
(21). This is because accommodation may produce enough additional plus power to bring
the parallel rays of light to focus on the retina. Thus young hyperopes may compensate and
see well until their accommodative power weakens and they begin to experience manifest
hyperopia in their mid to late 30s. LASIK corrects for hyperopia by removing a ring of tis-
sue around the center of the cornea, thereby making the cornea steeper.
Astigmatism refers to a refractive error in which the curvature of the cornea, or less
commonly the curvature of the lens, varies in different meridians. In other words, patients
with astigmatism have two focal lines formed by the convergence of rays of light. The first
focal line, created by the more powerful corneal meridian, is closer to the cornea. The sec-
ond focal line, formed by the less powerful meridian, is further away. Dioptrically midway
Refractive Errors and Their Treatment 3
between the two focal lines is the circle of least confusion. A proper refractive correction
will place the circle of least confusion on the retina.
While astigmatism is clinically detectable in up to 95% of eyes, astigmatism of less
than 0.50 diopters rarely requires optical correction (22). However, 10% of the general pop-
ulation has naturally occurring astigmatism greater than 1 D. Since an astigmatic refractive
error of 1.00 to 2.00 D can decrease uncorrected vision to the 20/30 to 20/50 level, this de-
gree of astigmatism causes an unacceptably poor quality of uncorrected visual acuity
(23,24).
Regular astigmatism refers to corneal curvatures that are different but symmetrical,
with principal meridians 90 degrees away from each other. For regular astigmatism, one
4 Glazer and Azar
Figure 1.2 Schematic diagrams of myopia and hyperopia. In myopia, the far point is in front of
the eye (top), and the secondary focal point (F
2
) is anterior to the retina, in the vitreous (middle). In
hyperopia (bottom), the rays of light are in focus at a point behind the retina. (From Ref. 115.)
can achieve a refractive correction with cylindrical or spherocylindrical lenses. LASIK can
correct regular astigmatism by removing more tissue from the steeper side of the cornea.
Irregular astigmatism refers to a condition in which the principal meridians change
from point to point across the pupil, or in which the amount of astigmatism changes from
one point to another. Examples of irregular astigmatism include keratoconus or traumatic
corneal scars. LASIK is contraindicated in eyes with irregular astigmatism. Often, rigid
contact lenses are the best way to improve the visual acuity for eyes with irregular astig-
matism. The use of Zernike polynomials to analyze the wavefront in patients with irregu-
lar astigmatism has allowed, at least in theory, the incorporation of this information into the
laser treatment algorithm in LASIK and paved the way for custom corneal treatments.
Presbyopia refers to the age-related loss of accommodative response. Presbyopia typ-
ically sets in at approximately 40 years of age. The condition results from either a loss of
lens elasticity or an anatomic change in the position of the lens equator to the ciliary body
position. Presbyopia is an important issue to discuss during the informed consent of pa-
tients seeking refractive surgery. Some physicians give their patient the option of monovi-
sion, in which one eye is corrected for near vision and the other for distance vision.
D. ETIOLOGY, EPIDEMIOLOGY, AND LASIK ALTERNATIVES
1. Corneal Subtractive Procedures
a. Lamellar Procedures
Lamellar refractive keratoplasty refers to the placement of a lenticule on or within the
cornea to change its refractive power, typically by altering its anterior curvature. As de-
scribed above and in Chap. 2, Jose Barraquer’s introduction of lamellar refractive surgery
and of cryolathe keratomileusis were followed by Ruiz’ introduction of the concept of in
situ keratomileusis (25). He introduced a microkeratome propelled by gears that could cre-
ate the corneal flap and allow one to perform intrastromal tissue subtraction. When ad-
vances in instrumentation allowed the lamellar keratoplasty to be performed more accu-
rately, the procedure became known as automated lamellar keratoplasty (ALK) (Figure
1.3). The initial results of ALK for myopia showed improvement over previous lamellar
techniques. Results described postoperative uncorrected visual acuities of 20/40 or better
in 86% of patients, with a 6% loss of two or more lines of best spectacle corrected visual
acuity (26,27). However, ALK outcomes were not significantly better than the results
achieved with contemporary radial keratotomy (RK) techniques (28,29). The accuracy of
ALK is limited by the imprecise nature of mechanical cutting.
Hyperopic ALK uses a very deep lamellar pass, cutting more than 70% of the stromal
depth (versus the 40% of stromal depth that is used for myopic ALK or for LASIK). The in-
traocular pressure causes the thinned cornea to bow forward, and thus the central cornea
steepens (Figure 1.4). As one may imagine, this procedure was often unpredictable and oc-
casionally led to progressive ectasia. Long-term results published in 1998 reported instabil-
ity of the postoperative refraction with a progressive myopic shift. Even worse, 26% of eyes
developed “iatrogenic keratoconus,” and 16.4% required penetrating keratoplasty (30,31).
Hyperopic ALK is now considered unsafe due to the risk of progressive corneal ectasia.
b. Surface Laser Ablation (PRK and LASEK)
Another form of refractive surgery that utilizes corneal subtractive procedures is photore-
fractive keratectomy (PRK). PRK is more accurate than ALK because it makes use of the
Refractive Errors and Their Treatment 5
193 nm argon fluoride (ArF) excimer laser. Like LASIK, PRK utilizes the excimer laser to
flatten the cornea by ablating the central cornea. Unlike LASIK, no corneal flap is made;
rather, the central corneal epithelium is simply removed with a spatula or with the laser it-
self (Fig. 1.5). While there are three distinct techniques for performing excimer laser PRK
for myopia, the most widely used technique applies wide-area surface ablation with a large-
diameter beam. Most of the published results of PRK are based on PRK performed in this
manner. It is primarily in Europe that PRK is performed with the two other techniques:
scanning slit and flying spot lasers. A modification of PRK, first performed at the Mas-
sachusetts Eye and Ear Infirmary by Azar and Abad in 1996, was the use of 20% alcohol
to create an epithelial flap which was repositioned to cover the stromal ablation bed. Since
then, several investigators have started to use this laser epithelial keratomileusis (LASEK)
technique, but it is not clear whether LASEK offers any advantages over PRK.
PRK has been shown to be safe and efficacious for the treatment of low to moderate
myopia. Several national studies of PRK demonstrated that 90 to 100% of patients had
20/40 or better uncorrected visual acuity, and 78 to 98% had postoperative refractions
within 1.0 D of the target outcome (32–34). Refractive results of PRK in patients with more
than 6 D of myopia tend to be highly variable and undergo more regression. Another dis-
advantage of PRK is a prolonged recovery time: while the corneal epithelium is healing,
6 Glazer and Azar
Figure 1.3 Myopic automated lamellar keratoplasty. (A) A microkeratome is used to create a
hinged flap in the cornea. (B) A second microkeratome is used to remove a parallel-faced disc of tis-
sue from the corneal bed. (C) The initial flap is replaced, leaving a flattened central cornea. (From
Ref. 116.)
patients may experience discomfort and blurry vision. In addition, potential complications
include postoperative haze, halos, induced astigmatism, diplopia, and keratitis (32,35–37).
Although PRK was the first widely accepted laser vision correction procedure, it has
been largely supplanted by LASIK as the refractive procedure of choice not just for high
myopia but even for low and moderate myopia. This is partly because LASIK provides
faster visual rehabilitation and decreased time for wound healing (38–43).
While LASIK is often the refractive procedure of choice for myopes, PRK may be
preferable to LASIK for patients who are hesitant to undergo incisional surgery, or for pa-
tients who have contraindications to LASIK. Such contraindications would include corneal
Refractive Errors and Their Treatment 7
Figure 1.4 Hyperopic automated lamellar keratoplasty. (A) A hinged flap, approximately 70% of
corneal thickness, is created with a microkeratome. (B) Intraocular pressure displaces the very thin
posterior layer and overlying flap anteriorly, causing central corneal steepening. The original shape
of the cornea is shown with dotted lines. (From Ref. 116.)
Figure 1.5 Schematic illustration of photorefractive keratectomy. The excimer laser is used to re-
move anterior stroma (left), causing corneal flattening (middle). The region of tissue subtraction is
designated by the shaded area. (From Ref. 115.)
thinning, in which less than 200 to 250 microns of tissue would be left in the corneal bed,
and epithelial anterior basement membrane dystrophy, because this carries the risk of ep-
ithelial ingrowth. PRK is also competitive with LASIK in the arena of treatment for low
hyperopia. A recent review of 65 eyes with ϩ1.00 to ϩ4.00 D of hyperopia documented
that 92% of the eyes were within Ϯ1.00 D of the intended manifest SE at 18 months fol-
low-up (44). Other reports support the use of hyperopic PRK (H-PRK) for treating low de-
grees of hyperopia (45–48). H-PRK for the treatment of higher degrees of hyperopia is less
predictable and may result in greater regression (45).
2. Biologic and Synthetic Tissue Addition: Epikeratoplasty and ICRS
One can alter the shape, and thus the refractive index, of the cornea by adding material ei-
ther to the surface of the cornea or into the corneal stroma. Epikeratoplasty involves adding
a lenticule with power (i.e., carved donor tissue) to the deepithelialized surface of Bow-
man’s layer. Also called epikeratophakia or lamellar keratoplasty, this technique has
proven problematical. First, the refractive results have been disappointing: only 33% of
eyes achieved 20/40 or better uncorrected visual acuity, 15% lost two or more lines of best
corrected visual acuity (BCVA), and regression was more than 2 D in 17.6% of patients at
5 months’ follow-up (49–51). Secondly, 2.5–3.5% of patients who underwent this proce-
dure had delayed reepithelialization, which in some cases led to infections and graft melt-
ing (49,52–55). Almost 8% of grafts had to be removed due to failure of epithelialization,
haze, glare, irregular astigmatism, epithelial ingrowth, epithelial interface cysts, melt, in-
fection, stromal infiltrates, and wound dehiscence (49,53–54,56–57). After the lenticules
were removed, many patients were left with central corneal scarring and loss of BCVA
(58,59). Because of the disappointing outcomes and unfortunate complications, epikerato-
plasty has been abandoned as a technique for the correction of myopia. However, epiker-
atoplasty is still occasionally used to diminish the myopia and irregular astigmatism of ker-
atoconus or other corneal thinning disorders (60). This procedure is also sometimes used
for the treatment of pediatric and adult aphakia.
Keratophakia is a procedure in which a lens is placed within the corneal stroma to al-
ter the cornea’s refractive power. While lenses used to be fashioned from donor human
corneas (homoplastic), these lenses had variable outcomes and a high incidence of compli-
cations (61). Keratophakic lenses are now fashioned exclusively from synthetic materials
(alloplastic).
The intrastromal corneal ring segment (ICRS), approved in the United States in April
1999, is a keratophakic technique to correct for low myopia (Ϫ1.00 to Ϫ3.00 D with an
astigmatic component of 1.00 D or less). The ICRS consists of two 160 degree polymethyl
methacrylate (PMMA) segments placed in two pockets of the peripheral stroma, thereby
inducing peripheral steepening and indirectly causing central corneal flattening (Fig. 1.6).
The procedure is unique in that it retains the potential to be adjusted or reversed.
Two-year results of the phase II and phase III FDA trial analyzed 358 patient eyes.
At 24 months after surgery, 97% of the eyes had an UCVA of 20/40 or better, 76% were
20/20 or better, and 55% were 20/16 or better. In addition, 93% were within Ϯ1.00 D range,
and 73% were within Ϯ0.50 D range (62).
Potential disadvantages with the procedure include accidental perforation into the an-
terior chamber, surface perforation of the epithelium anteriorly, and induced astigmatism,
which can result from postoperative movement of the intracorneal ring segments (63).
8 Glazer and Azar
3. Corneal Surface Remodeling: Incisional Surgery, Thermal Surgery,
and Orthokeratology
a. Incisional Surgery: Radial Keratotomy
Radial keratotomy (RK) is a procedure that treats myopia by making deep and radial
corneal stromal incisions to weaken the peripheral and paracentral cornea and thereby flat-
ten the central cornea (Figs. 1.7 and 1.8). Experimenting on rabbits’ corneas, Dutch oph-
thalmologist Lendert Jan Lans first demonstrated that nonperforating corneal incisions par-
allel to the limbus cause peripheral bulging and central flattening (Fig. 1.7) (64,65).
Japanese ophthalmologist Tsutomu Sato first performed anterior and posterior kerato-
tomies to treat myopia after observing that patients with keratoconus who had experienced
breaks in Descemet’s membrane and hydrops subsequently developed corneal flattening
(66–67). Sadly, the role of the corneal endothelium was not understood when Sato was per-
forming his keratotomies. Later follow-up showed that of 170 eyes that underwent a kera-
totomy by Sato’s group, 121 (71%) developed bullous keratopathy, and only 49 eyes (21%)
retained clear corneas. The average time between the procedure and the onset of edema was
20 years (68). The posterior keratotomy was abandoned for anterior keratotomies once the
physiological importance of the corneal endothelium had been established.
In 1960, Svyatoslav Fyodorov of the Soviet Union visited Japan, where Akiyama
taught him Sato’s technique of radial keratotomy (Sato himself had recently died). Fyo-
dorov improved upon Sato’s techniques by devising improved instrumentation and by de-
veloping mathematical formulas to improve the reproducibility of RK (69–70). Fyodorov
also showed that paracentral incisions were more effective than peripheral, scleral inci-
sions. In addition, he demonstrated that the diameter of the central clear zone is inversely
proportional to the magnitude of refractive correction.
In the United States, the first RK was performed by Bores at the Kresge Eye Institute
in November 1978. It was at about this time that the 20 year follow-up data of Sato’s pos-
terior keratotomy patients was revealing the 75% incidence of bullous keratopathy. Be-
cause the newer anterior procedure had scarce follow-up data, the National Eye Institute
chose to fund two large nationwide studies to examine RK: the Prospective Evaluation of
Radial Keratotomy (PERK) and the Analysis of Radial Keratotomy.
Refractive Errors and Their Treatment 9
Figure 1.6 Intrastromal corneal ring segments. (From Ref. 63.)
The PERK study showed that 10 years after surgery, 53% of the patients had 20/20
or better uncorrected vision, 85% had 20/40 or better, and 63% of patients younger than 40
years old were spectacle-independent. Low myopes did better than moderate myopes: 94%
of patients with low myopia (Ϫ2.00 to Ϫ3.12 D) had an uncorrected visual acuity (UCVA)
of 20/40 or better, while 79% of moderate myopes (Ϫ3.25 to Ϫ4.37) had 20/40 or better
UCVA. The 10-year PERK results also revealed a long-term instability of refractive errors:
10 Glazer and Azar
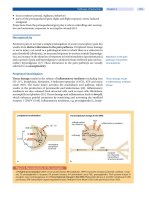
Figure 1.7 The development of radial and astigmatic keratotomy. Illustrations of Lans’s studies
showing peripheral corneal bulging (A) and subsequent scarring (B) after astigmatic keratotomy (C)
and radial keratotomy (D). (From Ref. 117.)