Ebook Sherlock’s diseases of the liver and biliary system (13/E): Part 2
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (11.98 MB, 1,259 trang )
Chapter 19
Autoimmune Hepatitis and Overlap
Syndromes
Ashnila Janmohamed and Gideon M. Hirschfield
Centre for Liver Research, NIHR Biomedical Research Centre,
University of Birmingham, Birmingham, UK
LEARNING POINTS
Autoimmune hepatitis (AIH) is an immune mediated liver
disease that can present in all ages and races and both sexes.
Disease presentation is variable, ranging from asymptomatic
disease to fulminant liver failure.
There is an absence of a specific diagnostic marker, hence
diagnosis is made by exclusion of alternative liver disease
and precipitants, for example, drugs and viruses.
Clinically, AIH is characterized by raised serum alanine
aminotransferase, hypergammaglobulinaemia,
autoantibodies, and interface hepatitis. Seropositivity for
autoantibody subclassifies disease into two distinct entities:
antinuclear and/or smooth muscle antibodies (type 1
disease) and anti liver kidney microsomal 1 and/or liver
cytosol 1 (type 2 disease).
The cornerstone of therapy is immunosuppression using
corticosteroids and azathioprine with the majority of patients
achieving remission, reflecting that AIH is normally
treatment responsive.
Liver transplantation is indicated in patients who present
with fulminant liver failure or have end stage liver disease.
Lack of response to immunosuppression should prompt
confirmation of compliance, exclusion of
alternative/additional aetiology such as primary biliary or
primary sclerosing cholangitis, and initiation of second line
967
therapy if appropriate.
Lack of standardization of second line therapy in AIH
demonstrates an ongoing need for new and more rational
therapies.
Introduction
Autoimmune hepatitis (AIH) is a heterogeneous immune
mediated liver disease that, in most cases, has effective treatment
when diagnosed promptly and treated judiciously with classical
immunosuppression focused on corticosteroids and azathioprine.
The spectrum of presentation alongside the absence of clear
pathophysiology and non specific diagnostic markers results in a
chronic, rare, and progressive immune mediated liver disease,
which has ongoing unmet needs. Clinically, in its classical form,
patients with AIH are characterized by raised serum alanine
transaminase (ALT) activity, hypergammaglobulinaemia, non
organ specific autoantibodies, and a chronic relapsing hepatitis,
associated with a plasma cell hepatic infiltrate. Exclusion of drug
precipitants (prescribed, ‘over the counter’, or herbal),
alternative aetiologies of liver disease and confirmation of negative
viral studies are always required (Fig. 19.1).
968
Fig. 19.1 Evaluating a patient with autoimmune liver disease. ALP,
alkaline phosphatase; ALT, alanine transaminase; AST, aspartate
transaminase;
ANAs,
antinuclear
antibodies;
AMAs,
antimitochondrial antibodies; MRCP, magnetic resonance
cholangiopancreatography; MRI, magnetic resonance imaging;
PBC, primary biliary cholangitis; PSC, primary sclerosing
cholangitis; γ GT, γ glutamyltransferase; SMAs, anti smooth
muscle antibodies.
Historical perspective
Appreciation for an autoimmune aetiology in chronic hepatitis
emerged in the 1940s as Waldenström recognized the relevance of
hypergammaglobulinaemia in chronic hepatitis, and Kunkel et al.
[1] described a persistent liver disease predominantly in young
females with hypergammaglobulinaemia, alongside extra hepatic
symptoms including rash, arthralgia, fever, and amenorrhoea.
Subsequent observations reporting the presence of lupus
erythematosus cells and antinuclear antibodies (ANAs) led to the
suggestion of an immune mediated disease prompting treatment
with cortisone, which resulted in marked symptomatic
improvement, suggesting an important inflammatory component to
969
this disease. Prolonged immunosuppressive therapy with
corticosteroids and azathioprine, offered to patients from the 1960s
onwards, proved effective and remains standard therapy [2–4]. The
term autoimmune hepatitis was applied in 1965 by Cowling and
Mackay, and endorsed globally in 1993.
Disease overview
Clinical manifestations
These range from asymptomatic through to fulminant hepatic
failure. Although most patients present when symptomatic (fatigue,
arthralgia, anorexia, jaundice), others are diagnosed incidentally.
The prevalence, clinical presentation, and outcome of AIH vary
between ethnic groups and geographical regions, and clinicians
should be mindful of this. AIH is more common and severe in North
American Aboriginals/First Nations populations compared with
predominately Caucasians, non First Nations populations [5].
Cirrhosis at presentation is more frequent in black North American
patients with AIH than white North Americans [6]. They are also
younger at presentation, similar to patients from Brazil and
Argentina, and have poorer prognosis [5]. Africans, Asians, and
Arabs have an earlier disease onset than people from Northern
Europe. Along with Alaskan natives, they additionally appear to
have a higher frequency of cholestatic laboratory findings and acute
icteric disease. Similarly, patients of Hispanic origin tend to present
aggressively both biochemically and histologically and have a very
high prevalence of cirrhosis and cholestatic features.
Serology
Serological patterns of autoreactivity permit two major
classifications: type 1, characterized by ANAs and/or anti smooth
muscle antibodies (SMAs), and type 2, characterized by anti
liver/kidney microsomal type 1 (anti LKM 1) and/ or liver cytosol
type 1 (anti LC 1) antibodies [5].
Epidemiology
Patients of all ages and races and both genders can develop AIH.
AIH is considered rare, as its prevalence ranges from 16 to 18 cases
970
per 100 000 inhabitants in Europe. Higher prevalence rates have
been reported in areas with stable ‘populations’ (where both growth
rates and relative age distribution do not change with time). A large
nationwide population study in Denmark, assessing the incidence
and prevalence of AIH over a 20 year period between 1994 and
2013, demonstrated that the incidence of AIH had increased
markedly over the study time period. Between 1994 and 2012, the
incidence rate had nearly doubled, reaching a point prevalence in
2012 of 23.9 per 100 000 [7].
Natural history
Sherlock’s follow up study, in which 44 patients, diagnosed
between 1963 and 1967, were randomly allocated into control and
treatment (prednisolone) groups, provides stark natural history
data for patients with severe disease. Ten year survival data
demonstrated significantly improved survival in the treatment
group, where 63% of patients were alive at 10 years (median
survival 12.2 years), compared with 27% (median survival 3.3 years)
in the control group (Fig. 19.2).
971
Fig. 19.2 Later results of the Royal Free Hospital trial of
prednisolone in chronic autoimmune hepatitis. Note the improved
survival in the treated group.
Source: Kirk et al. 1980 [36]. Reproduced with permission of BMJ
Publishing.
Biological determinants of disease
Immunobiology
Hepatic immunological tolerance is maintained in numerous ways,
including (a) antigen priming in the liver, (b) sinusoidal tolerance
and T regulatory cell (Treg) induction, and (c) hepatic stellate cell
induced effector T cell apoptosis and generation of myeloid
derived suppressor cells [8]. Loss of tolerance is precipitated
through various events, which collectively culminate in a common
final pathway towards liver injury. Evidence implies that both
972
innate and adaptive immune systems are involved in AIH.
Data demonstrate a genetic predisposition, strongly associated with
the HLA locus, systemic immunoregulatory changes notably
affecting Treg function, and immune restricted responses to target
antigens. Microscopic evaluation of the liver demonstrates an
autoaggressive cellular immune attack with lymphocytes,
macrophages, and plasma cells forming a dense portal mononuclear
cell infiltrate involving surrounding parenchyma to varying degrees
(interface hepatitis). Immunohistochemical studies have revealed a
predominance of αβ T cells, the majority being CD4 helper T cells
and a sizeable minority being CD8 cytotoxic suppressor T cells.
Other cells present include natural killer (NK) cells,
monocytes/macrophages, and γδ T and B cells.
Antigen restricted immune mediated injury is driven through a
combination of cellular and antibody mediated immunological
attack against liver specific targets. Th1, Th2, and Th17 cells
interact to generate disease. Th1 cells enhance HLA class I
expression and induce expression of HLA class II molecules on
hepatocytes, Th2 cells favour autoantibody production by B
lymphocytes and Th17 cells play a role in organ specific
autoimmune inflammation (Fig. 19.3).
973
Fig. 19.3 Diagrammatic representation of immunopathogenesis of
autoimmune hepatitis. A, a self antigenic peptide is presented to
an uncommitted T helper (Th0) lymphocyte within the HLA class II
molecule of an antigen presenting cell (APC). Th0 cells become
activated and, depending on the cytokine milieu in the
microenvironment, differentiate into Th1, Th2, and Th17 cells,
initiating a series of immune reactions. B, Th1 secrete IL2 and
IFN γ, which stimulate cytotoxic T lymphocytes (CD8), enhance
expression of class I, induce expression of class II HLA molecules
on hepatocytes, and activate monocytes/macrophages (M0/Mφ)
which in turn release TNF α and IL1. C, Th2 cells secrete IL4,
IL10, and IL13, which induce the maturation of B cells in to plasma
(P) cells. D, expansion of plasma cells results in excess production
of immunoglobulins, which bind to normal membrane constituents
of the hepatocytes, inducing complement activation, engagement of
natural killer (NK) cells, and hepatocyte death. E, Th17 cells release
IL17, IL23, and IL6. IL17 induces IL6 expression in hepatocytes,
which in turn further stimulates Th17 cells. Depending on the state
of the immune system and IL6 production, a reciprocal relationship
is thought to exist between Th17 cells and T regulatory (Treg) cells.
F, Tregs are derived from Th0 cells in the presence of transforming
growth factor (TGF β).
A considerable number of IL17 (a potent pro inflammatory
cytokine) producing cells can also be present in the inflammatory
infiltrate of the livers of patients with AIH. The role of Th17 cells in
AIH is incompletely understood. IL17 produced by Th17 cells has
been shown to induce IL6 (pro /anti inflammatory cytokine)
expression in hepatocytes, stimulating Th17 cells further, resulting
in a positive feedback loop between Th17 cells and hepatocytes and
exacerbating the inflammatory process. Depending on the state of
the immune system and IL6 production, a reciprocal relationship is
thought to exist between Th17 cells and Tregs in both development
pathways and function.
Studies have shown that Tregs in AIH are reduced in number and
function with decreased proliferative activity in response to
stimulation. Their ability to produce IL10 (anti inflammatory
cytokine) has been shown to be impaired, contributing to a
functional impairment [9]. Tregs were found to have both impaired
suppressive capacity and increased susceptibility to apoptosis
974
following contact with hepatic microenvironment that was rich in
pro inflammatory cytokines but deficient in IL2. Exogenous IL2
reversed these effects, suggesting a possible mechanism to explain
Treg dysfunction in inflamed tissue such as in AIH. IL2
supplementation in addition to Treg therapy is an experimental
paradigm being explored potentially to restore immune homeostasis
in autoimmune liver diseases (AILDs) [10]. In contrast, some
studies have demonstrated no differences in the frequency and
function of peripheral Tregs in AIH. In one study, intrahepatic
Tregs were noted to be increased in untreated type 1 AIH whereas
immunosuppression resulted in a disproportionate loss [11].
The role of B cells in AIH remains unclear. They are mainly
responsible for antibody production. Although AIH is characterized
by the presence of autoantibodies, they are not generally disease
specific, nor do they correlate with disease severity or outcome. B
cells can act as antigen presenting cells (APCs) and modulate
immune responses by cytokine production. Preclinical and clinical
studies investigating the effect of B cell depletion in AIH have
produced contradictory results, although in many patients a clear
response has been noted [12,13]. B cell activating factor (BAFF) is
a cytokine that influences B cell survival and maturation and plays
a role in immune regulation. Early conference proceedings have
shown that patients with AIH have significantly elevated serum
BAFF concentrations that correlate with transaminase activity,
whereas corticosteroid therapy markedly reduces BAFF levels.
Genetics and AIH
Genetic associations with AIH that are confirmed include common
genetic variation in (a) HLA and (b) SH2B3, alongside (c) very rare
coding variants in AIRE and GATA2.
HLA loci and AIH
The first genome wide association study (GWAS) of patients with
type 1 AIH in the Netherlands confirmed the strong involvement of
the major histocompatibility complex region [14] and identified
HLA DRB1*03:01 as a primary susceptibility genotype and HLA
DRB1*04:01 as secondary. In European and North American
Caucasians, HLA A1 B8, HLA DRB1*03:01, and HLA
DRB1*04:01 are associated with type 1 AIH ad DRB1*03, DRB1*07,
975
and DQB1*02:01 with type 2. Those over 60 years of age are more
likely to have the HLA DRB1*04 allele, which is associated with
less severe disease, whereas HLA A1 B8 DR3 haplotype is
over represented in men with AIH and strongly associated with
early disease onset and relapse [15]. The presence of HLA
DRB1*03 is associated with failure to respond to treatment and a
more severe disease course.
HLA associations vary around the globe and may explain some
disease presentation differences. For example, in Japan and
Argentina HLA DRB1*04:05 is associated with AIH, in Brazil
HLA DRB1*13:01 and DRB3*01 are associated with disease, and
among Mestizo Mexicans HLA DRB1*04:04 is predominant.
Non‐HLA loci and AIH
GWAS identified the first non HLA genetic AIH risk factor;
SH2B3, a protein that acts as a negative regulator of T cell
activation, tumour necrosis factor (TNF) and janus kinase 2 and 3
signalling, and also plays a critical role in hematopoiesis. It is also
associated with other autoimmune diseases such as primary
sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), type
1 diabetes mellitus, and coeliac disease.
Other non HLA genes associated with AIH susceptibility include
autoimmune regulator type 1 (AIRE 1), a transcription factor that
regulates clonal deletion of autoreactive T cells. A single coding
mutation of this transcription factor results in a severe autoimmune
polyglandular syndrome type 1 (APS1). Patients with APS1 suffer
from mucocutaneous candidiasis and a number of organ specific
autoimmune diseases, including AIH. Recently, a mutation in
GATA2, another haematopoietic transcription factor, has been
shown to be associated with AIH, further highlighting relevant
mechanisms of liver injury, notably Treg dysfunction [16].
Environmental and drug triggers
In patients with a presumed underlying genetic predisposition,
environmental toxins such as drugs and viral infections may be
presented immunologically in a manner that precipitates molecular
mimicry.
Drugs can induce both immunologically mediated hepatocellular
976
and cholestatic liver disease. Generally, liver injury results from the
bioactivation of drugs to reactive metabolites, which may interact
with cellular macromolecules, disrupt cellular signalling, and lead to
mitochondrial dysfunction.
Emerging evidence from studies relevant to our concepts underlying
AIH pathogenesis have shown that isoniazid (INH) induced liver
injury is immune mediated. Anti INH and cytochrome P450
antibodies were detected in the serum of patients who had INH
induced liver failure but not those with minimal liver biochemical
abnormality or normal serum ALT following INH treatment,
suggesting that INH induced liver failure has an immune
mediated mechanism [17].
Checkpoint inhibitors such as the cytotoxic T lymphocyte antigen
4 (CTLA 4) antibody ipilimumab and the programmed cell death
protein 1 (PD 1) antibodies pembrolizumab and nivolumab, used
for the treatment of metastatic melanoma, are known to result in an
autoimmune like drug induced liver injury presumed to be
related to the blockade of T cell inhibition [18]. Both CTLA 4 and
PD 1 promote immune tolerance via downregulation of T cell
activation. Antibodies against these immune checkpoints can result
in both tumour destruction and clinically relevant decreases in
self tolerance.
Viral triggers
Viruses have repeatedly been shown to trigger AIH, the best
descriptions being following hepatitis A. Sequence similarities
between viral and self proteins could trigger autoimmunity and
the simultaneous presence of inflammatory cytokines during virus
infection may add to the risk of developing self perpetuating
autoimmunity.
Disease presentation
General features
AIH should be considered in any patient who presents with raised
transaminase activity, particularly if the patient has features of
other autoimmune diseases (Table 19.1). Type 2 disease may present
more acutely, at a younger age (but not exclusively), and
977
immunoglobulin A deficiency is often noted without elevation of
immunoglobulin G (IgG) concentration, whereas symptoms, signs,
family history, and associated autoimmune diseases are similar for
both serological groups (Table 19.2).
Table 19.1 Differential diagnosis for elevated transaminase activity
Drug induced liver injury
Acute viral hepatitis
Chronic viral hepatitis (hepatitis B and C predominantly; consider
hepatitis E in the immunosuppressed)
Steatohepatitis (alcoholic and non alcoholic)
Autoimmune liver disease, including overlap presentations
Coeliac disease
Hypothyroidism/hyperthyroidism
Haemochromatosis
Alpha1 antitrypsin deficiency
Wilson disease
Ischaemia (including Budd–Chiari syndrome)
Hepatic infiltration (malignant and non malignant)
Table 19.2 Clinical differences between serological classifications
of autoimmune hepatitis
Type 1 AIH
>80%
Type 2 AIH
20% in Europe, 4% in USA
Relative
prevalence
Autoantibodies ANAs, SMAs
commonly
associated
LKM 1, LC 1
Gender
4:1
(female : male)
Geography
Worldwide
Age of onset
9:1
All ages. Median
age 40 years
Predominately common in
Northern Europe
Average 10 years old but seen
in adults, specifically in Europe
978
Other
commonly
associated
autoimmune
diseases
Presentation
Prevalence of 17–
48%: thyroid
disease, synovitis,
ulcerative colitis
Prevalence unclear: diabetes,
thyroid disease, vitiligo,
pernicious anaemia, IgA
deficiency
Variable. Acute
onset rare
Response to
treatment
Excellent
response
Likely to present acutely.
Frequently presents with
cirrhosis in children and more
aggressively
Likely to be more treatment
resistant, higher relapse rates,
inevitable need for long term
immunosuppression
Progression of 25% have
disease
cirrhosis at
diagnosis; 45%
develop cirrhosis
~80% develop cirrhosis
ANAs, antinuclear antibodies; LKM 1, anti liver kidney microsomal type 1;
LC 1, liver cytosol type 1; SMAs, anti smooth muscle antibodies.
About 30% of patients have cirrhosis at presentation, suggesting
that chronic hepatitis was probably present prior to diagnosis.
Pathologists must be careful not to mistake the acute collapse and
architectural change of acute severe AIH (bridging necrosis) for
cirrhosis; equally, imaging in the acute stage can suggest cirrhosis
when appearances in face reflect hepatic collapse.
Patterns of presentation
AIH has a variable presentation; most patients present with
insidious disease including 25% who are asymptomatic.
Approximately 25–30% of patients present with acute onset AIH,
rarely progressing to fulminant liver failure. Some patients
classified as having cryptogenic cirrhosis or seronegative fulminant
hepatitis are likely to have acute presentations of AIH. A
retrospective review of liver histology in the Acute Liver Failure
Registry found that 42 of 72 patients (58%) diagnosed with acute
liver failure of indeterminate nature had probable AIH [19].
The elderly may have an indolent progressive disease that is
979
asymptomatic or masked by other concurrent conditions.
Superimposed hepatitis E on the background of underlying chronic
liver disease, including AIH, should be sought, particularly in
elderly men with new onset chronic cholestatic hepatitis/liver
failure.
Symptoms and signs
Patients can present with a variety of non specific symptoms,
including jaundice, fatigue, lethargy, nausea, anorexia, weight loss,
abdominal pain, pruritus, arthralgia, arthritis, acne, and
amenorrhea. Acute presentations are often indistinguishable from a
viral illness, and hepatic discomfort, anorexia, and nausea may be
evident. Clinical features range from firm hepatomegaly and
splenomegaly (in which case a small liver is usually found) to other
features of chronic liver disease. In advanced stages, patients can
present with features of portal hypertension, including ascites,
encephalopathy, and oesophageal varices.
Associated autoimmune diseases
AIH is associated with other autoimmune diseases in as many as
one in three patients; exemplars include Hashimoto thyroiditis,
Graves disease, Sjörgen syndrome, autoimmune haemolysis,
rheumatoid arthritis, ulcerative colitis (UC), and idiopathic
thrombocytopenic purpura. Approximately 40% of patients have a
family history of autoimmune disease.
Laboratory features
Liver biochemistry and immunoglobulins
IgG concentrations commonly 1.2–3.0 times the upper limit are
found in 85% of patients accompanied by elevations in serum
transaminase activity ranging from minor elevations to values in the
thousands. A minority of patients, particularly those who present
acutely, may have normal IgG concentrations. In the authors’
experience, patients with type 2 disease often do not have
significant IgG elevations. Clinically, it is also relevant to look at the
absolute IgG concentration in a patient at presentation and to gauge
an individual’s response to therapy, as in some patients the IgG
980
concentration remains within the normal range, but still decreases
with therapy.
Elevated alkaline phosphatase (ALP) values can also be seen and, if
greater than threefold, should prompt additional investigation of
the biliary system. Jaundice, coagulopathy, and hypoalbuminaemia
may be noted in very acute presentations. Haemolysis (often
accompanied by a low serum ALP value and an increased AST : ALT
ratio) should prompt exclusion of haemolytic Wilsonian crisis.
Serology
Autoantibody positivity assists in diagnosis and allows
subclassification (Table 19.3). The usual titres of serum
autoantibodies are at or above 1 in 40–80, but found in isolation
they have low positive predictive values since the prevalence of
autoantibodies in healthy individuals exceeds the burden of disease;
their presence also increases with age. Lower titres, at or above 1 in
20, are of significance only in children and correlate with disease
activity.
Table 19.3 Autoantibodies commonly associated with chronic liver
disease
Autoantibody Target
Nucleus
Reported associations
ANAs
Nuclear membranes
and DNA (general)
Type 1 AIH, PBC, NAFLD,
chronic hepatitis B/C
Histones
pANCA
Microsomal
LKM 1
Nucleosomes
Neutrophil granules
Type 1 AIH, PBC
Type 1 AIH, PSC, PBC
Mitochondrial enzyme Type 2 AIH, hepatitis C
CYP450 2D6
Mitochondrial
AMAs
ATPase associated
antigens of inner
mitochondrial
membrane
Smooth muscle
981
PBC, AIH
SMAs
Fibroblast actin,
tubulin, and
intermediate
filaments (general)
Type 1 AIH, PSC, PBC,
chronic hepatitis B/C,
NAFLD
Actin
Cytosol
SLA/LP
F actin specifically
Type 1 AIH
UGA repressor
tRNA associated
protein
AIH; can be a marker of
patients with a very high
relapse risk, therefore
cessation of therapy is not
advisable if a patient is
SLA/LP positive
Liver cytosol 1
Formiminotransferase Type 2 AIH, chronic
cyclodeaminase
hepatitis C
AIH, autoimmune hepatitis; ANAs, antinuclear antibodies; AMAs,
antimitochondrial antibodies; SMAs, anti smooth muscle antibodies;
LKM 1, anti liver kidney microsomal type 1; LC 1, anti liver cytosol 1;
NAFLD, non alcoholic fatty liver disease; pANCA, perinuclear
antineutrophil cytoplasmic antibody; PBC, primary biliary cholangitis; PSC,
primary sclerosing cholangitis; SLA/LP, soluble liver and pancreas antigen.
Seronegative disease can occur (Fig. 19.4). Antibody titres and
specificity can vary throughout the disease course; seronegative
individuals may develop autoantibodies later in the disease, hence
repeat testing in these patients and those with low antibody titres at
presentation is recommended. Routine repeat serology is not
proven to be necessary, although in paediatrics serological
remission can be used as one of the treatment endpoints [20]. The
presence of autoantibodies without other features of AIH is not
diagnostic of AIH, and conversely their absence does not preclude a
diagnosis of AIH in the presence of other supporting features.
982
Fig. 19.4 Use of serology to distinguish patterns of autoimmune
liver disease. ANA, antinuclear antibody; AMA, antimitochondrial
antibody; ELISA, enzyme linked immunosorbent assay; LKM 1,
anti liver kidney microsomal type 1; PBC, primary biliary
cholangitis; PSC, primary sclerosing cholangitis; SMA, anti
smooth muscle antibody.
Antinuclear antibodies
ANAs are present alone (approximately 10%) or with SMAs
(approximately 50%) in two thirds of patients. Classic AIH is
associated with homogeneous, speckled and nucleolar ANA
patterns. ANAs in AIH are non specific.
Smooth muscle antibodies
SMAs are present in approximately 90% of AIH patients either in
isolation (approximately one third) or with ANAs (approximately
half). However, SMAs are very non specific since low titres are
frequent in healthy individuals, especially those over 60 years of age
and in viral, autoimmune, or malignant disease. Anti F actin
antibodies are more specific for type 1 AIH; however, assays are not
universally available [21]. ANA and SMA levels fluctuate during the
983
course of AIH and may disappear with corticosteroid therapy.
Neither their titre at diagnosis nor their fluctuation during the
course of illness predicts outcome in adult patients.
Microsomal antibodies
There are four subclassifications of LKM. LKM 1 reacts with the
mitochondrial enzyme cytochrome P450 2D6 subtype (CYP2D6),
inhibiting its activity in vivo. CYP2D6 metabolizes several known
medications, including antihypertensives and benzodiazepines, and
is genetically polymorphic. LKM 2 reacts with CYP450 2C9 and has
been associated with hepatitis caused by the drug ticrynafen (tienilic
acid), withdrawn from the US market in 1982. LKM 3 has affinity to
uridine diphosphate glucuronosyl transferase and was previously
associated with chronic hepatitis D. LKM 4 recognizes CYP1A2 and
CYP2A6 (with an immunofluorescence pattern indistinguishable
from that of LKM 1) and has been described in patients with AIH
associated with autoimmune polyendocrinopathy–candidiasis–
ectodermal dystrophy.
Anti LKM 1 is seemingly rare in the USA, occurring in only 4% of
adults with AIH. It has been described mainly in paediatric patients
in Europe, but 20% of patients with anti LKM 1 in France and
Germany are adults. In UK adult practice, 1–2% of patients with
AIH have type 2 AIH.
In paediatrics, the presence and titre of anti LC 1 have been shown
to correlate well with disease activity and may be associated with
aggressive, recurrent disease.
Both anti LKM 1 and anti LC 1 can occur either alone or together
in type 2 AIH. As with any autoantibody, neither is truly disease
specific, but both do have high sensitivity.
Soluble liver and pancreas antigen
Initially, individuals who were soluble liver and pancreas antigen
(SLA/LP) positive were classified as having type 3 AIH; however,
given that anti SLA/LP is also found in 50% of patients with type 1
AIH, the proposed type 3 classification of AIH has been abandoned.
About 10–30% of patients with type 1 AIH are SLA/LP positive.
Anti SLA/LP is a better marker of AIH since normal individuals
984
and those with non hepatic disorders are invariably anti LP
negative. It has a high diagnostic value, with 99% specificity for
AIH, and its presence at the time of diagnosis may identify patients
with more severe disease and outcome [22]. Anti SLA/LP
antibodies have been shown to be strongly associated with
DRB1*03:01.
In addition to conventional antibodies, anti SLA/LP may be
associated with antibodies to ribonucleoprotein/Sjogern syndrome
(SS) A antigen (anti Ro/SSA) and anti Ro52 conversely can be
the sole antibody detectable in patients.
Mitochondrial antibodies
Anti mitochondrial antibodies (AMAs) may be found in
approximately 20% of patients with AIH. They are usually lower in
titre (≤1 : 40) and can represent false positives misinterpreted by
indirect immunofluorescence. The presence of AMAs must not be
taken directly to imply an AIH–PBC overlap syndrome. A long
term study of AIH patients who were persistently AMA positive
showed that these individuals had the same laboratory, histological,
and clinical features and treatment outcomes as AMA negative
individuals [23].
Imaging
Imaging in AIH is useful in excluding important differential
diagnoses such as acute Budd–Chiari syndrome, infiltrative disease,
and unsuspected biliary processes. Doppler ultrasound is the initial
investigation method of choice. In those with an acute/subacute
presentation of AIH there is often marked histological architectural
collapse. Radiologically, this may give a pseudo cirrhotic
appearance in the absence of true cirrhosis. Furthermore, in
subacute liver failure, splenomegaly and ascites can be present
without true chronic liver disease.
Biliary overlap is variably reported, more commonly in those
presenting in childhood, suggesting that magnetic resonance
cholangiography should be routinely considered for those with AIH
presenting in childhood or as young adults, or who do not respond
classically to treatment [24]. Careful interpretation is needed, as
those with a cirrhotic liver may have peripheral secondary biliary
985
changes consequent on architectural distortion resembling those of
sclerosing cholangitis.
Cirrhosis regardless of aetiology is a risk factor for hepatocellular
carcinoma (HCC) [25], and those with biopsy proven cirrhosis or
imaging highly suggestive of this after initial presentation may be
considered for HCC surveillance programmes, although HCC in
cirrhotic AIH likely occurs at a frequency of only just over 1% per
year.
On treatment, imaging periodically is appropriate. A change in
spleen size, alongside thrombocytopenia, is a useful parameter to
evaluate the need for variceal surveillance. The use of transient
elastography to stage and monitor disease is evolving and is
discussed later.
Liver biopsy and histological features
Few would be confident enough to diagnose and manage AIH
without histology. Occasionally, a presumptive diagnosis is made
without a liver biopsy, for example when contraindications to
percutaneous biopsy exist or transjugular biopsy is unavailable.
Generally, however, a liver biopsy should always be performed to
identify features suggestive of AIH, exclude alternative liver disease
(in particular viral inclusions, vascular disease, non alcoholic
steatohepatitis, alcoholic hepatitis, infiltration by lymphoma or
adenocarcinoma, and Wilson disease), grade inflammatory activity,
and estimate fibrosis.
Those with access to laparoscopic biopsy are aware that the disease
is not necessarily homogeneous. To minimize sampling error,
adequately sized liver specimens (≥2.5 cm) are essential as both
parenchymal and biliary evaluations are important. Pretreatment
histological findings may help predict outcomes [26], and during
treatment liver biopsy is used to confirm resolution of histological
activity before therapy cessation. Histological activity commonly
lags behind biochemical response by at least 3–6 months. Portal
plasma cell infiltrates, while the patient is on immunosuppressant
therapy, are associated with relapse upon stopping treatment.
However, an inactive biopsy does not equate with an absent risk of
relapse.
986
Histological features
Although there are certain characteristic features, none are specific
for AIH diagnosis. Indeed, features such as lymphoplasmacytic
interface hepatitis (mononuclear cell infiltrate invading the limiting
plate, i.e. the sharply demarcated hepatocyte boundary surrounding
the portal triad), lobular hepatitis and centrilobular necrosis, can
also occur in chronic hepatitis of other causes (Fig. 19.5, Fig. 19.6,
Fig. 19.7). It is therefore usual for reports to conclude that features
are consistent with AIH, but viral and drug induced liver injury
cannot be excluded. The degree of plasmacytosis can be useful in
discriminating AIH from viral hepatitis. A paucity of plasma cells
can occur in 33% of AIH patients and their absence or the presence
of low numbers does not exclude AIH. The presence of
emperipolesis (active penetration by one cell into and through a
larger cell) is also frequently seen in AIH but is not truly disease
specific. Close attention must be paid to biliary features, as it is not
infrequent for patients with PBC to have interface activity and
initially reported as having AIH. Finally, giant cell changes can be
seen, and may be a reflection of aggressive disease.
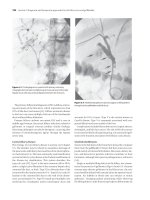
Fig. 19.5 Gross histological features of autoimmune hepatitis. (H &
E, ×50.)
Image provided by Dr Oyedele Adeyi, Staff Pathologist, Ass. Professor,
Department of Laboratory Medicine & Pathobiology, University of
Toronto.
987
Fig. 19.6 Plasma cell rich hepatitis in acute autoimmune
hepatitis. (H & E, ×100.)
Image provided by Dr Oyedele Adeyi, Staff Pathologist, Ass. Professor,
Department of Laboratory Medicine & Pathobiology, University of
Toronto.
988
Fig. 19.7 Interface hepatitis, a characteristic lesion in autoimmune
hepatitis. (H & E, ×200.)
Image provided by Dr Oyedele Adeyi, Staff Pathologist, Ass. Professor,
Department of Laboratory Medicine & Pathobiology, University of
Toronto.
Simplified grading
In a simplified scoring system, histology is graded atypical,
compatible with AIH, and typical. Interface hepatitis,
lymphocytic/lymphoplasmacytic infiltrates in portal tracts and
extending into the lobule, emperipolesis, and hepatic rosette
formation are regarded as typical for AIH [27]. To be considered
typical, all features of classic AIH histology need to be present.
Compatible features are chronic hepatitis with lymphocytic
infiltration without all the features considered usual. Histology is
considered atypical when showing signs of another diagnosis, such
as steatohepatitis, a condition that increasingly coexists with AIH
and confounds evaluation. One study found emperipolesis and
hepatocyte rosette formation to be superior independent
989
histological predictors of AIH in comparison with interface hepatitis
and plasmacytosis [28].
Classical histological features of AIH may be absent or less
pronounced in patients presenting acutely; instead, centrilobular
haemorrhagic and massive/submassive liver necrosis predominates
in 86% of patients. Central perivenulitis in addition to
lymphoplasmacytic interface hepatitis supports a diagnosis of AIH
in 50–90% of patients with acute liver failure.
Biliary lesions
The portal lesion generally spares the biliary tree but approximately
10% of biopsies may show duct destruction (not associated with
detectable AMAs), and additionally approximately 10% show
lymphocytic infiltration of bile duct epithelium without ductopenia
[29]. The histological pattern of injury may be indistinguishable
from that in PBC. Just as the presence of AMAs does not mean that
the patient has PBC, biliary changes are not synonymous with a
diagnosis of an AIH–PBC overlap syndrome. All features need to be
considered in the context of the presentation of the patient,
including severity of the underlying liver disease (Fig. 19.8 and Fig.
19.9).
990
Fig. 19.8 Trichrome staining demonstrating marked interface
hepatitis in acute autoimmune hepatitis, with plasma cell
infiltration and incidental duct injury. (×200.)
Image provided by Dr Oyedele Adeyi, Staff Pathologist, Ass. Professor,
Department of Laboratory Medicine & Pathobiology, University of
Toronto.
991