Ebook Evidence-Based dermatology (3rd edition): Part 2
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (32.81 MB, 345 trang )
S E C T I O N 3
Infective skin diseases, exanthems, and infestations
Masutaka Furue and Yuping Ran, editors
CHAPTER 38
Local treatments for cutaneous warts
Juping Chen1 and Yan Wu2
1
Department of Dermatology, Second Clinical Medical College of Yangzhou University, Yangzhou, China
Department of Dermatology, No.1 Hospital of China Medical University, Shenyang, China
2
Background
Definition
Cutaneous warts are extremely common, benign, and usually selflimiting. Infection of epidermal cells with the human papilloma
virus (HPV) results in cell proliferation and a thickened, warty
papule on the skin. The most common sites involved are hands and
feet, though any area of skin can also be infected. Genital warts are
also common and frequently sexually transmitted, but are not discussed in this chapter.
Prevalence
There are few reliable, population-based data on the prevalence of
cutaneous warts. The prevalence rate varies according to different
ages, populations, and periods of time, and it is the highest in children and young adults. Two large population-based studies revealed
prevalence rates of 0.84% and 12.9% [1,2]. Studies in school populations showed rates of 12% in 4–6-year-olds and 24% in 16–18-yearolds [3,4].
Etiology and risk factors
Warts are caused by HPV, of which there are over 70 different types.
Lesions are most common at sites of trauma, and probably result
from inoculation of virus into minimally damaged areas of epithelium. Plantar warts are often acquired from common bare-foot
areas; severe hand warts showed an occupational risk in butchers
and meat handlers [5,6].
Prognosis
Non-genital warts in immunocompetent people are harmless and
usually resolve spontaneously as a result of natural immunity
within months or years. The rate of resolution is highly variable and
probably depends on a number of factors, including host immunity,
age, HPV type, and site of infection. One frequently cited study of
an institutionalized population showed that two-thirds of warts
resolved within a 2-year period [7]. Evaluation of clearance rates in
control groups in randomized controlled trials (RCTs) may also
give some indication of natural clearance tendency, although a nonspecific benefit of vehicle bases may make interpretation difficult.
Twenty-three RCTs discussed in this chapter showed an average
cure rate of 18% (range 0–73%) with placebo preparations after an
average period of 10 weeks (range 4–24 weeks).
Diagnostic tests
Warts are frequently diagnosed clinically. Microscopic examination
obtained surgically can confirm the diagnosis if there is doubt. HPV
typing is used in research laboratories and occasionally in medicolegal cases to investigate child abuse.
Aims of treatment
To clear warts completely and permanently.
Relevant outcomes
• Cure rate (total clearance rate);
• recurrence;
• adverse reactions, such as pain and blistering.
Methods of search
Data sources and search strategy
All RCTs on the topical treatments for extragenital warts were identified, without limitation of language or publication status. The
Medline, Embase, Cochrane Library, the Meta Register of Controlled
Trials, CBM, and CNKI were searched. Reference lists of prior
reviews, systematic reviews, and trials were also checked. The most
recent searches were completed in June 2012.
Study selection and data extraction
Two investigators independently screened studies for inclusion,
retrieved potentially relevant studies, and determined eligible
studies. Disagreements were resolved by consensus. Two investigators independently extracted data from the included studies using
Evidence-based Dermatology, Third Edition. Edited by Hywel C. Williams, Michael Bigby, Andrew Herxheimer, Luigi Naldi, Berthold Rzany, Robert P. Dellavalle, Yuping Ran,
and Masutaka Furue.
© 2014 John Wiley & Sons, Ltd. Published 2014 by John Wiley & Sons, Ltd.
Companion Website: www.evidencebasedseries.com/dermatology
320
Local treatments for cutaneous warts 321
custom-made standardized forms and a third investigator was
assigned with the checking process.
active treatment group developed cellulitis. Minor skin irritation
was noted occasionally in some trials.
Quality assessment
Comments
The criteria recommended by the Cochrane Collaboration Handbook
were used to assess the methodological quality of included trials. It
mainly focused on description of randomization (sequence generation progress), allocation concealment, blinding, addressing of
incomplete outcome data, reporting of selective outcome, and other
potential threats to validity [8]. The judgment for each entry was
based on the answer of a question, with “yes” indicating “low risk
of bias,” “no” indicating “high risk of bias,” and “unclear” indicating
“either lack of information or uncertainty over the potential for
bias” [8]. Disagreements were resolved by group consensus.
Outcomes measurement
The primary outcome is the cure rate (total clearance rate). The
secondary outcome is adverse reactions.
Statistical analysis
All statistical analyses were performed using the duplicate data
entry facility of Revman 5.0.25 (evidence-based medicine software)
by two investigators [9]. In addition to 95% confidence intervals
(CIs), relative risks (RRs) and mean differences (MDs) were respectively used for dichotomous and continuous outcomes. The χ2 (chisquare test) statistic was calculated to determine the proportion of
between-study variation due to heterogeneity. The value ranged
from 0 to 100%, and high values indicated strong heterogeneity. If
heterogeneity was low (P > 0.1, χ2 < 50%), a fixed-effect model
was used, otherwise a random-effect model was used. Intention to
treat (ITT) was done for efficacy evaluation, and per-protocol (PP)
analysis was done for adverse reaction evaluation.
Results
Salicylic acid or topical products
containing salicylic acid
Efficacy
Salicylic acid versus placebo
Five RCTs [10–14] compared salicylic acid (SA) with placebo. SA
preparations gave a higher cure rate of 73% versus that of 48% with
placebo. The RR was 1.60, 95% CI was 1.16–2.23, number needed
to treat (NNT) was 4 (95% CI, 3–7).
Salicylic acid versus cryotherapy
Three RCTs [15–17] compared SA with cryotherapy. The cure rates
were respectively 60% and 70% in the two groups, and no significant difference was seen between the two treatments (RR, 0.85; 95%
CI, 0.59–1.21).
There is reservation about the validity of pooled data from the different RCTs, because of the generally low quality of trials and the
heterogeneity of their design and methodology. For instance, the
RCTs included used different topical SA products, or some RCTs
included patients with refractory warts whereas others excluded
them. Despite this, we consider that there is good evidence for a
modest benefit of SA in treating non-genital warts.
Cryotherapy with liquid nitrogen
Efficacy
Cryotherapy versus placebo or no treatment
Two small RCTs compared cryotherapy with either placebo cream
[22] or no treatment [23]. One of the two trials [22] reported a very
low cure rate for cryotherapy (one of 11), while the other trial [23]
showed a high cure rate in the placebo group (eight of 20). The
pooled data of cure rates did not demonstrate a significant difference between the cryotherapy group and control group: 35% versus
34% (RR, 0.88; 95% CI, 0.26–2.95).
Cryotherapy versus salicylic acid
Three RCTs [15–17] compared SA with cryotherapy. The results
have already been mentioned in the section entitled “Salicylic acid
versus cryotherapy.”
Cryotherapy versus bleomycin
Two RCTs compared cryotherapy with intralesional bleomycin
[24,25]. Because of heterogeneity of methodology and design, the
two trials are described separately. One trial [24], which used 1 mg/
mL bleomycin, obtained cure rates of 76.5% in the cryotherapy
group and 94.9% in the bleomycin group (RR, 0.18; 95% CI, 0.03–
0.90, P = 0.04). Another RCT [25], using 0.5 mg/mL bleomycin,
had a cure rate of 68.2% in the cryotherapy group and 100% in the
bleomycin group (RR, 1.27 (1 < RR < 1.6); P < 0.05).
Length of freeze
Four RCTs [26–29] compared aggressive and gentle cryotherapy in
592 adults and children. However, the definitions of “aggressive”
and “gentle” used differed, and some studies included refractory
warts whereas others did not. Overall, cure rates were 52% with
aggressive cryotherapy and 31% with gentle cryotherapy (RR, 1.90;
95% CI, 1.15–3.15; NNT, 5; 95% CI, 3–7).
Interval between freezes
Three RCTs [15,30,31] showed no significant difference in cure
rates among 2-week, 3-week, and 4-week intervals. Cure was generally achieved more quickly with shorter treatment intervals.
Other comparisons
Seven RCTs [15,18–21] compared different products containing SA
or compared SA with other topical treatments, such as glutaraldehyde and dithranol. The heterogeneous trials showed no convincing
advantage of any particular delivery system for SA.
Optimum number of freezes
Only one RCT [32] examined this question in 115 adults and
children who did not cure 3 months after 3-weekly cryotherapy
and showed no benefit of prolonging cryotherapy for a further 3
months. The cure rates were 43% and 38% in the treated and
untreated groups, respectively (no data available for calculating the
odds ratio).
Drawbacks
Drawbacks
Generally, topical SA was reported to have no significant harmful
effects. In one RCT that compared a mixture of monochloroacetic
acid and 60% SA with placebo [13], one of the 29 patients in the
Only two RCTs included precise data on adverse events. Pain or
blistering was reported by 64 of 100 participants (64%) treated with
an “aggressive” (10 s) regimen, in comparison with 44 of 100
322 The evidence
participants (44%) treated with a “gentle” (brief freeze) regimen
(RR, 1.45; 95% CI, 1.12–2.31). Five participants withdrew from the
aggressive-regimen group and one from the gentle-regimen group
because of pain and blistering [26]. Pain or blistering was reported
in 29%, 7%, and 0% of those treated at 1-week, 2-week, and 3-week
intervals, respectively (no data available for the odds ratio) [30].
The rate of reported adverse affects was relatively high with a
shorter interval, but this is likely to be a reporting artifact, as these
participants were seen sooner after each treatment.
Comments
The evidence from the available RCTs of cryotherapy for warts is
limited and contradictory. Just as the RCTs on topical SA, the heterogeneities of study designs, methods, and populations make it
difficult to draw firm conclusions from the pooled data. For
instance, some trials included all types of warts on the hands and
feet in all age groups, whereas others were more selective and
simply focused on hand warts, or excluded certain groups such as
those with mosaic plantar warts or refractory warts. Of particular
note is the likelihood that the “populations” in these studies may
have very different characteristics at different periods of time. For
example, studies conducted in the 1970s in the UK would have
included a higher proportion of participants with incident warts,
which have a greater chance of cure or spontaneous resolution. In
the 1980s and 1990s, more people with warts were treated in
primary care. Thus, clinics would have had a more selected population with a higher proportion of refractory warts and correspondingly lower cure rates.
Occlusive treatment with duct tape
Efficacy
Duct tape versus placebo
Two published trials did this comparison and did not show good
cure rates with duct tape [33,34]. The trial by De Haen et al. [33]
included 103 primary-school children who were randomly assigned
to continuous duct tape and a no occlusive corn pad once a week.
After 6 weeks, recurrence occurred in eight of 51 (16%) versus three
of 52 (6%) of the trial warts. Curiously, other untreated warts
resolved in 21% and 27% of the children, respectively. Wenner et
al. [34] conducted a trial comparing occlusive duct tape (obscured
by a sticky-backed fabric called moleskin) with the moleskin alone
in 90 adult patients (the trial population had an unusually high age
of 54 years). After 8 weeks, relapse occurred in eight of 39 (21%)
versus nine of 41 (22%), respectively. Among the cured patients, six
of eight (75%) and three of 10 (30%), respectively, had relapsed
again after 6 months.
Duct tape versus cryotherapy
One trial compared occlusive treatment with duct tape and cryotherapy in 61 children and young adults [35]. The duct tape was
applied for 6.5 days every 7 days, cryotherapy was given for 10 s
every 2–3 weeks up to a maximum of six times. The cure rates were
22 of 30 (71%) and 15 of 31 (46%), respectively (RR, 1.52; 95% CI,
0.99–2.31). There were limitations to the trial: the number of
patients was relatively small, 10 s of cryotherapy was inadequate, an
unspecified number of outcome assessments were carried out over
the phone, and it was not entirely clear how long after the treatment
period was done.
Drawbacks
Duct tape is simple, safe, and cheap. No significant adverse events
were mentioned in any of these trials.
Comments
None of these three trials are strong methodologically; thus, the
results are somewhat contradictory and difficult to summarize
meaningfully.
Contact immunotherapy with
dinitrochlorobenzene
Efficacy
Two small RCTs [22,36] of dinitrochlorobenzene (DNCB) in 80
children and adults achieved a cure rate of 80% (32/40) in comparison with 38% (15/40) in the placebo/no treatment groups (RR, 2.12;
95% CI, 1.38–3.26; NNT, 2; 95% CI, 2–4).
Drawbacks
One trial [36] commented that six of 20 participants treated with
2% DNCB were sensitized only after the second application. All of
them subsequently experienced significant local irritation, with
blistering, when they were treated with 1% DNCB. None withdrew
from the study.
Comments
DNCB, a potent contact allergen, can cause significant local irritation and dermatitis, which probably precludes its use outside specialist centers.
Photodynamic therapy
Efficacy
Photodynamic therapy versus placebo
One trial [37] randomized active (proflavine+black light) and
placebo (picric acid + black light) treatments for recalcitrant symmetrical verrucae vulgaris, on the left and right sides of the body.
The result showed no significant difference. Three trials [38–40]
compared 5-aminolevulinic acid (ALA)–photodynamic therapy
(PDT) with placebo–PDT, and all patients locally used keratolytic.
Meta-analysis showed no significant difference (RR, 1.53; 95% CI,
0.86–2.73).
Photodynamic therapy versus salicylic acid
One RCT [41] including 120 adults and children compared methylene blue/dimethyl sulfoxide PDT with a mixture of SA and creosote. The cure rates were 8% and 15%, respectively.
Photodynamic therapy versus cryotherapy
In one RCT [42], four different types of light source for PDT were
compared with cryotherapy. Topical SA was used for all patients.
More warts were completely healed after white light–PDT than after
red/blue light–PDT and cryotherapy.
5-Aminolevulinic acid–photodynamic therapy versus highfrequency electrocautery
One RCT [43] including 60 patients with plantar warts did this
comparison. The cure rates of ALA treatment group were 37.5% (12
of 32 patients) and 17.86% (five of 28 warts), which were higher
than those of the high-frequency electrocautery treatment group.
5-Aminolevulinic acid–photodynamic therapy+salicylic acid
versus microwave therapy
One trial [44] including 126 plantar warts patients showed the cure
rates were similar between two groups, while the recurrence rate
was lower in the ALA treatment group than that in the microwave
treatment group.
Local treatments for cutaneous warts 323
5-Aminolevulinic acid–photodynamic therapy versus
semiconductor laser
One RCT [45] reported that ALA–PDT is superior to semiconductor laser (cure rates 25/28 vs 18/28).
Different concentrations of bleomycin
One RCT [54] in 26 adults, comparing 0.25, 0.5, and 1.0 IU/mL
bleomycin, showed cure rates of 73%, 88%, and 90% of warts,
respectively; the differences were not statistically significant.
Methyl aminolevulinate–photodynamic therapy+chemical
keratolytic treatment versus chemical keratolytic treatment
One trial [46] evaluated methyl aminolevulinate (MAL)–PDT with
or without chemical keratolytic treatment for hand warts in a population of renal transplant patients. The number of vanished warts
showed no significant difference between two treatments.
Drawbacks
5-Aminolevulinic acid–photodynamic therapy+CO2 laser versus
CO2 laser
In one trial [47], a total of 70 patients with plantar warts were randomly divided into two groups. The cure rates were 48.6% (17 of
35 patients) and 20% (seven of 35 warts) in ALA–PDT+CO2 laser
treatment group, higher than those of CO2 laser treatment group.
Side effects
One RCT [48] focused on the pain induced by PDT of warts, by
filling in questionnaires about pain immediately and 24 h after each
treatment. Forty-five patients were enrolled in a randomized,
placebo-controlled trial with six consecutive ALA– and placebo–
PDT treatments for recalcitrant foot and hand warts. Severe or
unbearable pain was reported from a median of 17% (6–31%) of
the ALA-treated warts and from a median of 2% (0–15%) from the
placebo-treated warts immediately after the treatments. With
increasing treatments, no significant change in pain intensity was
observed, no significant relation was found between the pain intensity and the relative change in wart area. The pain was primarily
characterized as burning and shooting. The pain lasted about 30 h
(range: 1–96 h).
Nine RCTs [38–40,42–47] reported the side effects during or
after the PDT treatment. Pain, burning, itching, local anesthesia,
swelling, and erythema were common; most were mild/moderate
and acceptable. No treatments were suspended because of pain.
Comments
The fact that most trials used different protocols of PDT (different
photosensitizer, different light sources, different treatment intervals, and so on) makes it difficult to draw a definite conclusion. So
far, PDT appears to offer no particular advantages in cure rates or
adverse effects than other simpler and cheaper local treatments
available. But for recalcitrant warts, it might be an alternative
approach, which should be proved by further research.
Intralesional bleomycin
Efficacy
Bleomycin versus placebo
Conflicting results were reported in five RCTs of intralesional bleomycin. Three trials [49–51] reported higher cure rates with bleomycin than with placebo, one [52] showed that placebo was
associated with higher cure rates than bleomycin, and one [53]
showed no significant differences between bleomycin and placebo.
The pooled results showed that bleomycin was more effective than
placebo in the treatment of cutaneous warts (RR, 3.84; 95% CI,
2.19–6.71).
Bleomycin versus cryotherapy
Two RCTs [24,25] compared liquid-nitrogen cryotherapy with intralesional bleomycin. The results were shown in the section
“Cryotherapy with liquid nitrogen.”
No precise data on adverse effects were provided in any of the RCTs.
One RCT [52] reported “adverse events” in 19/62 (31%) participants, but the nature of the adverse events and the proportions in
the active treatment and placebo groups were not specified. Three
of the trials [49,50,54] reported that most participants experienced
pain. In two trials [50,51], local anesthetic was used routinely
before the injection of bleomycin. One trial [54] reported pain was
seen in most participants, which was irrespective of dose. In one
trial [49] of 24 participants who received bleomycin, two patients
withdrew because of the pain of the injections and pain in the
period after the injection.
Comments
Again, methodological and statistical heterogeneity (different outcomes, trial periods, units of analysis, numbers of injections, vehicles, and concentrations) make it impossible to organize the data
from these trials.
5-Fluorouracil
Efficacy
5-Fluorouracil versus placebo
One RCT [55] compared 5-fluorouracil with placebo. Altogether,
60 patients (including 40 children) were randomized to be treated
with topical 5-fluorouracil for 4 weeks and placebo. During the
procedure, 31 patients were lost to the active treatment and the cure
rate was 60%, versus three lost to the placebo treatment and the
cure rate was 17%. There were statistically significant difference
between the two treatments (RR, 7.44; 95% CI, 2.86–19.35).
5-Fluorouracil cryotherapy versus cryotherapy+placebo
One RCT [56] compared cryotherapy combination with 5-
fluorouracil and cryotherapy combination with placebo; the cure
rates were 58.57% and 65.29% respectively. There was no significant
difference (RR, 0.55; 95% CI, 0.22–1.40).
5-Fluorouracil+duct tap versus duct tape
One RCT [57], including 40 patients, treated with 5-fluorouracil
combined with duct tape and duct tape alone for 12 weeks. The cure
rates were 95% (19/20) and 10% (2/20) respectively (RR, 9.50; 95%
CI, 2.54–35.51).
Drawbacks
Only one trial [57] mentioned topical 5-fluorouracil side effects.
Eleven cases of fingertip or periungual warts had nail separation
after topical 5-fluorouracil treatment; the rate was 22.9% out of 48
cases in total. However, the nature and proportion of the side effects
of the treatment were not elaborated.
Comments
Methodological and statistical heterogeneity (different outcomes,
trial periods, units of analysis, vehicles, and concentrations) make
it impossible to organize the data from these trials.
Localized heat therapy
Efficacy
Localized heat therapy versus placebo
Two RCTs [58,59] compared localized heat therapy with placebo;
because of heterogeneity in methodology and design, the two trials
324 The evidence
are described separately. One trial [58] including 13 patients (29
warts) used local hyperthermia at 50 °C lasting 30 min. The cure
rates were 86% (25/29) in the treatment group and 41% (7/17) in
the control group, the difference was statistically significant (RR,
8.93; 95% CI, 2.14–37.34). Another RCT [59] used local hyperthermia at 44° lasting 30 min once a day for three consecutive days. Two
weeks later, patients received similar treatments for two consecutive
days. Patients in the control group received a red spot on the targeted lesion without experiencing a heating sensation. Three
months later, the cure rates were 53.57% (15/28) and 11.54% (3/26),
respectively. The difference was statistically significant (RR, 8.85;
95% CI, 2.15–36.37).
Drawbacks
One trial [59] showed that 80% of patients (12/15) in the treatment
group who had initial complaints of load-bearing pain reported a
decreased sensation of pain, one experienced an increased sensation of pain, and two remained stable. In the control group, 14.3%
of patients (2/14) reported a decreased sensation of pain, five experienced an increased sensation of pain, and three remained stable.
There was a significant difference between the two groups (RR, 24;
95% CI, 3.38–170.38).
Comments
Methodological and statistical heterogeneity (different outcomes,
trial periods, units of analysis, vehicles, and concentrations) make
it impossible to organize the data from these trials.
Light and laser treatment
Efficacy
Intense pulsed light
In one trial [60], 79 patients with recalcitrant hand and foot warts
were included and randomized into treatments with either paring
of warts followed by intense pulsed light (IPL) or paring of warts
alone. No significant difference in cure rate was found between the
two intervention groups, but the pain intensity after paring plus IPL
was significantly higher than that after paring alone.
Pulsed dye laser
One trial [61] compared the efficacy and safety of pulsed dye laser
(PDL) (595 nm PDL + cooling pulses) with a placebo (cooling
pulses alone) in the treatment of patients presenting palmoplantar
warts. For both groups, hyperkeratosis was removed manually with
a scalpel before each session. The results showed that 64% (48/75)
of warts in the laser group resolved completely compared with 13%
(4/30) in the placebo group (P < 0.001). Three trials [62–65]
(including adults and children) compared PDL therapy with cryotherapy and no superior efficacy was found (RR, 1.02; 95% CI,
0.85–1.21). One controlled study [66] included 66 lesions from 19
patients. PDL was applied to 33 lesions following 30% SA application twice a day for 5 days; the remaining 33 lesions underwent
PDL therapy alone. PDL was administered in both groups at 4-week
intervals varying from one to five sessions. Complete clearance was
observed after 2.2 sessions in the SA+PDL group versus 3.1 sessions in the PDL group (P < 0.05). Although the clearance rate
showed no difference between the SA+PDL group and the PDL
group after all the sessions, adding SA to PDL decreased the number
of sessions to an extent.
Q-switched laser
Two trials [67,68] (including 212 adults and children with verruca
planar) compared Q-switched laser therapy with cryotherapy. All
patients took transfer factor capsules; 92 patients were injected with
immnunoenhancement for 3 weeks. Q-switched laser therapy
obtained higher cure rates (RR, 1.26; 95% CI, 1.12–1.43). The other
two trials [69,70] compared Q-switched laser therapy with combination treatments (retinoic acid cream, α-2b interferon (IFN) ointment, ceramic grinding treatment). The combination treatments
were superior to single laser treatment.
CO2 laser
Three trials [71–73] (including 453 adults) compared CO2 laser
therapy with cryotherapy. A significant difference was found
between the two treatments (RR, 1.36; 95 % CI, 1.01–1.83). Two
trials [74,75] (including 575 adults) compared CO2 laser therapy
with microwaves therapy. CO2 laser therapy did not give higher
cure rates (RR, 1.05; 95% CI, 0.78–1.41). Another nine trials
[64,72,76–82] compared topical treatments (α-2b IFN ointment,
semiconductor laser, different types of CO2 laser, retinoin acid
drugs, etc.) combined with or without CO2 laser therapy. The results
showed that combination treatments were superior to single laser
treatment.
Holmium laser
One trial [83] including 120 children and adult patients with
plantar warts compared holmium laser with cryotherapy. The cure
rate was 97.5% (78/80) versus 55% (22/40). A significant difference
was found between the two treatments, but high risk of bias was
found in this trial.
Drawbacks
Ten RCTs [60,63,65,66,69,76–80] reported the side effects caused
by light or laser therapy, including pain, erythema, swelling, hyperpigmentation, burning, itching, desquamation, crusts, and scar.
Most of them were mild and tolerable.
Comments
PDL appears to be an effective treatment in non-genital cutaneous
warts, but the efficacy seems to be no superior to that of traditional
treatments (cryotherapy). Other laser treatments (Q-switched laser,
CO2 laser, etc.) seem to be more effective than cryotherapy, but
more placebo-controlled trials should be undertaken to prove the
results. The combination treatment could be considered in clinical
practice.
Imiquimod
Efficacy
Imiquimod versus retinoic acid drugs
Four RCTs [84–87] (including 308 adults and children) did this
comparison. Topical 5% imiquimod cream did not give higher cure
rates (RR, 1.26; 95% CI, 0.98–1.63).
Imiquimod+retinoic acid drugs versus retinoic acid drugs
Three trials [84,88,89] (including 216 adults and children) did this
comparison. The cure rate was 42.99% versus 24.04%. A significant
difference was found between the two treatments (RR, 1.80; 95%
CI, 1.20–2.70).
Imiquimod+retinoic acid drugs versus imiquimod
Three trials [84,90,91] (including 229 adults and children) did this
comparison. The cure rate was 52.63% versus 22.61%. A significant
difference was found between the two treatments (RR, 2.33; 95%
CI, 1.59–3.42).
Local treatments for cutaneous warts 325
Other comparisons
Nine trials [92–100] compared topical treatments (α-2b IFN ointment, traditional Chinese medicine, cryotherapy, microwave, hot
water immersion, etc.) with or without imiquimod cream. The
limited evidence showed the combination treatments were superior
to single treatment.
or topical treatments in 16 trials [91,97,111–124], seven trials
[84,89,90,125–128], five trials [120,129–132], and one trial [133].
The limited evidence showed that the combination treatments were
superior to the single treatment. However, all the trials had a high
risk of bias, so the results were less worthy of belief.
Drawbacks
All RCTs except one trial [120] reported the side effects caused by
retinoic acid drugs. The most common drawbacks were erythema,
itching, and desquamation. Most patients could get better after
treatment cessation, using vitamin E ointment, reducing treatment
frequency, or even keeping on treatment. Other side effects were
burning, swelling, photosensitive, tight skin, tingling, pigmentation, and so on.
Sixteen RCTs reported the side effects caused by imiquimod cream,
including erythema, burning, tingle, swelling, erosion, itching,
edema, desquamation, and pigmentation. No systemic side effects
were reported.
Comments
No evidence showed good efficacy of 5% imiquimod cream on
non-genital cutaneous warts. Although the pooled data showed
combined treatment with retinoic acid drugs to be effective in
verruca planar, the results should be verified by high-quality RCTs.
Retinoic acid drugs
Efficacy
Retinoic acid
Retinoic acid versus α-2b interferon Two RCTs [101,102] (including 218 adults and children) compared retinoic acid ointment with
α-2b IFN ointment. Topical retinoic acid ointment did not give
higher cure rates (RR, 0.87; 95% CI, 0.59–1.31).
Retinoic acid+α-2b interferon versus retinoic acid Three RCTs
[101–103] (including 324 adults and children) did this comparison.
A significant difference was found between the two treatments (RR,
1.99; 95% CI, 1.53–2.57).
Retinoic acid+α-2b interferon versus α-2b interferon Four RCTs
[101,102,104,105] compared retinoic acid cream combined with
α-2b IFN ointment in 350 adults and children. The pooled data
showed a significant difference between the two treatments (RR,
1.84; 95 % CI, 1.45–2.32).
Tazarotene
Tazarotene+α-2b interferon versus tazarotene Two RCTs [106,107]
did this comparison in 68 patients with verruca planar and 104
patients with plantar warts. The combination treatment group had
a higher cure rate than the tazarotene ointment only group (RR,
2.79; 95% CI, 1.62–4.81).
Tazarotene+α-2b interferon versus α-2b interferon In one RCT
[108], 98 adults and children with verruca planar were randomized
into two groups (topical tazarotene cream combined with α-2b IFN
ointment group and topical α-2b IFN ointment alone group). After
4 weeks, the cure rates were 48.08% (25/52) versus 23.91% (11/46).
The former had greater efficacy than the latter on treatment of
verruca planar.
Tazarotene versus ftibamzone Two RCTs [109,110] (including 347
adults and children with verruca planar) compared tazarotene
cream with ftibamzone ointment. The pooled data did not demonstrate a significant difference in cure rates (RR, 1.15; 95% CI,
0.80–1.63).
Other comparisons
Retinoic acid ointment, tazarotene cream, adapalene gel, and
isotretinoin gel were respectively compared with different products
Drawbacks
Comments
It was hard to draw a definite conclusion on the efficacy of retinoic
acid drugs because high-quality placebo-controlled trials were
absent. Although the pooled data showed that retinoic acid drugs
combined with α-2b IFN treatment were effective in verruca planar,
the generally low quality of trials means there are reservations.
Furthermore, clinical heterogeneity made it impossible to organize
the data from most trials. So there was no compelling evidence to
give retinoic acid drugs to non-genital cutaneous warts patients.
Other local treatments for warts
Intralesional IFN as a treatment for warts is more of historical interest. Six trials [134–139], most dating from the 1970s and 1980s,
were found using this treatment; evidence provided by all the trials
was severely limited by heterogeneity of the methodology and
design, and overall did not suggest any striking efficacy.
In one RCT [140] of intralesional antigen therapy, a form of local
immunotherapy was designed to elicit an immune reaction in
warts, and patients were injected with Candida, mumps, or
Trichophyton antigens. Unfortunately, the design of the trial was
made more complex by the addition of intralesional IFN, resulting
in four treatment arms (antigen with or without IFN and placebo
with or without IFN) rather than two, which would have given
much clearer data. Up to five injections were given at 3-weekly
intervals into the largest wart in each patient. Blinding involved
only the patients and not the investigators, introducing a source of
potentially significant bias. The main outcome reported was a
reduction of more than 75% in the wart surface area at the end of
treatment – an outcome of no relevance to patients (who naturally
want their warts cleared). No long-term follow-up appears to have
been carried out. A total of 201 patients with refractory warts completed the trial; 57 of 95 patients (60%) injected with antigen with
or without additional IFN experienced the resolution of at least one
wart, in comparison with 25 of 106 patients (24%) injected with
saline or IFN alone. The number of patients who experienced complete clearance of all warts is a little difficult to ascertain from the
paper, but it appears to have been 21 of 95 (22%) in the treatment
groups and 11 of 106 (10%) in the “placebo” groups. For a fairly
painful and expensive treatment, this does not appear to offer any
striking advantages.
One RCT [141] compared 20% zinc oxide and 15% SA complex,
involving 44 patients who were randomized to the two treatments
twice a day for 3 months. The results showed that the cure rates of
zinc oxide and SA complex group were respectively 50% and 42%,
and there was no statistically difference between the two treatments
(RR, 1.44; 95% CI, 0.44–4.76). Topical zinc oxide is a possible treatment of warts, but needs more RCTs to support.
326 The evidence
One RCT [142] compared topical α-lactalbumin-oleic acid with
placebo. The trial employed an unusual hybrid molecule (consisting
of a combination of α-lactalbumin from human breast milk and
oleic acid), which was said to be lethal to a wide range of transformed cells but harmless to normal ones. The trial was properly
randomized and double blinded, and the analysis focused on the
main outcome of a more than 75% reduction in wart volume, rather
than the more relevant complete clearance of warts. Unfortunately,
the trial defaulted to an open-label design after 3 months, making
the long-term follow-up data unconvincing. Although 100% of
patients in the treatment group were reported to have experienced
a reduction in wart volume of more than 75%, only 21% of the
lesions in the treatment group resolved completely. Nine of 20
patients (45%) with active treatment experienced the resolution of
at least one wart, in comparison with three of 20 (15%) in the
placebo group (RR, 3.0; 95% CI, 0.95–9.48). Given the size of
the trial, and consequently the wide CIs, the results were
unconvincing.
One RCT [143] compared smoke from leaves of Populus euphratica Olivier and conventional cryotherapy, once a week, for 10
times. After 22 weeks, the cure rates were 68% (16/24) and 46%
(13/28) respectively (RR, 2.31; 95% CI, 0.75–17.13).
No RCTs were identified that studied the efficacy of the following
treatments: surgical excision, curettage and cautery, formaldehyde,
podophyllin, and podophyllotoxin.
Key points
• Topical preparations containing SA are generally effective and safe
for treating warts.
• The available, rather limited, evidence shows that no significant
difference was seen between cryotherapy and topical treatments
containing SA.
• Contact immunotherapy with DNCB appears to be a promising
treatment, but is probably best reserved for highly refractory
warts.
• PDT and PDL therapy do not appear to have any particular
advantage in terms of higher cure rates or fewer adverse effects
than the other simpler and cheaper local treatments available.
• There is no compelling evidence for the efficacy of intralesional
bleomycin.
References
1. Johnson M, Roberts J. Skin Conditions and Related Need for Medical Care among
Persons 1–74 Years. United States, 1971–1974. Vital and Health Statistics, Series
11, No. 212. Washington, DC: US Department of Health, Education and Welfare/
National Centre for Health Statistics, 1978: 1–72.
2. Beliaeva TL. The population incidence of warts. Vestn Dermatol Venereol
1990;2:55–8 (in Russian).
3. Williams HC, Pottier A, Strachan D. The descriptive epidemiology of warts in
British schoolchildren. Br J Dermatol 1993;128:504–11.
4. Kilkenny M, Merlin K, Young R, et al. The prevalence of common skin conditions
in Australian school students: 1. Common, plane and plantar viral warts. Br J
Dermatol 1998;138:840–5.
5. Johnson LW. Communal showers and the risk of plantar warts. J Fam Pract 1995;
40:136–8.
6. Keefe M, al-Ghamdi A, Coggon D, et al. Cutaneous warts in butchers. Br J
Dermatol 1994;130:9–14.
7. Massing AM, Epstein WL. Natural history of warts. Arch Dermatol 1963;87:
306–10.
8. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews
of Interventions Version 5.1.0, [updated March 2011]. The Cochrane Collab
oration, 2011. Available from www.cochrane-handbook.org [accessed March 10,
2014].
9. Review Manager (RevMan) [Computer Program]. Version 5.0.25 for Windows.
Oxford: The Cochrane Collaboration, 2010.
10. Bart BJ, Biglow J, Vance JC, et al. Salicylic acid in karaya gum patch as a treatment
for verruca vulgaris. J Am Acad Dermatol 1989;20:74–6.
11. Felt BT, Hall H, Olness K, et al. Wart regression in children: comparison of
relaxation imagery to topical treatment and equal time interventions. Am J Clin
Hypn 1998;41:130–7.
12. Spanos NP, Williams V, Gwynn MI. Effects of hypnotic, placebo, and salicylic
acid treatments on wart regression. Psychosom Med 1990;52:109–14.
13. Steele K, Shirodaria P, O’Hare M, et al. Monochloroacetic acid and 60% salicylic
acid as a treatment for simple plantar warts: effectiveness and mode of action. Br
J Dermatol 1988;118:537–43.
14. Bunney MH, Hunter JA, Ogilvie MM, et al. The treatment of plantar warts in
the home. A critical appraisal of a new preparation. Practitioner 1971;207:
197–204.
15. Bunney MH, Nolan MW, Williams DA. An assessment of methods of treating
viral warts by comparative treatment trials based on a standard design. Br J
Dermatol 1976;94:667–79.
16. Steele K, Irwin WG. Liquid nitrogen and salicylic/lactic acid paint in the treatment of cutaneous warts in general practice. J R Coll Gen Pract 1988;38:256–8.
17. Cockayne S, Curran M, Denby G, et al. EVerT: cryotherapy versus salicylic acid
for the treatment of verrucae-a randomised controlled trial. Health Technol Assess
2011;32:1–170.
18. Auken G, Gade M. Pilgaard CE. Treatment of warts of the hands and feet with
Verucid. Ugeskr Laeger 1975;137:3036–8.
19. Flindt-Hansen H, Tikjob G, Brandrup F. Wart treatment with anthralin. Acta
Derm Venereol 1984;64:177–9.
20. Parton AM, Sommerville RG. The treatment of plantar verrucae by triggering
cell-mediated immunity. Br J Pod Med 1994;131:883–6.
21. Veien NK, Madsen SM, Avrach W, et al. The treatment of plantar warts with a
keratolytic agent and occlusion. J Dermatol Treat 1991;2:59–61.
22. Gibson JR, Harvey SG, Barth J, et al. A comparison of acyclovir cream versus
placebo cream versus liquid nitrogen in the treatment of viral plantar warts.
Dermatologica 1984;168:178–81.
23. Wilson P. Immunotherapy v cryotherapy for hand warts; a controlled trial. Scott
Med J 1983;28:191 [abstract].
24. Dhar SB, Rashid MM, Islam A, et al. Intralesional bleomycin in the treatment of
cutaneous warts: a randomized clinical trial comparing it with cryotherapy.
Indian J Dermatol Venereol Leprol 2009;75:262–7.
25. Adalatkhah H, Khalilollahi H, Amini N, et al. Compared therapeutic efficacy
between intralesional bleomycin and cryotherapy for common warts: a randomized clinical trial. Dermatol Online J 2007;13:4.
26. Connolly M, Basmi K, O’Connell M, et al. Efficacy of cryotherapy is related to
severity of freeze. Br J Dermatol 1999;141:31 [abstract].
27. Sonnex TS, Camp RDR. The treatment of recalcitrant viral warts with high dose
cryosurgery under local anaesthesia. Br J Dermatol 1988;199:38–9 [abstract].
28. Berth-Jones J, Bourke J, Eglitis H, et al. Value of a second freeze-thaw cycle in
cryotherapy of common warts. Br J Dermatol 1994;131:883–6.
29. Hansen JG, Schmidt H. Plantar warts. Occurrence and cryosurgical treatment.
Ugeskr Laeger 1986;148:173–4.
30. Bourke JF, Berth-Jones J, Hutchinson PE. Cryotherapy of common viral warts at
intervals of 1, 2 and 3 weeks. Br J Dermatol 1995;132:433–6.
31. Larsen PO, Laurberg G. Cryotherapy of viral warts. J Dermatol Treat 1996;7:
29–31.
32. Berth-Jones J, Hutchinson PE. Modern treatment of warts: cure rates at 3 and 6
months. Br J Dermatol 1992;127:262–5.
33. De Haen M, Spigt MG, van Uden CJ, et al. Efficacy of duct tape vs. placebo in
the treatment of verruca vulgaris (warts) in primary school children. Arch
Paediatr Adolesc Med 2006;160:1121–5.
34. Wenner R, Askari S, Cham P, et al. Duct tape for the treatment of common warts
in adults. Arch Dermatol 2007;143:309–13.
35. Focht DR, Spicer C, Fairchok MP. The efficacy of duct tape vs. cryotherapy in the
treatment of verruca vulgaris. Arch Paediatr Adolesc Med 2002;156:971–4.
36. Rosado-Cancino MA, Ruiz-Maldonado R, Tamayo L, et al. Treatment of multiple
and stubborn warts in children with 1-chloro-2,4-dinitrobenzene (DNCB) and
placebo. Dermatol Rev Mex 1989;33:245–52.
37. Veien NK, Genner J, Brodthagen H, et al. Photodynamic inactivation of verrucae
vulgares. II. Acta Derm Venereol 1977;57:445–7.
38. Fuchs SM, Fluhr JW, Bankova L, et al. Photodynamic therapy (PDT) and water
filtered infrared A (wIRA) in patients with recalcitrant common hand and foot
warts. Ger Med Sci 2004;2:1612–3174.
39. Stender IM, Na R, Fogh H, et al. Photodynamic therapy with 5-aminolaevulinic
acid or placebo for recalcitrant foot and hand warts: randomised double-blind
trial. Lancet 2000;355:963–6.
Local treatments for cutaneous warts 327
40. Fabbrocini G, Di Costanzo MP, Riccardo AM, et al. Photodynamic therapy with
topical delta-aminolaevulinic acid for the treatment of plantar warts. J Photochem
Photobiol B 2001;61:30–4.
41. Stahl D, Veien NK, Wulf HC. Photodynamic inactivation of virus warts: a controlled clinical trial. Clin Exp Dermatol 1979;4:81–5.
42. Stender IM, Lock-Anderson J, Wulf HC. Recalcitrant hand and foot warts successfully treated with photodynamic therapy with topical 5-aminolaevulinic acid:
a pilot study. Clin Exp Dermatol 1999;24:154–9.
43. Chen R, Chen H, Wu Z, et al. Comparison of therapeutic effect of 5-ALAphotodynamic therapy and high frequency electrocautery on plantar warts. J
Guangdong Med Coll 2008;26:522–4 (in Chinese).
44. Wang Q, Zhu J. Clinical efficacy of photodynamic therapy with 5-aminolaevulinic
acid on the treatment of patients with multi plantar warts. People’s Mil Surg 2012;
55:433–4 (in Chinese).
45. Xia Y, Xu S, Liu M, et al. Photodynamic therapy with 5-aminolaevulinic acid on
plantar warts. Dermatology 2007;40:646.
46. Sparsa A, Blaise S, Tack B, et al. Photodynamic therapy can improve warts’ discomfort in renal transplant patients prospective multicenter study. Photochem
Photobiol 2012;88:1023–6.
47. Wang Y, Wang Z, Ruan Y. Topical treatment using photodynamic therapy with
5-aminolaevulinic acid combined with CO2 laser on plantar warts. Chinese J Laser
Med Surg 2010;19:307–9 (in Chinese).
48. Stender IM, Borgbjerg FM, Villumsen J, et al. Pain induced by photody
namic therapy of warts. Photodermatol Photoimmunol Photomed 2006;22:
304–9.
49. Bunney MH, Nolan MW, Buxton PK, et al. The treatment of resistant warts
with intralesional bleomycin: a controlled clinical trial. Br J Dermatol 1984;111:
197–207.
50. Rossi E, Soto JH, Battan J, et al. Intralesional bleomycin in verruca vulgaris.
Double-blind study. Dermatol Rev Mex 1981;25:158–65.
51. Shumer SM, O’Keefe EJ. Bleomycin in the treatment of recalcitrant warts. J Am
Acad Dermatol 1983;9:91–6.
52. Munkvad M, Genner J, Staberg B, et al. Locally injected bleomycin in the treatment of warts. Dermatologica 1983;167:86–9.
53. Perez Alfonzo R, Weiss E, Piquero Martin J. Hypertonic saline solution vs. intralesional bleomycin in the treatment of common warts. Dermatol Venez 1992;30:
176–8.
54. Hayes ME, O’Keefe EJ. Reduced dose of bleomycin in the treatment of recalcitrant warts. J Am Acad Dermatol 1986;15:1002–6.
55. Hursthouse MW. A controlled trial on the use of topical 5-fluorouracil on viral
warts. Br J Dermatol 1975;92:93–6.
56. Luk NM, Tang WY, Tang NL, et al. Topical 5-fluorouracil has no additional
benefit in treating common warts with cryotherapy: a single-centre,
double-blind, randomized, placebo-controlled trial. Clin Exp Dermatol 2006;3:
394–7.
57. Salk RS, Grogan KA, Chang TJ, et al. Topical 5% 5-fluorouracil cream in the
treatment of plantar warts: a prospective, randomized, and controlled clinical
study. J Drugs Dermatol 2006;5:418–24.
58. Stern P, Levine N. Controlled localized heat therapy in cutaneous warts. Arch
Dermatol 1992;128:945–8.
59. Huo W, Gao XH, Sun XP, et al. Local hyperthermia at 44 degrees C for the treatment of plantar warts: a randomized, patient-blinded, placebo-controlled trial. J
Infect Dis 2010;201:1169–72.
60. Akarsu S, Ilknur T, Demirtaşoglu M, et al. Verruca vulgaris: pulsed dye laser
therapy compared with salicylic acid + pulsed dye laser therapy. J Eur Acad
Dermatol Venereol 2006;20:936–40.
61. Katrine TB, Christian G, Per W, et al. Paring and intense pulsed light versus
paring alone for recalcitrant hand and foot warts: a randomized clinical trial with
blinded outcome evaluation. Lasers Surg Med 2010;42:179–84.
62. Robson KJ, Cunningham NM, Kruzan KL, et al. Pulsed-dye laser versus conventional therapy in the treatment of warts: a prospective randomized trial. J Am
Acad Dermatol 2000;43:275–80.
63. Passeron T, Sebban K, Mantoux F. 595 nm pulse dye laser therapy for viral warts:
a single-blind randomized comparative study versus placebo. Ann Dermatol
Venereol 2007;134:135–9.
64. Yang B, Chen D, Hu D, et al. Clinical efficacy of Q-switched laser and/or ceramic
grinding on the treatment of patients with verruca plana. Chin J Derm Venereol
2004;18:547–8 (in Chinese).
65. Li Z, Qiao G, Liu L. Clinical efficacy of CO2 laser combined with You-Jing-An
ointment on the treatment of verruca plana (68 cases report). Harbin Pharm
2005;25:43–4 (in Chinese).
66. Ling L, Ling W, Ye H, et al. Comparison of the effect of microwave and laser
therapy on verruca vulgaris of hand. Mod Hosp 2006;6:26–7 (in Chinese).
67. Huang Y, Lin Q, Xie Y, et al. Clinical effects of carbon dioxide laser combined
with semiconductor laser on the treatment of patients with plantar warts. China
Trop Med 2007;7:1154–5 (in Chinese).
68. Kang H, Xiao H. Comparison of the effect of laser and cryotherapy treatment on
common warts. Chin J Derm Venereol 2007;21:376 (in Chinese).
69. Li L, Yan D, Zhong G. Comparison of the effect of cryotherapy and holmium
laser treatment on plantar warts. Chin J Derm Venereol 2007;21:376–7 (in
Chinese).
70. Lin Q, Tang Y, Zhu G, et al. Comparison of therapeutic effect of pulse CO2 laser
and continuous CO2 laser on flat wart patients. China Trop Med 2007;7:235–6 (in
Chinese).
71. Yang R, Liu Y, Xu W. Effects of combined therapies on verruca vulgaris. Chin J
Dem Venereol 2007;21:677–8 (in Chinese).
72. Bi X. Clinical effects of 585nm pulsed dye laser on the treatment of 72 children
with verruca vulgaris. Shanxi Med J 2008;37:1359–60 (in Chinese).
73. Ma Z, Gai L. Clinical effects of ultra pulse CO2 laser combined with tazarotene
cream on the treatment of verruca plana on the face. China J Lepr Skin Dis
2008;24:842–3 (in Chinese).
74. Yuan J, Li J, Wang L, et al. Clinical effects of ultra pulse CO2 laser combined with
α-2b interferon gel on the treatment of verruca plana. J South Med Univ 2008;28:
2198–9 (in Chinese).
75. Zou X. Comparison of the effect of cryotherapy and CO2 laser treatment on
plantar warts. Chin J Dem Venereol 2008;2:30–1 (in Chinese).
76. Xu B, Chen D, Li H, et al. Comparison of the efficacy of Q-switched laser therapy
and liquid nitrogen cryotherapy on flat warts. J Luzhou Med Coll 2009;32:369–70
(in Chinese).
77. Wang C, Peng Y. Clinical effects of CO2 laser and cryotherapy on the treatment
of flat warts. Shandong Pharm 2010;50:85–6 (in Chinese).
78. Jin C. Clinical study on optimization of CO2 laser therapy for plantar warts. Clin
J Derm Venereol 2011;25:245–6 (in Chinese).
79. Liu Z, Lin J. Clinical effects of CO2 laser combined with topical MEBO on the
treatment of 60 patients with flat warts. J China Tradit Chin Med Inf 2011;3:209–
11 (in Chinese).
80. Pi C, Liang Y, Gao J. Comparison of the efficacy of Q-switched Nd:YAG laser and
cryotherapy on the treatment of flat warts. Lab Med Clin 2011;8:2895–6 (in
Chinese).
81. Wen J, Mei C. Observation of the clinical efficacy of CO2 laser therapy or filiforin
warts. Chin J N Clin Med 2011;4:872–4 (in Chinese).
82. Lei J, Chen M. Therapeutic effect of microwaves therapy combined with CO2 laser
on 193 cases of verruca plantaris. Hainan Med J 2012;23:43–4 (in Chinese).
83. Yang F, Huang X, Chen D. Observation on the efficacy of Q-switch Nd:YAG laser
combination with local drug therapy for flat warts. Chin J Derm Venereol
2011;25:693–4 (in Chinese).
84. Zhang J, Zhou Z, Shao Q. The clinical efficacy of imiquimod cream combination
with tazarotene gel for verruca plana. Chin J Derm Venereol 2010;24:255–6 (in
Chinese).
85. Chen G. Observation on the efficacy of 5% imiquimod cream for verruca plana.
Zhejiang J Integr Tradit Chin West Med 2008;18:208–52 (in Chinese).
86. Wang X, Yang G. Observation on the efficacy of topical 5% imiquimod cream for
verruca plana. J Pract Med Tech 2006;133:3981 (in Chinese).
87. Huang Q. Observation on the efficacy of topical 5% imiquimod cream for verruca
plana. J Chin Physician 2006;8:995 (in Chinese).
88. Ruan J, Zhu R, Zhu H, et al. Observation on the efficacy of topical 5% imiquimod
cream combined with tretinoin cream for verruca plana. J Gannan Med Coll
2005;25:641–2 (in Chinese).
89. Yang H, Su Y, Sun R, et al. The clinical observation on treatment of verruca plana
with imiquimod and tazarotene gel. J Heilongjiang Pharm Sci 2011;34:42–3 (in
Chinese).
90. Yin H. Clinical observation on the efficacy of topical Imiquimod cream combined
with Tazarotene gel in the Treatment of verruca plana. Chin J Derm Venereol
2010;124:588–9 (in Chinese).
91. Zhang X, Zhao Y, Xu H. Observation of the efficacy of imiquimod cream combined with vitamin-A acid cream for treating flat warts. J Baotou Med Coll
2009;25:58–9 (in Chinese).
92. Lu T, Jiang Y, Du X, et al. Observation on the efficacy of imiquimod cream
combination with corn plaster for multi plantar warts. J Dermatol Diagn Ther
2011;18:177–8 (in Chinese).
93. Liu J. The clinical efficacy of imiquimod cream combination with topical interferon ointment for 40 patients with flat warts. Chin J Derm Venereol 2011;33:182
(in Chinese).
94. Li W, Guo M, Sun Y, et al. Observation on the efficacy of topical Chinese and
foreign herbal washing on the treatment of verruca plana. Chin J Dermato
Venereol Integr Trad West Med 2011;10:321 (in Chinese).
95. Zhang J, Zhou Z, Shao Q. The clinical efficacy of imiquimod cream combination
with tazarotene gel for verruca plana. Chin J Derm Venereol 2010;24:255–6 (in
Chinese).
96. Yin H. Clinical observation on the efficacy of topical Imiquimod cream combined
with tazarotene gel in the treatment of verruca plana. Chin J Derm Venereol 2010;
124:588–9 (in Chinese).
328 The evidence
97. Zhang X, Zhao Y, Xu H. Observation of the efficacy of imiquimod cream combined with vitamin-A acid cream for treating flat warts. J Baotou Med Coll 2009;
25:58–9 (in Chinese).
98. Ye J, Lu M, Zhou W. Observation on the efficacy of topical Chinese herbal
washing and imiquimod cream for 62 patients with verruca plana. Med J West
China 2008;20:351 (in Chinese).
99. Wang J. Observation of the efficacy of 5% imiquimod cream combined with
microwave therapy on the treatment of multi plantar warts. China Mod Doct
2008;46:150–1 (in Chinese).
100. Ruan J, Zhu R, Zhang X, et al. Observation of the efficacy of imiquimod cream
combined with hot water immersion on the treatment of multi plantar warts.
South China J Dermatol Venereol 2008;115:92–3 (in Chinese).
101. Wu XI, Yu X, Xu X, et al. Therapeutic observation on the treatment of verruca
plana with You-Jing-An ointment and Di-Wei cream. China J Lepr Skin Dis
2006;22:190 (in Chinese).
102. Huang Y, Lv Y, Wang J. Therapeutic observation on the treatment of verruca plana
with tretinoin liniment and You-Jing-An ointment. J Qiqihar Med Coll 2009;1:
2144 (in Chinese).
103. Xu Y, Xia Y, Lv H. Observation on the treatment of 53 verruca plana patients with
α-2b interferon ointment and tretinoin cream. J Dermatol Venereol 2007;29:44
(in Chinese).
104. Jiang Y, Li F, Dong C. Therapeutic observation on the treatment of 86 verruca
plana patients with tretinoin liniment and α-2b interferon gel. China Mod Med
2009;16:68–9 (in Chinese).
105. Chen X. Observation on the treatment of 46 verruca plana patients with topical
α-2b interferon gel and tretinoin cream. Med Inf 2008;21:2345 (in Chinese).
106. Xiang J, Song N, Ma X. Effect of tazarotene cream and restitution human interferon α-2b cream on refractoriness verruca plana. J Community Med 2009;7:10–1
(in Chinese).
107. He Q, Yin G. Therapeutic observation on the treatment of plantar warts with
tazarotene gel and α-2b interferon ointment. Chronic Pathematol J 2010;12:623
(in Chinese).
108. Li H. Therapeutic observation on the treatment of verruca plana with tazarotene
gel and α-2b interferon gel. Mod J Integr Tradit Chin West Med 2008;17:4770 (in
Chinese).
109. Jin L, Feng H. Therapeutic observation on the treatment of verruca plana with
Tazarotene gel and Ftibamzone ointment. Acta Acad Med Nei Mongol 2008;30:38–
9 (in Chinese).
110. Shao B. The curative effect observation of 0.1%tazarotene gel in combination with
ribavirin injection for treatment of flat wart. Chin J Mod Drug Appl 2011;5:18–9
(in Chinese).
111. Wang Y, Wang L, Bo L, et al. Clinical therapeutic observation on the treatment
of verruca plana with traditional Chinese medicines and 0.1% tretinion Cream.
Clin J Derm Venereol 2002;16:315–6 (in Chinese).
112. Hu F, Zhang J. Sun C. Clinical therapeutic observation on the treatment of
verruca plana with 0.05% Tretinion Cream. Clin J Derm Venereol 2004;18:315–6
(in Chinese).
113. Liu H. Therapeutic observation on the treatment of 53 verruca plana patients
with Wei-Te-Ming cream and acyclovir cream. China J Lepr Skin Dis 2004;20:437
(in Chinese).
114. Wang H, Ren Y. Observation on the efficacy of topical Chinese herbal and 0.1%
Di-Wei cream for verruca plana. Mod J Integr Tradit Chin West Med 2004;13:193–
4 (in Chinese).
115. Xing F, Xie H, Zheng M, et al. Topical treatment of all-trans retinoic acid cream
combined with penciclovir cream for verruca plana: a multiple centre, doubleblind and control study. Chin J Aesthet Med 2005;14:473–5 (in Chinese).
116. Qiao M. Therapeutic observation on the treatment of verruca plana with
Mu-Xiang-Ye and 0.1% tretinoin cream. China J Lepr Skin Dis 2005;21:670–1 (in
Chinese).
117. Ding Z, Zhuang W. Therapeutic observation on the different treatment frequency
of verruca plana with 0.1% tretinoin cream. China J Lepr Skin Dis 2005;21:978
(in Chinese).
118. Bao H, Bian E, Wu X. Observe the therapeutic effects of vitamin-A acid cream
combined with ftibamzone cream about verruca plana. Chin J Aesthet Med 2006;
15:446–7 (in Chinese).
119. Li P. Therapeutic observation on the treatment of 52 verruca plana patients with
α-2b interferon ointment and 0.1% tretinoin cream. J Dermatol Venereol 2007;29:
29 (in Chinese).
120. Cui Y. Observation of the efficacy of Chinese herbal combined with tretinoin
cream on the treatment of multi plantar warts. J Chin Community Doct 2008;10:
120 (in Chinese).
121. Wan J, Xiong L. Therapeutic observation on the treatment of 86 verruca plana
patients with tretinoin liniment and α-2b interferon gel. J Chin Community Doct
2009;11:105–6 (in Chinese).
122. Wang L, Gan C, Zhang X. Therapeutic observation on the treatment of verruca
plana with tretinoin liniment and ftibamzone ointment. China Med Her 2009;6:56
(in Chinese).
123. Xing F, Xia N. Therapeutic observation on the treatment of 87 verruca plana
patients with tretinoin liniment and Mu-Zei-Tang. Mod Med Health 2010;26:3777
(in Chinese).
124. Pu X, Ni J, Tang Q. Study on the treatment of flat wart with fire-needle and
tretinoin. Shanghai J Acu-mox 2011;30:170–2 (in Chinese).
125. Lu J, Zhang C, Chen Y, et al. Topical treatment of tazarotene gel combined with
Xiao-You-Ling for verruca plana. J Suzhou Univ 2005;25(4):591–604 (in Chinese).
126. Huang F, Lu C, Fang J, et al. Therapeutic observation on the treatment of verruca
plana with tazarotene gel and penciclovir cream. China J Lepr Skin Dis 2007;23:125
(in Chinese).
127. Liu X. Therapeutic observation on the treatment of 120 verruca plana patients
with tazarotene gel. Med J Chin People’s Health 2008;20:2101–13 (in Chinese).
128. Dong Y. Therapeutic observation on the treatment of verruca plana with tazarotene gel and Chinese herbal. China Mod Med 2009;16:43–4 (in Chinese).
129. Yang M, Zeng H, Lin W, et al. Therapeutic observation on the treatment of
verruca plana with adapalene gel and α-2b interferon gel. J Clin Dermatol 2004;
44:343 (in Chinese).
130. He T. Therapeutic observation on the treatment of 72 verruca plana patients with
Chinese herbal and 0.1% adapalene gel. J Dermatol Venereol 2007;29:37–8 (in
Chinese).
131. Wang Z. Therapeutic observation on the treatment of verruca plana with Chinese
herbal and adapalene gel. China Med Herald 2009;6:85 (in Chinese).
132. Wang Y, Tian F. Observation on the treatment of verruca plana with adapalene
gel and cryotherapy. J Med Forum 2012;33:104–6 (in Chinese).
133. Wu X. Therapeutic observation on the treatment of verruca plana with isotretinoin gel. J Pract Clin Med 2008;8:61 (in Chinese).
134. Berman B, Davis-Reed L, Silverstein L, et al. Treatment of verrucae vulgaris with
alpha 2 interferon. J Infect Dis 1986;154:328–30.
135. Lee SW, Houh D, Kim HO, et al. Clinical trials of interferon-gamma in treating
warts. Ann Dermatol Venereol 1990;2:77–82.
136. Niimura M. Application of beta-interferon in virus-induced papillomas. J Invest
Dermatol 1990;95:149–51S.
137. Pazin GJ, Ho M, Haverkos HW, et al. Effects of interferon-alpha on human warts.
J Interferon Res 1982;2:235–43.
138. Vance JC, Bart BJ, Hansen RC, et al. Intralesional recombinant alpha-2 interferon
for the treatment of patients with condyloma acuminatum or verruca plantaris.
Arch Dermatol 1986;122:272–7.
139. Varnavides CK, Henderson CA, Cunliffe WJ. Intralesional interferon: ineffective
in common viral warts. J Dermatol Treat 1997;8:169–72.
140. Horn TD, Johnson SM, Helm RM, et al. Intralesional immunotherapy of warts
with mumps, Candida and Trichophyton skin test antigens: single-blinded, randomized, and controlled trial. Arch Dermatol 2005;141:589–94.
141. Khattar JA, Musharrafieh UM, Tamim H, et al. Topical zinc oxide vs. salicylic
acid–lactic acid combination in the treatment of warts. Int J Dermatol 2007;46:
427–30.
142. Gustafsson L, Leijonhufvud I, Aronsson A, et al. Treatment of skin papillomas
with topical alphalactalbumin–oleic acid. N Engl J Med 2004;350:2663–72.
143. Rahimi AR, Emad M, Rezaian GR, et al. Smoke from leaves of Populus euphratica
Olivier vs. conventional cryotherapy for the treatment of cutaneous warts: a pilot,
randomized, single-blind, prospective study. Int J Dermatol 2008;47:393–7.
CHAPTER 39
Molluscum contagiosum
Minh L. Lam
Dermatology Department, Queen’s Medical Centre, Nottingham, UK
Background
Definition
Molluscum contagiosum is a benign infection of the skin and
mucous membranes caused by the molluscum contagiosum pox
virus. Most people with mollusca have multiple lesions, which typically begin as small papules that enlarge to around 3–6 mm and
rarely to more than 1 cm in diameter. Fully developed papules typically have a central umbilication or depression that contains a
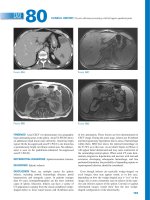
white, waxy, curd-like core. Most lesions resolve without scarring,
but they may cause discomfort and/or itching (Figure 39.1) [1].
Mollusca can be associated with a surrounding dermatitis and occasionally a reactive skin eruption similar to Gianotti–Crosti syndrome [2].
Incidence
Infections with the molluscum contagiosum virus occur throughout the world, but the incidence varies considerably, with higher
rates in areas with warm climates [1]. Children are most often
affected [3–5]. In a British study, the incidence was 243 per 100 000
person-years (py) in males and 231 per 100 000 py in females.
Ninety percent of cases were reported in children aged 0–14 years
(incidence 1265 per 100 000 py) [4]. In New Guinea, the annual
attack rate was 6% in children aged 0–9 years [6]. Incidence rates
are higher in American Indians and Alaska Natives than in the
general US population [7]. Patients with weakened immune system
are particularly prone to molluscum infection and have increased
difficulty in clearing lesions (point prevalence in patients with
human immunodeficiency virus/acquired immune deficiency syndrome (AIDS): 5–18%) [8–11]. A history of eczema was found in
62% of children with molluscum contagiosum in Australia [12].
Another Japanese study found that lifetime molluscum was
increased in young children with atopic eczema [13].
Etiology
Molluscum contagiosum, also known as Molluscipoxvirus, is a
member of the pox virus (Poxviridae) family [14,15]. Transmission
of molluscum occurs during contact with infected lesions, contami-
nated fomites, or sexual contact [16]. Autoinoculation through
scratching is also suspected, especially as lesions can develop along
scratchmarks (koebnerization) [17]. Outbreaks have occurred
among children attending swimming pools [18,19].
Prognosis
After a variable incubation period (2–6 months), the lesions usually
persist for several months and resolve as a result of an inflammatory
response which may develop spontaneously, following trauma (e.g.,
scratching) or secondary bacterial infection [1,20,21]. In immunocompromised people – for example, patients with AIDS – molluscum contagiosum usually does not resolve spontaneously and is
often refractory to treatment [1,22].
Aims of treatment
Molluscum contagiosum is self-limiting in immunocompetent
individuals. Many parents of children with molluscum may seek
treatment because of concerns about the appearance of the lesions,
lesions becoming sore due to friction from clothing and in skin
folds, persistence of lesions, spread of lesions, concern about secondary infection, or because of other people’s comments. The aims
of treatment are to shorten the duration of the condition, to resolve
discomfort (e.g., itching), to limit spread, and to prevent secondary
bacterial infection [1].
Relevant outcomes
Clinical cure of all infected lesions is the clinically most relevant
outcome. Treatment success is defined as the proportion of patients
completely cleared of all molluscum contagiosum lesions. Treatment
success should ideally be assessed 4–8 weeks after the discontinuation of treatment because of the tendency of molluscum to heal
spontaneously in healthy children. Secondary outcomes include the
time to clearance of all lesions and the cosmetic result.
Methods of search
We included randomized controlled trials (RCTs) of all interventions for cutaneous, nongenital infection with molluscum contagiosum in immunocompetent patients. We updated the previous
Evidence-based Dermatology, Third Edition. Edited by Hywel C. Williams, Michael Bigby, Andrew Herxheimer, Luigi Naldi, Berthold Rzany, Robert P. Dellavalle, Yuping Ran,
and Masutaka Furue.
© 2014 John Wiley & Sons, Ltd. Published 2014 by John Wiley & Sons, Ltd.
Companion Website: www.evidencebasedseries.com/dermatology
329
330 The evidence
RCT we have identified that provides supporting evidence for a
physical destructive method in the treatment of molluscum contagiosum [26]. The clearance rate of 100% (37 out of 37 subjects) was
the highest out of all studies, though clearly tolerability and scarring
are major drawbacks. This study also found a 92% (34/37) clearance
rate with imiquimod after 16 weeks, in contrast to the previously
discussed Papadopoulos studies [25], which found clearance rates
of 24% (112/470 – pooled data) after 18 weeks.
Cantharidin, a blister beetle extract, has been widely used to treat
viral warts as well as molluscum contagiosum. However, the only
RCT we found for the treatment of mollusca suggests that there was
no significant improvement compared with placebo, though it was
a small study that may have been underpowered [27].
Despite previous studies suggesting no benefit in treating mollusca with potassium hydroxide compared with placebo [28, 29],
there have been two further studies published, by Rajouria et al.
[30] and Uçmak et al. [31], showing a greater reduction in mean
lesion count and improved complete clearance with potassium
hydroxide. However, in both studies, patient numbers were small
and no control groups were present.
Sodium nitrite
Figure 39.1 Molluscum contagiosum.
edition’s chapter by searching the Cochrane Controlled Trials
Register (May 2013), the Cochrane Library for systematic reviews
(May 2013), and Medline (June 2006–May 30, 2013).
Question
In immunocompetent patients with cutaneous,
nongenital molluscum contagiosum, what is the
efficacy (defined as the proportion of patients
completely cleared of all lesions at 4–8 weeks
after discontinuation of treatment) of physical
destructive methods, topical and
systemic treatments, or waiting for
spontaneous resolution?
Evidence summary
A Cochrane review on the interventions for cutaneous molluscum
contagiosum was published in 2006 and subsequently updated to
include studies published up to June 2009 [23]. Only 11 studies,
with a total number of 495 participants, could be analysed. Study
limitations included no blinding (four studies), high drop-out rates
(three studies), and no intention-to-treat analysis, and the small
study sizes meant inadequate power to detect possible important
differences. The overall conclusion from the authors was that no
single intervention has been shown to be convincingly effective in
the treatment of molluscum contagiosum [23].
We identified another seven relevant studies, including two of
the largest RCTs ever conducted in the intervention of molluscum
contagiosum. These two studies, identified by Katz and Swetman
[24], suggest that imiquimod 5% is no more effective than placebo
in achieving clearance of mollusca, yet these studies have never
been published in a peer-reviewed journal [25]. A study by Mutairi
et al. comparing 5% imiquimod cream with cryotherapy is the only
Efficacy
After 3 months of treatment with sodium nitrite 5%–salicylic acid
5% cream with occlusion on each lesion overnight, 75% of patients
(12/16) completely cleared, in comparison with 25% of children
(4/16) treated with salicylic acid 5% cream [32].
Drawbacks
Brown staining of the skin was recorded in six patients out of 16
with active treatment, but in none in the control group. The treatment is awkward and time consuming, and causes irritation in
some patients (frequency not reported) [32].
Comment
Because of potential staining, sodium nitrite is not recommended
for facial molluscum contagiosum. It appears to be beneficial for
lesions on the trunk, although larger trials are needed to confirm
the results.
Salicylic acid, phenol
Efficacy
Salicylic acid 12%, lactic acid 4% gel (Salatac) applied once to twice
weekly, and 10% phenol in a 70% alcohol solution applied once
daily had similar clearance rates to those of the vehicle (Salatac 57%
(21/37) versus phenol 42% (17/41) versus placebo 44% (16/36);
completely cleared, P = 0.38) [33].
Drawbacks
Significantly more patients discontinued treatment due to stinging
in the salicylic acid group in comparison with phenol and vehicle.
No serious adverse events occurred [33].
Comment
Because an efficacy greater than that of the vehicle was not demonstrated in this study, neither salicylic acid nor phenol are recommended. The sample size of the study was quite small, and important
treatment differences might have been missed. In contrast to the
full-text paper, in which the results were based on an intention-totreat analysis, the authors reported results after excluding drop-outs
in a previously published abstract. Salicylic acid appears to be ben-
Molluscum contagiosum 331
eficial in the per-protocol analysis, highlighting the potential of
misleading inferences due to inappropriate statistical methods
[33,34].
Imiquimod versus vehicle and cryotherapy
Efficacy
Theos et al. found imiquimod 5% cream applied three times a week
for 12 weeks was not superior to the vehicle in inducing complete
clearance (imiquimod 33.3% completely cleared (4/12) versus
vehicle 9.1% (1/12); P = 0.32); partial responses (defined as at least
a 30% reduction in the lesion count) were more frequent in patients
treated with imiquimod [35]. An RCT by Al Mutairi et al. demonstrated imiquimod 5% cream applied five times a week led to complete cure at 16 weeks in 34/37 participants (22/37 at week 6),
compared with complete clearance in all 37 patients receiving onceweekly cryotherapy after 3 weeks [26]. Interestingly, a commentary
by Katz and Swetman [24] in 2013 on the deficiencies of US law in
failing to mandate that important negative studies are published in
full in peer-reviewed journals highlighted two unpublished RCTs
(by Papadopoulos in 2006) conducted in comparing imiquimod 5%
used three times weekly for up to 16 weeks against placebo [25].
Both studies found no statistical difference in complete clearance
rates of those treated with imiquimod, 52/217 and 60/253 (both
24%) compared with placebo, 60/253 and 35/216 (24% and 16%).
Drawbacks
Tolerability did not differ significantly between imiquimod and the
vehicle in the study by Theos et al. with local skin reactions and
pruritus commonly reported in both groups [35]. The Al Mutairi
et al. study showed significantly more adverse effects with cryotherapy compared with imiquimod, including blistering (9/37),
pigmentary changes (15/37), and scarring (8/37). Together with the
treatment-associated pain of cryotherapy, this represents a major
drawback of the use of this modality in young children. Pain during
application (27/37) and erythema (28/37) were also common side
effects reported with imiquimod [26]. A pharmacokinetic study
suggested that percutaneous absorption in imiquimod was low;
however, leukopenia and neutropenia were commonly noted [36].
Comment
With a total of 23 participants included, the first study was not
sufficiently powered to show small differences [35]. The Al Mutairi
et al. study reported high clearance at 16 weeks, for both imiquimod
and cryotherapy, but given the self-limiting nature of molluscum,
the lack of a placebo arm in this trial was a significant flaw, as was
the lack of description of method of randomization and subsequent
allocation concealment and blinding. The unpublished trials cited
by Katz and Swetman highlight the problem of publication bias
against studies with negative results. The original manufacturer of
imiquimod and clinicians involved in their pivotal studies chose
not to publish the results of these studies in a journal, yet they are
available on the US Foods and Drugs Administration (FDA)
website. As a result, these key studies have not been picked up and
included in dermatology textbooks, reference guides, and even the
Cochrane review on the interventions for cutaneous molluscum
contagiosum.
Australian lemon myrtle (Backhousia citriodora)
Efficacy
The essential oil of the Australian lemon myrtle (Backhousia citriodora) (Herbal BioScience, Oakvale, California, USA), applied once
daily for 21 days, induced partial remission (defined as at least a
90% reduction in the lesion count) in 56% (9/16) of patients, in
comparison with 0% (0/15) treated with the vehicle. Complete
response rates are not reported [37].
Drawbacks
None. The treatment was well tolerated in the study [37].
Comment
Australian lemon myrtle appears to beneficial against molluscum
contagiosum. However, complete response rates are not reported,
and complete clearance is probably of most relevance for the patient
[37]. Further evidence has suggested cytotoxic effects associated
with the use of this oil [38,39], which has led the research group
(Centre for Biomedical Research Inc.) to evaluate another formulation of lemon myrtle to improve safety and efficacy [40].
Combination of essential oil of Melaleuca alternifolia
with iodine
Efficacy
The same company that evaluated Backhousia citriodora went on to
test another essential plant oil obtained from Melaleuca alternifolia,
more widely known as tea tree oil. They compared melaleuca oil
against melaleuca plus iodine versus iodine alone in a three-armed
RCT of 53 children and found that 16 out of 19 children (84%) had
a greater than 90% reduction of lesions in the combination group,
compared with one out of 16 (6%) and three out of 18 (17%) for
the iodine and melaleuca-only groups (P < 0.01) by the end of day
30 using an intention-to-treat analysis [40]. Complete response
rates are not reported.
Drawbacks
Adverse effects were limited to application site redness that did not
result in any children withdrawing from the study. Long-term scarring after resolution of the lesions was not assessed.
Comment
This study suggests that a specially formulated combination of tea
tree oil and iodine is more effective than either product used alone,
although the absence of a vehicle-only group is a limitation given
the tendency of molluscum to resolve spontaneously. The internal
validity of this company-sponsored trial is questionable given that
the method of randomization and subsequent allocation concealment is not described, and although parents and the evaluating
physicians were reported to have been blinded to the treatment
allocation, it is unclear whether assessment was truly blinded given
that iodine stains the skin. Plant extracts could have a beneficial
effect in inducing local clearance of mollusca, and an independent
trial of tea tree oil plus iodine versus a vehicle of similar colour and
smell with blinded assessment of complete clinical response might
be worthwhile.
Povidone iodine solution combined with salicylic
acid plaster
Efficacy
Ohkuma found that 10% povidone iodine solution and 50% salicylic acid plaster was effective in curing 20/20 participants (100%)
compared with 3/5 who received povidine iodine alone (60%) and
7/10 who received salicylic acid plaster alone (70%). Thus, the combination treatment cured more participants than either component
alone (and more rapidly – 26 days, 86 days, and 47 days were mean
332 The evidence
time to cure for each group respectively), though this failed to reach
statistical significance [41].
Drawbacks
All participants developed local redness within 3–7 days of starting
treatment. The duration of redness was variable, and the more
marked the inflammation the earlier the cure was [41].
Comment
Based on the small number in the comparator groups and lack of
statistical difference between the groups there is insufficient evidence to recommend this treatment.
Benzoyl peroxide
Efficacy
In total, 73% (11/15) of patients treated with benzoyl peroxide 10%
cream twice daily for a total of 4 weeks had complete remission of
all lesions at week 6, in comparison with 33% (5/15) of patients
treated with tretinoin 0.05% cream (relative risk [RR], 2.20; 95%
confidence interval [CI], 1.01–4.79; P = 0.05) [42].
Drawbacks
Side effects were limited to mild dermatitis in both treatment
groups (exact numbers not reported) [42].
Comment
Benzoyl peroxide 10% cream appears to be beneficial for molluscum contagiosum. However, owing to poor reporting quality and
methodological shortcomings (i.e., failure to carry out an intentionto-treat analysis) bias cannot be ruled out [42], and better studies
are needed.
Cantharidin versus placebo
Efficacy
Cantharidin is a topical vesicant that has been used to treat molluscum for several decades. It is an extraction from blister beetles,
Cantharis vesicatoria, and when applied to the skin it produces a
small intraepidermal blister that usually heals without scarring. In
this randomized double-blind study, 15% (2/13) of those who
received cantharidin and 6% (1/16) of those who received vehicle
achieved complete clearance after 8 weeks, which was not statistically different [27].
Drawbacks
Although the placebo vehicle had an identical texture and smell to
the active drug, there were significant differences in reported
adverse effects in that blistering was reported in 12/13 of those who
received cantharidin, but in only 8/16 receiving placebo. The active
group also experienced more pigmentary changes (6/13 vs 0/16).
Three patients in the cantharidin group were excluded because they
were later found not to meet the inclusion criteria [27].
Comment
Despite anecdotal evidence supporting the use of cantharidin, this
study is the first prospective, placebo-controlled, randomized trial
evaluating the safety and efficacy of cantharidin for molluscum
contagiosum. Although the performance of cantharidin was similar
to that of placebo in this study, it was probably too small to be
conclusive.
Potassium hydroxide versus saline and tretinoin cream
Efficacy
Bazza and Ryatt reported a study where treatments were randomized to the right or left side of the body. Application of 5%
potassium hydroxide was compared with 0.9% saline. In both
groups, 85% (17/20) of patients were cured at 12 weeks [28]. The
same comparison was made by Short et al., where treatment with
10% potassium hydroxide was successful after 3 months in 70%
(7/10) compared with 20% (2/10) in the saline group [29]. Pooling
these data showed no significant benefit from potassium hydroxide. Rajouria et al. compared 5% potassium hydroxide with tretinoin 0.05% cream in a nonrandomized controlled study, with 25
patients allocated to each treatment, and found at 4 weeks a mean
reduction in lesion count from 9.48 (±3.00 standard deviation
[SD]) to 1.67 (±0.58 SD) and from 8.35 (±2.82 SD) to 2.00 (±1.00
SD) for potassium hydroxide and tretinoin respectively [30]. A
study by Uçmak et al. compared treatment with potassium hydroxide at concentrations of 2.5% and 5%. Complete clearance was
reported in 23% (3/13) and 67% (8/12) at 60 days after commencing treatment with potassium hydroxide twice daily in each group
respectively [31].
Drawbacks
Four patients (two in each group) dropped out of the Rajouria
et al. study due to noncompliance, and a table of side effects for the
remainder of the participants showed erythema (14/23), edema
(5/23), and burning (4/23) reported in those treated with 5% potassium hydroxide, though erosions (6/23) and ulceration (1/23) were
also reported. Side effects from potassium hydroxide were common
in the study by Uçmak et al., with at least one side effect reported
in 86.4% and 91.7% of those treated with 2.5% and 5% potassium
hydroxide respectively. The exact number and nature of side effects
were not reported. Tretinoin 0.05% cream appeared to produce
milder adverse effects.
Comment
The studies by Bazza and Ryatt and by Short et al. showed no significant benefit of potassium hydroxide in clearing molluscum. The
Rajouria et al. study focused on a reduction in lesion count rather
than clearance at 4 weeks. Although there was a significant reduction in mean lesion count with both treatments, there was no significant difference between the treatments and no placebo arm to
assess the mean reduction in those without any treatment. Therefore,
the study suggests potassium hydroxide (and tretinoin cream)
improve the resolution of mollusca, but how much better than
spontaneous resolution is unclear. This study was also nonblinded
and nonrandomized, which is at high risk of selection and information bias, and it is unclear whether an intention-to-treat analysis
was performed. Although the Uçmak et al. study was reported as
randomized, allocation concealment was unclear. Despite efforts to
blind study participants, it is unclear whether assessors had been
blinded, and in fact the same observer was used for all follow-up
visits, risking significant information bias. This study would also
have benefitted from a control arm.
Calcarea carbonica
Efficacy
This homeopathic drug given daily for 15 days resulted in improvement in 93% (13/14) participants in the treatment arm and 17%
(1/6) in the placebo arm of the trial (RR, 5.57; 95% CI, 0.93–33.54;
not statistically significant) [43].
Placebo
Melaleuca
alternifolia
alone
Iodine alone
Salicylic acid 12%,
lactic acid 4% gel
(Salatac)
Imiquimod 5%
cream
Essential oil of
Australian lemon
myrtle
Benzoyl peroxide
10% cream
Cimetidine 35 mg/
kg/day
Melaleuca
alternifolia + iodine
5% imiquimod
cream
10% povidone
iodine solution and
50% salicylic acid
plaster
Leslie (2005)
[33]
Theos (2004)
[35]
Burke (2004)
[37]
Saryazdi
(2004) [42]
Antony
(2001) [44]
Markum
(2012) [40]
Al Mutairi
(2010) [26]
Ohkuma
(1990) [41]
10% povidone
iodine solution
alone, 50%
salicylic acid
plaster alone
Cryotherapy
Tretinoin 0.05%
cream
Vehicle
Vehicle
(a) 10% phenol
in 70% alcohol,
(b) 70% alcohol
(placebo)
Salicylic acid
5% cream
alone
Sodium nitrite 5%–
salicylic acid 5%
cream
Ormerod
(1999) [32]
Comparators
Intervention
First author,
ref.
Children (2–9
years)
Children (2–8
years)
Children
(6.3 ± 5.1
years)
Children (1–16
years)
Children (not
reported)
Children
(4.6 ± 3.1
years)
Children
(4.7 ± 1.9
years)
Children (1–15
years)
Children
(median age 6
years)
Study
population
35
74
53
38
30
31
23
114
32
Sample
size (n)
Table 39.1 Summary of RCTs identified on molluscum contagiosum in otherwise healthy individuals.
3-arm RCT
Nonblinded
RCT
3-arm RCT
Double-blind
RCT
Investigatormasked RCT
Double-blind
RCT
Double-blind
RCT
Nonblinded
RCT
Double-blind
RCT
Study design
Unclear
16 weeks
30 days
3 months
4 weeks
3 weeks
12 weeks
6 months
3 months
Duration
of active
treatment
Complete
clearance
Time to cure
Complete
clearance rate
after 16 weeks
Proportion of
patients with
reduction of
lesions >90%
Complete
clearance rate
after 4 months
Complete
clearance rate
at week 6
Proportion of
patients with
reduction of
lesions >90%
Complete
clearance rate
at week 12
Complete
clearance rate
after 6 months
Complete
clearance rate
after 3 months
Outcome
measures
10% povidone iodine solution and
50% salicylic acid plaster 20/20
completely cleared at 26 days vs 10%
povidone iodine solution alone 3/5 at
68 days vs salicylic acid plaster alone
7/10 at 47 days (P > 0.05)
Imiquimod 92% (34/37) vs cryotherapy
100% (37/37) completely cleared
(P = 0.3)
Melaleuca alternifolia+iodine 84%
(16/19) vs Melaleuca alternifolia alone
17% (3/18) vs iodine alone 6% (1/16)
met efficacy outcome (P < 0.01)
Cimetidine 50% (4/8) vs placebo 46%
(5/11); completely cleared (P > 0.05)
Benzoyl peroxide 73% (11/15) vs
tretinoin 0.05% 33% (5/15);
completely cleared (P = 0.05)
Essential oil of Australian lemon myrtle
56% (9/16) vs vehicle 0% (0/15); met
efficacy outcome (P < 0.05)
Imiquimod 33.3% (4/12) vs vehicle
9.1% (1/12); completely cleared
(P = 0.32)
Salatac 57% (21/37) vs phenol 42%
(17/41) vs placebo 44% (16/36);
completely cleared (P = 0.38)
Sodium nitrite 5%–salicylic acid 5%
cream 75% (12/15) vs salicylic acid 5%
cream 25% (4/15); completely cleared
(P = 0.01)
Main results
Continued
Inadequate
Inadequate
Unclear
Inadequate
Inadequate
Adequate
Inadequate
Inadequate
Adequate
Inadequate
Adequate
Inadequate
Inadequate
Inadequate
Inadequate
Adequate
Adequate
Adequate
Inadequate
Inadequate
Adequate
Adequate
Not applicable
Adequate
Adequate
Adequate
Inadequate
Study quality:
Randomizationa
Blindingb
Statistical
analysisc
Molluscum contagiosum 333
Cantharidin
Imiquimod cream
5%
5% potassium
hydroxide
10% potassium
hydroxide
5% potassium
hydroxide
5% potassium
hydroxide
Calcarea carbonica
Coloe Dosal
(2012) [27]
Papadopoulos
(2006) [25]
Bazza (2007)
[28]
Short (2006)
[29]
Rajouria
(2011) [30]
Uçmak (2013)
[31]
Manchanda
(1997) [43]
Placebo
2.5%
potassium
hydroxide
0.05% tretinoin
cream
Saline
0.9% normal
saline
Vehicle
Vehicle
Comparators
Children and
adults (0–30
years)
Children (15
months–18
years)
Children (6
months–14
years)
Children (2–12
years)
Children (2–12
years)
Children (2–12
years)
Children (5–10
years)
Study
population
20
25
46
20
30
323
379
29
Sample
size (n)
RCT
Patient-blinded
RCT
Nonrandomized
controlled trial
Double-blind
RCT
Double-blind
RCT
RCT
Double-blind
RCT
Study design
15 days
60 days
4 weeks
Unclear
3 weeks
16 weeks
8 weeks
Duration
of active
treatment
RCT, randomized controlled trial.
a
Adequate, if clear description of method of randomization and concealment of allocation of randomization given.
b
Adequate, if assessors and participants were completely blinded to the study interventions.
c
Adequate, if an intention-to-treat analysis was carried out; that is, all patients originally randomized were included in the final main analysis.
Intervention
First author,
ref.
Table 39.1 Continued
Improvement
(not clear after
what period)
Complete
clearance after
60 days
Mean
reduction in
lesion count
after 4 weeks
Complete
clearance up
to 90 days
Complete
clearance after
12 weeks
Complete
clearance after
18 weeks
Complete
clearance after
8 weeks
Outcome
measures
Calcarea carbonica 93% (13/14) vs
placebo 17% (1/6) improvement
(P > 0.05)
5% potassium hydroxide 67% (8/12)
vs 2.5% potassium hydroxide 23%
(3/13) completely cured (P < 0.047)
5% potassium hydroxide: 9.48 ± 3.00
SD to 1.67 ± 0.58 SD vs 0.05%
tretinoin: 8.35 ± 2.82 SD to
2.00 ± 1.00 SD
10% potassium hydroxide 70% (7/10)
vs saline 20% (2/10) completely
cleared (pooled with data above
P > 0.05)
5% potassium hydroxide 85% (17/20)
vs 0.9% normal saline 85% (17/20)
completely cleared
Imiquimod 24% (52/217) and (60/253)
vs vehicle 26% (28/106) and 28%
(35/126) completely cleared (P > 0.05)
Cantharidin 15% (2/13) vs vehicle 6%
(1/16) completely cleared (P > 0.05)
Main results
Inadequate
Adequate
Inadequate
Inadequate
Inadequate
Inadequate
Inadequate
Inadequate
Inadequate
Unclear
Adequate
Unclear
Unclear
Adequate
Inadequate
Unclear
Unclear
Unclear
Adequate
Inadequate
Inadequate
Study quality:
Randomizationa
Blindingb
Statistical
analysisc
334 The evidence
Molluscum contagiosum 335
Drawbacks
The study duration, time to resolution, dosing regimen, and adverse
events were not reported and the study was not analyzed by an
intention-to-treat principle. The number of dropouts (20/104 for
the whole trial, including other skin conditions) is unclear for the
molluscum participants.
Comment
For the above reasons, calcarea carbonica is not recommended as
a treatment for molluscum.
Cimetidine
Efficacy
Oral cimetidine 35 mg/kg per day for 3 months added no notable
benefit compared with placebo treatment: cimetidine 50% (4/8)
versus placebo 46% (5/11), completely cleared after 4 months
(P > 0.05) [44].
Drawbacks
Half of the patients (19 of 38) dropped out. The reasons for withdrawal are not reported [44].
Comment
Cimetidine is not recommended in the treatment of molluscum
contagiosum.
Destructive methods such as cryotherapy,
electrodessication, physical squeezing
Apart from the Al Mutairi et al. study of the trial that compared
imiquimod with cryotherapy [26], we were not able to identify any
trials demonstrating the efficacy of these commonly used physical
destructive treatments against each other or against no treatment.
Although this study demonstrated high cure rates within a short
period (37/37 completely cleared at 3 weeks), like most destructive
methods this benefit must be balanced against tolerability in young
children and adverse effects such as scarring.
Implications for clinical practice
Molluscum contagiosum is a self-limiting disease in the immunocompetent host. Treatment is not necessary in most cases, since
natural resolution occurs. Topical treatments that do not leave scars
and are not associated with severe adverse events may be tried in
order to limit the spread, shorten the course of the disease, for
cosmetic reasons, and/or to suppress accompanying symptoms. The
decision to treat must take in to account these issues as well as
patient factors, such as a child’s age, history of atopy, secondary
infection, and so on. It is unclear whether treatment of molluscumassociated dermatitis can limit spread of lesions [2].
Treatment options for mollusca that can be recommended on the
basis of the existing scant evidence are 5% sodium nitrite with 5%
salicylic acid cream for nonfacial lesions and cryotherapy. Salicylic
acid preparations, phenol, cantharidin, imiquimod 5% cream, povidine iodine solution with salicylic acid plaster, oral calcarea carbonica, and cimetidine are not recommended on the basis of the
data available. Most studies have small sample sizes and may, therefore, be underpowered to detect differences in clinical outcomes. A
summary of trials for the treatment of molluscum contagiosum is
presented in Table 39.1.
The evidence base for treatment and prevention of spread of
mollusca is very poor considering it is such a common condition.
Future studies need to be much larger, randomized, include blinded
assessment of responses, and should be prospectively registered and
fully reported. The unit of analysis should be number of people and
not numbers of lesions, and placebo or no treatment arms should
be included. The importance of publishing studies with negative
results should also be emphasized in order to avoid persistent use
of treatments that have been proven not to work any better than
placebo.
Key points
• Molluscum contagiosum in otherwise healthy people is
self-limiting.
• Treatment is not necessary in most people.
• Many popular treatments, including physical destruction, have not
been evaluated properly with well-designed, prospective,
double-blinded randomized control trials.
• Topical sodium nitrite, cryotherapy, and possibly benzoyl peroxide
preparations appear to shorten the duration of the disease.
• Current evidence suggests that salicylic acid preparations, phenol,
cantharidin, imiquimod, and oral cimetidine are no more effective
than placebo.
Acknowledgments
I would like to thank Jochen Schmitt and Thomas L. Diepgen, the
authors of this chapter in the second edition, for their
contribution.
References
1. Lowy DR, Androphy EJ. Molluscum contagiosum. In: Freedberg KA, Eisen AZ,
Wolff K, et al., eds. Fitzpatrick’s Dermatology in General Medicine. New York:
McGraw-Hill, 2003: 2114–7.
2. Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum
and associated inflammatory reactions in a pediatric dermatology practice: the
bump that rashes. Arch Dermatol 2012;148:1257–64.
3. Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in
children. J Am Acad Dermatol 2006;54:47–54.
4. Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum,
scabies and lichen planus. Epidemiol Infect 2005;133:985–91.
5. Sladden MJ, Johnston GA. Common skin infections in children. BMJ 2004;329:
95–9.
6. Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West
Sepik District of New Guinea. Med J Aust 1971;2:751–4.
7. Reynolds MG, Holman RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS ONE
2009;4(4):e5255.
8. Goodman DS, Teplitz ED, Wishner A, et al. Prevalence of cutaneous disease in
patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related
complex. J Am Acad Dermatol 1987;17:210–20.
9. Gottlieb SL, Myskowski PL. Molluscum contagiosum. Int J Dermatol 1994;33:
453–61.
10. Tyring SK. Molluscum contagiosum: the importance of early diagnosis and treatment. Am J Obstet Gynecol 2003;189:S12–6.
11. Erdmann SM, Rubben A, Frank J, et al. Mollusca contagiosa in an infant with
atopic eczema. A therapeutic challenge. Hautarzt 2004;55:991–4 (in German).
12. Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr
Dermatol 2005;22:287–94.
13. Hayashida S, Furusho N, Uchi H, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis?
J Dermatol Sci 2010;60:173–8.
14. Diven DG. An overview of poxviruses. J Am Acad Dermatol 2001;44:1–16.
15. Myskowski PL. Molluscum contagiosum. New insights, new directions. Arch
Dermatol 1997;133:1039–41.
16. Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J 2003;9:2.
336 The evidence
17. Stulberg DL, Hutchinson AG. Molluscum contagiosum and warts. Am Fam
Physician 2003;67:1233–40.
18. Bader RE. Multiple occurrence of molluscum contagiosum in the zone of a swimming pool. Attempt at an epidemiological analysis. Arch Hyg Bakteriol 1967;151:
388–402 (in German).
19. Niizeki K, Kano O, Kondo Y. An epidemic study of molluscum contagiosum.
Relationship to swimming. Dermatologica 1984;169:197–8.
20. Waugh MA. Molluscum contagiosum. Dermatol Clin 1998;16:839–41, xv.
21. Hawley TG. The natural history of molluscum contagiosum in Fijian children. J
Hyg (Lond) 1970;68:631–2.
22. Schwartz JJ, Myskowski PL. Molluscum contagiosum in patients with human
immunodeficiency virus infection. A review of twenty-seven patients. J Am Acad
Dermatol 1992;27:583–8.
23. Van der Wouden JC, van der Sande R, van Suijlekom-Smit LWA, et al. Interventions
for cutaneous molluscum contagiosum. Cochrane Database Syst Rev 2009;(4):
CD004767.
24. Katz KA, Swetman GL. Imiquimod, molluscum, and the need for a better “Best
Pharmaceuticals for Children” Act. Pediatrics 2013;132:1–3.
25. Papadopoulos EJ. Clinical Executive Summary [Imiquimod]. 2006. Available at:
/>DevelopmentResources/UCM162961.pdf (accessed December 6, 2012).
26. Al Mutairi N, Al Doukhi A, Al Farag S, et al. Comparative study on the efficacy,
safety, and acceptability of imiquimod 5% cream versus cryotherapy for molluscum contagiosum in children. Pediatr Dermatol 2010;27:388–94.
27. Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of
molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial.
Pediatr Dermatol 2014; in press. doi: 10.1111/j.1525-1470.2012.01810.x [Epub
ahead of print].
28. Bazza MA, Ryatt KS. Sterile normal 0.9% saline as a effective 5% potassium
hydroxide in treatment of molluscum contagiosum, and safer. 2007. Unpublished
trial, cited in [23].
29. Short KA, Fuller LC, Higgins EM. Double-blind, randomized, placebo-controlled
trial of the use of topical 10% potassium hydroxide solution in the treatment of
molluscum contagiosum. Pediatr Dermatol 2006;23:279–81.
30. Rajouria EA, Amatya A, Karn D. Comparative study of 5 % potassium hydroxide
solution versus 0.05% tretinoin cream for molluscum contagiosum in children.
Kathmandu Univ Med J 2011;36(4):291–4.
31. Uçmak D, Akkurt MZ, Kacar SD, et al. Comparative study of 5% and 2.5%
potassium hydroxide solution for molluscum contagiosum in children. Cutan
Ocul Toxicol 2013;in press. doi: 10.3109/15569527.2013.796479 [Epub ahead of
print].
32. Ormerod AD, White MI, Shah SA, et al. Molluscum contagiosum effectively
treated with a topical acidified nitrite, nitric oxide liberating cream. Br J Dermatol
1999;141:1051–3.
33. Leslie KS, Dootson G, Sterling JC. Topical salicylic acid gel as a treatment for
molluscum contagiosum in children. J Dermatolog Treat 2005;16:336–40.
34. Leslie KS, Dootson G, Sterling JC. Does treatment of molluscum contagiosum
affect clearance? [Abstract PA-3, 84th BAD Annual Meeting 6–9 July 2004, Belfast,
UK.] Br J Dermatol 2004;151:67.
35. Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream
5% for treating childhood molluscum contagiosum in a double-blind, randomized
pilot trial. Cutis 2004;74:134–2.
36. Myhre PE, Levy ML, Eichenfield LF, et al. Pharmacokinetics and safety ofimiquimod 5% cream in the treatment of molluscum contagiosum in children. Pediatr
Dermatol 2008;25(1):88–95.
37. Burke BE, Baillie JE, Olson RD. Essential oil of Australian lemon myrtle
(Backhousia citriodora) in the treatment of molluscum contagiosum in children.
Biomed Pharmacother 2004;58:245–7.
38. Hayes AJ, Markovic B. Toxicity of Australian essential oil Backhousia citriodora
(lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem
Toxicol 2002;40(4):535–43.
39. Hayes AJ, Markovic B. Toxicity of Australian essential oil Backhousia citriodora
(lemon myrtle). Part 2. Absorption and histopathology following application to
human skin. Food Chem Toxicol 2003;41(10):1409–16.
40. Markum E, Baillie J. Combination of essential oil of Melaleuca alternifolia and
iodine in the treatment of molluscum contagiosum in children. J Drugs Dermatol
2012;11(3):349–54.
41. Ohkuma M. Molluscum contagiosum treated with iodine solution and salicylic
acid plaster. Int J Dermatol 1990;29(6):443–5.
42. Saryazdi S. The comparative efficacy of benzoyl peroxide 10% cream and tretinoin
0.05% cream in the treatment of molluscum contagiosum. [Abstract, 10th World
Congress on Pediatric Dermatology.] Pediatr Dermatol 2004;21:399.
43. Manchanda RK, Mehan N, Nahl R, et al. Double blind placebo controlled clinical
trials of homeopathic medicines in warts and molluscum contagiosum. CCRH Q
Bull 1997;19:25–9.
44. Antony F, Cliff S, Ahmad A, et al. Double-blind placebo-controlled study of
oral cimetidine treatment for molluscum contagiosum. Br J Dermatol 2001;
145:126.
CHAPTER 40
Impetigo
Sander Koning1, Renske van der Sande1, Lisette W.A. van Suijlekom-Smit2, and Johannes C. van der Wouden3
1
Department of General Practice, Erasmus MC-University Medical Center Rotterdam, Rotterdam, Netherlands
Department of Paediatrics, Erasmus MC-University Medical Center Rotterdam, Rotterdam, Netherlands
3
Department of General Practice, VU University Medical Center, Amsterdam, Netherlands
2
Background
Definition
Impetigo (Figure 40.1) is a contagious superficial skin infection,
characterized by superficial erosions covered with honey-colored
crusts, most often on the face. A distinction is made between
bullous and nonbullous impetigo. Impetigo may be primary or
secondary to other skin diseases, such as atopic eczema.
Incidence
Impetigo is most frequent in children; the incidence rates peak at
1–8 years of age. Population-based incidence rates are unknown.
Impetigo is common in general practice, with incidence rates of
around 20 episodes per 1000 children per year seen by general
practitioners in the UK and the Netherlands [1–3].
Etiology
In moderate climates, the primary pathogen in nonbullous impetigo
is Staphylococcus aureus. However, in warm and humid climates,
Streptococcus pyogenes or a combination of S. pyogenes and S. aureus
are often isolated. The relative frequency of S. aureus infections has
also changed with time. It was predominant in the 1940s and 1950s,
and then group-A streptococci became more prevalent. In the last
decade of the 20th century, S. aureus has been reported to have
become more common again [4]. Bullous impetigo is a staphylococcal disease.
Prognosis
Impetigo is believed to be self-limiting, taking several weeks to cure
without intervention. However, no research is available to substantiate this statement. Prompt resolution usually occurs with adequate treatment. The course of the disease is usually mild, but
sometimes general symptoms such as fever and lymphadenopathy
occur. Streptococcal impetigo can be complicated by nephritis.
Aims of treatment
Impetigo is treated to accelerate cure and to prevent spreading of
the infection.
Relevant outcomes
Clinical cure (clearance of crusts, blisters, and redness) is the most
relevant outcome. Criteria such as relief of pain, itching, and soreness, and bacteriological cure can be considered as secondary
outcomes.
Methods of search
We found four systematic reviews [5–8], the Cochrane review published in 2012 [8] being an extended update of earlier versions [5,7].
As the Cochrane review, with 68 included randomized controlled
trials (RCTs), is the most recent and most comprehensive one, it
has provided the basis for discussing treatments in this chapter.
The Cochrane review included randomized trials of all interventions for impetigo by using the following search terms in Medline:
impetigo (Medical Subject Headings, MeSH) or (MeSH) or impetigo
(in title or abstract) or pyoderma (in title or abstract), in combination with the standard search strategy for identifying randomized
trials. For this Cochrane review update, the literature was searched
up to July 2010. Owing to space limitations, we only report here on
nonbullous impetigo, and on the most relevant comparisons and
outcomes.
Questions
What are the effects of treatments on the
clearance of impetiginous lesions after 1 week?
Disinfecting treatments
Efficacy
Versus placebo We found one small randomized trial comparing
hexachlorophene with placebo [9]. Scrubbing with hexachlorophene added no notable benefit to placebo treatment.
Versus topical antibiotic treatment One multicenter RCT compared
hydrogen peroxide cream with fusidic acid cream/gel [10]. There
was no significant difference in treatment effect, but there was a
tendency towards a better effect of fusidic acid cream/gel. There was
Evidence-based Dermatology, Third Edition. Edited by Hywel C. Williams, Michael Bigby, Andrew Herxheimer, Luigi Naldi, Berthold Rzany, Robert P. Dellavalle, Yuping Ran,
and Masutaka Furue.
© 2014 John Wiley & Sons, Ltd. Published 2014 by John Wiley & Sons, Ltd.
Companion Website: www.evidencebasedseries.com/dermatology
337
338 The evidence
All other studies comparing topical antibiotics were small and
each studied a unique comparison of two antibiotics.
Versus oral antibiotics When 10 RCTs that compared topical mupirocin with oral erythromycin were pooled [4,22–30], mupirocin
was found to be significantly better than erythromycin [7]. In a
small RCT, cephalexin and mupirocin were both significantly more
effective than bacitracin cream [31]. A trial in patients with secondarily infected dermatitis found no difference between oral
cephalexin and topical retapamulin ointment [32].
Figure 40.1 A child with impetigo (reproduced with permission of A.P.
Oranje).
no significant difference between hexachlorophene and bacitracin
ointment in a small and older study [9].
Versus oral antibiotic treatment There was no significant difference
between hexachlorophene scrubbing and oral treatment with penicillin [9].
Drawbacks
Eleven percent of the patients using hydrogen peroxide cream
reported mild side effects (not specified). No patient was withdrawn from the study because of side effects [10]. No adverse effects
of scrubbing with hexachlorophene were recorded [9].
Comment
Disinfectants, such as povidone iodine and chlorhexidine advised
in some guidelines, have not been compared with a placebo.
Hydrogen peroxide cream showed good treatment results in a relatively large trial. However, the procedure for blinding in this trial
was considered inappropriate.
Implications for topical disinfectants in clinical practice
There is no good evidence for the value of disinfecting measures in
the treatment of impetigo.
Topical antibiotics
Efficacy
Versus placebo Six studies compared a topical antibiotic with placebo
treatment. Mupirocin has been studied in three placebo-controlled
trials, all of which found a better effect with mupirocin [11–13].
One other RCT showed that fusidic acid was much more effective
than placebo (55% of patients cured vs 13%) [14]. A result of similar
magnitude was found for retapamulin versus placebo (86% vs 52%)
[15].
Versus each other Several topical antibiotics have been compared
directly. Mupirocin and fusidic acid were compared in four studies
[16–19], none of which showed a significant difference in treatment
effect. In a large study (>500 patients), the difference between
retapamulin and fusidic acid was not statistically significant [20].
An old study found better results for gentamycin than for neomycin
[21].
Drawbacks
RCT reports usually note few, if any, side effects with local antibiotics. The two studies comparing mupirocin with placebo reported
none [11,12]. In studies comparing mupirocin with fusidic acid, the
greasy nature of mupirocin was reported as a side effect in 7% of
patients versus 1% [16]; minor itching/burning occurred in 5%
versus 4%, respectively [18]. No side effects were reported in
Gilbert’s study [17]. Studies comparing erythromycin with mupirocin recorded gastrointestinal side effects in 23% versus 8% [4],
none in either group [29], and an equal distribution between the
two groups [23]. Hydrocortisone/potassium hydroxyquinoline
caused two cases (3%) of mild staining [33]. In general, resistance
rates against topical antibiotics such as fusidic acid and mupirocin
will rise when the antibiotic is used excessively. Retapamulin caused
itching in 7% of cases in one study [15] but only 1% in another
study [20].
Comments
Most studies date back 20 years or more. Many RCTs deal with a
range of (skin) infections, including impetigo. Only trials that
reported separate results for the group of impetigo patients were
included here. The follow-up periods and definitions of “cure” and
“improvement” differ and are often not clear, making comparison
difficult. There is a lack of placebo-controlled studies.
Implications for topical antibiotics in clinical practice
Although they are traditionally considered less effective than oral
therapy, there is good evidence that local treatments are equal to or
more effective than oral treatment. In general, oral antibiotics have
more side effects, especially gastrointestinal side effects. Fusidic
acid, mupirocin, and retapamulin are equally effective. Resistance
patterns have changed since then. Contemporary and local characteristics and resistance patterns of the causative bacteria should
always be taken into account when choosing treatment. When a
large area is affected, or when the patient has general symptoms
such as fever, oral therapy seems more appropriate. However, this
assumption has never been tested properly.
Systemic antibiotics
Efficacy
Versus placebo Only one small and inconclusive trial was found,
comparing systemic antibiotics with placebo [9].
Versus topical antibiotics Discussed under topical antibiotics above.
Versus each other Two RCTs compared penicillin and erythromycin, both finding erythromycin to be more effective [34,35].
Cloxacillin was significantly superior to penicillin in two studies
[36,37]. All other comparisons were each made in only one study,
Impetigo 339
and none of these showed a relevant difference between
treatments.
Drawbacks
The incidences of side effects were:
• azithromycin – 17%, mainly mild gastrointestinal [38];
• cephalexin – 11%, mainly mild gastrointestinal [38], 11% mainly
diarrhea [39], no adverse effects in one study [34];
• cefdinir – 16%, mainly diarrhea [39];
• erythromycin – up to 17% gastrointestinal side effects, mainly
diarrhea [40].
Comments
Many RCTs have been carried out on a range of “soft-tissue” infections, with a subset of impetigo patients. Only trials reporting separate results for the group of impetigo patients were considered in
this chapter, although it should be pointed out that most trials in
patients with “soft-tissue” infections did not provide results for
impetigo separately.
There is a lack of placebo-controlled studies. Resistance rates of
the bacteria were determined in some studies, and differed from
study to study. The follow-up periods differ widely between the
studies, making comparison difficult.
Implications for use of systemic antibiotics in clinical practice
There is good evidence that local treatment is equal to or more
effective than oral treatment. Macrolide antibiotics provide better
treatment results than penicillin. In general, oral antibiotics have
more side effects than topical treatments, especially gastrointestinal
side effects. Most studies date back 20 years or more, and resistance
patterns have changed since then. Contemporary local characteristics and the resistance patterns of the causative bacteria should
always be taken into account when choosing treatment.
When a large area is affected, or when the patient has general
symptoms such as fever, oral therapy seems preferable. As mentioned above, this assumption has never been tested properly.
Key points
• The natural history of impetigo is not known.
• Few placebo-controlled studies have been done.
• Many different antibiotic treatments have been studied against
each other, often in small studies showing no significant
differences.
• There is no evidence supporting the value of disinfecting
treatments.
• Topical antibiotics such as mupirocin and fusidic acid are equal to
or more effective than oral antibiotics such as erythromycin and
have fewer side effects.
• Macrolide and cephalosporin antibiotics are more effective than
penicillins that are not resistant to beta-lactamase.
• For extensive infections accompanied by general symptoms such
as fever, oral antibiotics may be preferable.
• Resistance patterns in causative bacteria change over time, and
this should be taken into account when choosing a therapy for
impetigo.
References
1. Koning S, Mohammedamin RSA, van der Wouden JC, et al. Impetigo: incidence
and treatment in Dutch general practice in 1987 and 2001; results from two
national surveys. Br J Dermatol 2006;154:239–43.
2. Van der Ven-Daane I, Bruijnzeels MA, van der Wouden JC, et al. Occurrence and
treatment of impetigo vulgaris in children. Huisarts Wet 1993;36:291–3.
3. McCormick A, Fleming D, Charlton J. Morbidity Statistics from General Practice:
Fourth National Study 1991–1992. 1995. London: Department of Health, Office
of Population Censuses and Royal College of General Practitioners.
4. Dagan R, Bar-David Y. Double-blind study comparing erythromycin and
mupirocin for treatment of impetigo in children: implications of a high prevalence
of erythromycin-resistant Staphylococcus aureus strains. Antimicrob Agents
Chemother 1992;36:287–90.
5. Van Amstel L, Koning S, van Suijlekom-Smit LWA, et al. The treatment of impetigo
contagiosa, a systematic review. Huisarts Wet 2000;43:247–52.
6. George A, Rubin G. A systematic review and meta-analysis of treatments of
impetigo. Br J Gen Pract 2003;53:480–7.
7. Koning S, Verhagen AP, van Suijlekom-Smit LW, et al. Interventions for impetigo.
Cochrane Database Syst Rev 2004;(2):CD003261.
8. Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo.
Cochrane Database Syst Rev 2012;(1):CD003261.
9. Ruby RJ, Nelson JD. The influence of hexachlorophene scrubs on the response to
placebo or penicillin therapy in impetigo. Pediatrics 1973;52:854–9.
10. Christensen OB, Anehus S. Hydrogen peroxide cream: an alternative to topical
antibiotics in the treatment of impetigo contagiosa. Acta Derm Venereol 1994;74:
460–2.
11. Eells LD, Mertz PM, Piovanetti Y, et al. Topical antibiotic treatment of impetigo
with mupirocin. Arch Dermatol 1986;122:1273–6.
12. Gould JC, Smith JH, Moncur H. Mupirocin in general practice: a placebocontrolled trial. In: Wilkinson DS, Price JD, eds. Mupirocin: A Novel Topical
Antibiotic. International Congress and Symposium Series, No. 80. London: Royal
Society of Medicine, 1984:85–93.
13. Rojas R, Eells LD, Eaglstein W, et al. The efficacy of Bactroban ointment and
its vehicle in the treatment of impetigo: a doubleblind comparative study. In:
Dobson RL, Leyden JJ, Nobel WC, eds. Bactroban (Mupirocin): Proceedings of an
International Symposium, Nassau, Bahama Islands, 21–22 May 1984. Amsterdam:
Excerpta Medica, 1985: 96–102.
14. Koning S, van Suijlekom-Smit LWA, Nouwen JL, et al. Fusidic acid cream in the
treatment of impetigo in general practice: double blind randomised placebo controlled trial. BMJ 2002;324:203–6.
15. Koning S, van der Wouden JC, Chosidow O, et al. Efficacy and safety of retapamulin ointment as treatment of impetigo: randomized double-blind multicentre
placebo-controlled trial. Br J Dermatol 2008;158:1077–82.
16. Morley PAR, Munot LD. A comparison of sodium fusidate ointment and
mupirocin ointment in superficial skin sepsis. Curr Med Res Opin 1988;11:
142–8.
17. Gilbert M. Topical 2% mupirocin versus 2% fusidic acid ointment in the treatment
of primary and secondary skin infections. J Am Acad Dermatol 1989;20:1083–7.
18. White DG, Collins PO, Rowsell RB. Topical antibiotics in the treatment of superficial skin infections in general practice: a comparison of mupirocin with sodium
fusidate. J Infect 1989;18:221–9.
19. Sutton JB. Efficacy and acceptability of fusidic acid cream and mupirocin ointment
in facial impetigo. Curr Ther Res 1992;51:673–8.
20. Oranje AP, Chosidow O, Sacchidanand S, et al. Topical retapamulin ointment, 1%,
versus sodium fusidate ointment, 2%, for impetigo: a randomized, observerblinded, noninferiority study. Dermatology 2007;215:331–40.
21. Farah FS, Kurban AK, Malak JA, et al. The treatment of pyoderma with gentamicin. Br J Dermatol 1967;79:85–8.
22. Barton LL, Friedman AD, Sharkey AM, et al. Impetigo contagiosa III. Comparative
efficacy of oral erythromycin and topical mupirocin. Pediatr Dermatol 1989;6:
134–8.
23. Britton JW, Fajardo JE, Krafte-Jacobs B. Comparison of mupirocin and erythromycin in the treatment of impetigo. J Pediatr 1990;117:827–9.
24. Dux PH, Fields L, Pollock D. 2% topical mupirocin versus systemic erythromycin
and cloxacillin in primary and secondary skin infections. Curr Ther Res 1986;40:
933–40.
25. Esterly NB, Markowitz M. The treatment of pyoderma in children. JAMA 1970;
212:667–70.
26. Goldfarb J, Crenshaw D, O’Horo J, et al. Randomized clinical trial of topical
mupirocin versus oral erythromycin for impetigo. Antimicrob Agents Chemother
1988;32:1780–3.
27. Gratton D. Topical mupirocin versus oral erythromycin in the treatment of
primary and secondary skin infections. Int J Dermatol 1987;26:472–3.
28. McLinn S. Topical mupirocin vs. systemic erythromycin treatment for pyoderma.
Pediatr Infect Dis J 1988;7:785–90.
29. Mertz PM, Marshall DA, Eaglstein WH, et al. Topical mupirocin treatment
of impetigo is equal to oral erythromycin therapy. Arch Dermatol 1989;125:
1069–73.
30. Rice TD, Duggan AK, DeAngelis C. Cost-effectiveness of erythromycin versus
mupirocin for the treatment of impetigo in children. Pediatrics 1992;89:210–4.
340 The evidence
31. Bass JW, Chan DS, Creamer KM, et al. Comparison of oral cephalexin, topical
mupirocin, and topical bacitracin for treatment of impetigo. Pediatr Infect Dis J
1997;16:708–9.
32. Parish LC, Jorizzo JL, Breton JJ, et al. Topical retapamulin ointment (1% wt/wt)
twice daily for 5 days versus oral cephalexin twice daily for 10 days in the treatment
of secondarily infected dermatitis; results of a randomized controlled trial. J Am
Acad Dermatol 2006;55:1003–13.
33. Jaffe GV, Grimshaw JJ. A clinical trial of hydrocortisone/potassium hydroxyquinoline sulphate (Quinocort) in the treatment of infected eczema and impetigo in
general practice. Pharmatherapeutica 1986;4:628–36.
34. Demidovich CW, Wittler RR, Ruff ME, et al. Impetigo: current etiology and comparison of penicillin, erythromycin, and cephalexin therapies. Am J Dis Child 1990;
144:1313–5.
35. Barton LL, Friedman AD. Impetigo: a reassessment of etiology and therapy. Pediatr
Dermatol 1987;4:185–8.
36. Gonzalez A, Schachner LA, Cleary T, et al. Pyoderma in childhood. Adv Dermatol
1989;4:127–41.
37. Pruksachatkunakorn C, Vaniyapongs T, Pruksakorn S. Impetigo: an assessment of
etiology and appropriate therapy in infants and children. J Med Assoc Thai 1993;
76:222–9.
38. Kiani R. Double-blind, double-dummy comparison of azithromycin and
cephalexin in the treatment of skin and skin structure infections. Eur J Clin
Microbiol Infect Dis 1991;10:880–4.
39. Tack KJ, Keyserling CH, McCarty J, et al. Study of use of cefdinir versus cephalexin
for treatment of skin infections in pediatric patients. Antimicrob Agents Chemother
1997;41:739–42.
40. Faye O, Hay RJ, Diawara I, et al. Oral amoxicillin vs. oral erythromycin in the
treatment of pyoderma in Bamako, Mali: an open randomized trial. Int J Dermatol
2007;46(Suppl 2):19–22.
CHAPTER 41
Athlete’s foot
Inajara Rotta1, Michel F. Otuki1, and Cassyano J. Correr2
1
Pharmaceutical Sciences Postgraduate Program, Federal University of Paraná, Curitiba, Paraná, Brazil
Pharmacy Department, Federal University of Parana, Ponta Grossa, Paraná, Brasil
2
Background
Definition
Athlete’s foot, or tinea pedis, is commonly caused by dermatophyte
invasion of the skin of the feet. It is clinically presented as interdigital, moccasin, or vesicobullous forms, with interdigital being the
most common. Moccasin infection affects soles, heels, and sides of
the feet, characterized by dry and hyperkeratotic skin. With less
frequency, vesicobullous is the most severe form of athlete’s foot. It
appears as an acute and intense inflammation with vesicles, pustules, and bubbles, usually on the soles of feet [1–3] (Figure 41.1).
Incidence/prevalence
It has been estimated that 15% of the general population have athlete’s foot. It is thought to occur when individuals regularly use
communal changing rooms and swimming pools. There are studies
indicating higher prevalence in male and elderly people [4,5].
Etiology
Athlete’s foot is usually caused by Trichophyton rubrum, Trichophyton
mentagrophytes var. interdigitale, and Epidermophyton floccosum.
Individuals with athlete’s foot may be susceptible to secondary bacterial infection with, for instance, group A streptococcus [6].
Diagnostic tests
Diagnosis of dermatophytosis is based on clinical and laboratory
data. Clinical diagnostic procedure must include physical exam of
the lesions and epidemiological history survey, whereas mycological tests consist of microorganisms visualization in microscopy and
culture growth [2,7,8].
Aims of treatment
The management of dermatophytosis consists of topical formulations, oral therapy, or a combination of them. An oral drug is
needed when the affected area is wide, the patient is immunocompromised, or the disease is chronic or recurrent with failure on
topical treatment [2,9].
The aim of treatment is to decrease signs and symptoms, such as
redness, itching, and flaking, and also to eradicate the infection,
being that the course of treatment is partially determined by the
severity level [1].
Topical antifungals are available as over-the-counter drugs in
most countries and are divided into two main classes: azoles, in
which the mechanism of action is fungistatic, and alylamines,
acting as fungicides. Other “non-azoles” and “non-allylamines,”
such as tolnaftate and undecenoic acid, are also commercialized
over the counter and, commonly, cost less than the others [2,10].
Relevant outcomes
The efficacy outcomes evaluated were mycological cure at the end
of treatment (MCET) and sustained cure (SC), defined as cure
obtained up to 7 days after therapy conclusion and cure maintained
for at least 14 days following the end of treatment, respectively.
Efficacy of treatment was evaluated by meta-analysis, and the
mycological cure was the main outcome considered, evidenced by
microscopic negative or absence of growth of dermatophytes in
culture [11].
Methods of search
Systematic reviews and randomized clinical trials (RCTs) were
identified using a search strategy published elsewhere [11]. This was
updated to June 2012 using the same strategy. We included studies
that compared the use of antifungals among themselves or with
placebo in the treatment of any clinical form of athlete’s foot. Only
studies that had patients diagnosed mycologically with the disease
were included.
Questions
How effective are allylamine creams in the
treatment of athlete’s foot?
Two meta-analysis published comparing allylamines (terbinafine
and naftifine 1%) with placebo, used for 1–4 weeks, show that the
two antifungals are similarly effective and better than placebo
[5,10].
In a third meta-analysis were identified 12 RCTs (n = 1418)
comparing naftifine 1% and terbinafine 1% or 3% with placebo,
Evidence-based Dermatology, Third Edition. Edited by Hywel C. Williams, Michael Bigby, Andrew Herxheimer, Luigi Naldi, Berthold Rzany, Robert P. Dellavalle, Yuping Ran,
and Masutaka Furue.
© 2014 John Wiley & Sons, Ltd. Published 2014 by John Wiley & Sons, Ltd.
Companion Website: www.evidencebasedseries.com/dermatology
341
342 The evidence
Figure 41.1 Athlete’s foot.
used for 1 day (single-dose) to 28 days. For both efficacy outcomes,
allylamines were better than placebo. In terms of MCET, metaanalysis of data from nine RCTs estimated the pooled odds ratio
(OR) as 5.87 (95% confidence interval [CI], 2.46–14.01) and
number needed to treat (NNT) of 3. For SC outcome, combined
data from 11 studies, with follow-up period lasting from 2 to 12
weeks after the cessation of treatment, resulted in an OR of 14.22
(95% CI, 9.49–21.32) and NNT of 2 [12].
How effective are azole creams in the treatment
of athlete’s foot?
Two systematic reviews were found comparing the azoles clotrimazole 1%, tioconazole 1%, bifonazole 1%, econazole 1%, and miconazole 2% with placebo, used for 4–6 weeks. The antifungals were
similarly effective and better than placebo [5,10].
Another systematic review (n = 1223) compared econazole 1%,
miconazole 2%, oxiconazole 1%, sertaconazole 2%, and clotrimazole 1% with placebo, used for 28–42 days [12]. For MCET, the data
interpolation from seven RCTs resulted in an OR of 5.54 (95% CI,
3.01–10.19) and NNT of 3. For SC outcome, pooled data of four
trials with follow-up period lasted from 2 to 4 weeks after the cessation of treatment shows an OR of 6.64 (95% CI, 4.65–9.48) and
NNT of 2 [12].
How do allylamine creams compare with azole
creams in curing athlete’s foot?
A meta-analysis of nine RCTs (n = 1003) indicate better mycological cure rate with allylamines cream (terbinafine and naftifine 1%)
in comparison with azoles cream (clotrimazole 1–2% and bifonazole 1%), used for 4–6 weeks. The relative reduction in treatment
failure was 37%, favoring allylamines (relative risk [RR], 0.63; 95%
CI, 0.42–0.94) [5].
Considering different treatment regimens, data collected at 6
weeks from five trials (n = 962), which compared terbinafine 1%
used for 1 week with clotrimazole or miconazole 1% used for 4
weeks, did not show a statistically significant difference in treatment failure (RR, 0.75; 95% CI, 0.33–1.72). However, there was
considerable variation in the results of the individual trials [5].
In the study of Patel et al. [13], terbinafine was more effective
than clotrimazole after 1 week of therapy (RR, 1.51; 95% CI, 1.16–
1.98), but there were no differences between the classes in the following weeks.
We performed a meta-analysis of two studies (n = 955) [14,15],
which compared terbinafine 1% cream twice daily for 1 week and
clotrimazole 1% cream twice daily for 4 weeks. Terbinafine was
better than clotrimazole for the MCET outcome (OR, 0.28; 95% CI,
0.09–0.85), without differences for the SC outcome, 6 weeks after
the end of the treatment (OR, 0.40; 95% CI, 0.07–2.38). A result
favoring the use of allylamines (RR, 0.88; 95% CI, 0.78–0.99) was
also obtained by Hart et al. [10], although some language bias was
detected.
In another systematic review, eight trials (n = 1300) comparing
oxiconazole 1%, clotrimazole 1%, miconazole 2%, or bifonazole 1%
with naftifine and terbinafine, both 1%, were pooled. The OR of
0.55 obtained for MCET was statistically favorable to allylamines
(95% CI, 0.33–0.92) with an NNT of 41. The results of nine RCTs
(n = 1523) were interpolated for the SC outcome, giving an OR of
0.39 (95% CI, 0.22–0.67) and NNT of 13, in favor of the allylamines
[12].
How effectively do creams that can be bought in
the supermarket cure athlete’s foot?
Meta-analysis data indicates that tolnaftate (RR, 1.56; 95% CI,
1.05–2.31) and undecenoic acid (RR, 2.83; 95% CI, 1.91–4.19) are
more effective than placebo [5]. We performed a meta-analysis of
RCTs comparing ciclopiroxolamine (0.77% and 1%) used for 4
weeks with placebo (three trials, n = 654) [16–18]. The ORs
obtained were statistically favorable to the antifungal: 6.67 (95% CI,
3.47–12.80) for MCET and 8.98 (95% CI, 2.27–35.53) for the SC
outcome. Other studies have similar results [5,10].
Finally, we performed a meta-analysis of four trials (n = 711)
[19–22] comparing butenafine 1% cream with placebo, used for 1–4
weeks. A result favoring the use of butenafine was obtained for
MCET (OR, 7.25; 95% CI, 2.25–23.38) and SC (OR, 12.33; 95% CI,
6.16–24.71) outcomes.
Are oral drugs more effective than topical
formulations in the treatment of athlete’s foot?
The major advantage of oral drugs is a decrease on the duration
of the treatment, which can improve a patient’s compliance [4]. On
the other hand, its cost is higher than the treatments based on
topical agents, including the costs with medical care.
One RCT (n = 137) compared the efficacy of interdigital tinea
pedis treatment with oral (terbinafine 250 mg once daily for 1 week)
and topical antifungal (clotrimazole 1% cream twice daily for 4
weeks). At week 4, the mycological cure rates were similar for the
two drugs (72% for allylamine and 71% for clotrimazole). Although
at week 1 the patients treated with terbinafine showed more rapid
clinical improvement, higher levels of relapse were observed
between weeks 4 and 12 after treatment with this drug (17% vs 5%).
Both treatments were well tolerated, with incidence of adverse
events equal between the groups [23].
Considering the absence of more studies, it is cautious to reserve
treatment with oral antifungals only for several or relapsing cases
of athlete’s foot, when there is no topical treatment response [24].
Athlete’s foot 343
What are the most effective oral drugs in the
treatment of athlete’s foot?
The efficacy of oral drugs is not influenced by the type of tinea pedis
[4].
A meta-analysis of 12 RCTs (n = 700) showed no significant
differences between terbinafine (250 mg/day for 2 weeks) and itraconazole (100 mg/day for 4 weeks); between fluconazole 50 mg and
either itraconazole 100 mg or ketoconazole 200 mg, used for 6
weeks; or between griseofulvin 1000 mg and ketoconazole 200 mg,
used for 4 weeks [4].
For the same treatment length (2 weeks), terbinafine 250 mg was
more effective than itraconazole 100 mg. Moreover, terbinafine
250 mg cures 52% more patients than griseofulvin 500 mg, when
used once daily for 4–6 weeks [4].
Adverse effects were reported for all drugs, with gastrointestinal
effects, such as diarrhea and nausea, being the most commonly
reported. There were no long-duration or harming adverse events
[4]. The oral therapy with terbinafine, itraconazole, and fluconazole
was associated with low incidence of adverse events in an immunocompetent population [25].
What are the most effective topical drugs in the
treatment of athlete’s foot?
We applied a random-effects Bayesian mixed treatment comparisons (MTC) model to combine placebo-controlled and direct
topical antifungals comparison trials. Our analysis included 41
clinical trials published until June 2012 retrieved through a systematic review of the same databases used for conventional metaanalysis [11].
Our analysis did not show statistically significant differences
among all pairwise antifungals assessed (bifonazole, butenafine,
clotrimazole, ciclopiroxolaminae, econazole, flutrimazole, ketoconazole, miconazole, naftifine, oxiconazole, sertaconazole, and terbinafine) for the MCET outcome.
For the SC outcome, the ORs for butenafine, naftifine, and terbinafine were statistically superior to oxiconazole: 5.10 (95% CI, 1.16–
22.47), 4.61 (95% CI, 1.23–17.33), and 6.05 (95% CI, 1.74–21.05),
respectively. Terbinafine also showed better response when compared with clotrimazole (OR, 2.88; 95% CI, 1.27–6.53). Terbinafine,
butenafine, and naftifine, which occupied the first three places in
the ranking, might be the best strategies for cure maintenance.
Drawbacks
Our analysis of published clinical trials has showed no differences
in safety in all direct comparisons made between antifungals and
placebo and among antifungal class. Few serious adverse events
were reported, and the most common adverse events reported were
burning, stinging, and itching, all confined to the site of application
[11,12].
All oral antifungals were associated with the occurrence of
adverse events. The lowest prevalence of events wase obtained with
fluconazole (11%) and the highest with terbinafine (18%) [4].
Comments
There is consistent evidence regarding the superiority of antifungals
in comparison with placebo. For athlete’s foot, allylamines are better
than azoles in providing and maintaining mycological cure.
Oral antifungals are not more effective than topical ones in the
management of athlete’s foot. Additionally, higher rates of infection
relapse, adverse events, and costs of the treatment were observed
in patients treated with oral agents [7].
Implications for clinical practice
All topical antifungals (azoles, allylamines, butenafine, ciclopiroxolamine, tolnaftate, and undecenoic acid) can be recommended to
the treatment of athlete’s foot.
Allylamines are superior to azoles in obtaining and maintaining
cure, with the advantage of requiring a shorter period of time that
can be associated with greater rates of compliance. Nevertheless,
owing to their higher cost compared with other antifungals, an
individual analysis for each patient must be done.
Oral therapy must be considered only for severe infections or in
relapses cases.
Key points
• All topical antifungals are better than placebo in the treatment of
athlete’s foot.
• Direct comparisons of allylamines versus azoles used in the
treatment of athlete’s foot show allylamines to be generally more
efficacious than azoles.
• MTC results do not show statistical differences among the
antifungals considering the MCET outcome. Terbinafine,
butenafine, and naftifine might be the best strategies for cure
maintenance.
• There is evidence to suggest that oral drugs are no more effective
than creams in producing a cure for athlete’s foot and still
produce more adverse events.
• No differences were found in safety in all direct comparisons
established between antifungals and placebo and between
antifungals with each other.
References
1. Gupta AK, Ryder JE, Chow M, et al. Dermatophytosis: the management of fungal
infections. Skinmed 2005;4(5):305–10.
2. Hainer BL. Dermatophyte infections. Am Fam Physician 2003;67(1):101–8.
3. Thomas B. Clear choices in managing epidermal tinea infections. J Fam Pract
2003;52(11):850–62.
4. Bell-Syer SE, Hart R, Crawford F, et al. Oral treatments for fungal infections of the
skin of the foot. Cochrane Database Syst Rev 2002;(2):CD003584.
5. Crawford F, Hollis S. Topical treatments for fungal infections of the skin and nails
of the foot. Cochrane Database Syst Rev 2007;3:1–157.
6. Garber G. An overview of fungal infections. Drugs 2001;61(Suppl 1):1–12.
7. Gupta AK, Einarson TR, Summerbell RC, et al. An overview of topical antifungal
therapy in dermatomycoses. A North American perspective. Drugs 1998;55(5):
645–74.
8. Severo LC, Londero AT, eds. Tratado de Infectologia. ed. São Paulo: Atheneu, 2002.
9. Gupta AK, Cooper EA. Update in antifungal therapy of dermatophytosis.
Mycopathologia 2008;166(5–6):353–67.
10. Hart R, Bell-Syer SE, Crawford F, et al. Systematic review of topical treatments for
fungal infections of the skin and nails of the feet. BMJ 1999;319(7202):79–82.
11. Rotta I, Sanchez A, Goncalves PR, et al. Efficacy and safety of topical antifungals
in the treatment of dermatomycosis: a systematic review. Br J Dermatol 2012;166(5):
927–33.
12. Rotta I, Sanchez ACC, Otuki MF, et al. Efficacy of topical antifungals in different
dermatomycosis: a systematic review with metaanalysis. Rev Assoc Med Bras
2012;58(3):308–18.
13. Patel A, Brookman SD, Bullen MU, et al. Topical treatment of interdigital tinea
pedis: terbinafine compared with clotrimazole. Australas J Dermatol 1999;40(4):
197–200.
14. Evans EGV, Bodman B, Williamson DM, et al. Comparison of terbinafine and
clotrimazole in treating tinea pedis. Br Med J 1993;307(6905):645–7.
15. Schopf R, Hettler O, Brautigam M, et al. Efficacy and tolerability of terbinafine 1%
topical solution used for 1 week compared with 4 weeks clotrimazole 1% topical
solution in the treatment of interdigital tinea pedis: a randomized, double-blind,
multi-centre, 8-week clinical trial. Mycoses 1999;42(5–6):415–20.
16. Aly R, Fisher G, Katz HI, et al. Ciclopirox gel in the treatment of patients with
interdigital tinea pedis. Int J Dermatol 2003;42(Suppl 1):29–35.
17. Gupta AK, Skinner AR, Cooper EA. Evaluation of the efficacy of ciclopirox 0.77%
gel in the treatment of tinea pedis interdigitalis (dermatophytosis complex) in a
344 The evidence
randomized, double-blind, placebo-controlled trial. Int J Dermatol. 2005;44(7):
590–3.
18. Kligman AM, Bogaert H, Cordero C. Evaluation of ciclopirox olamine cream for
the treatment of tinea pedis: multicenter, double-blind comparative studies. Clin
Ther 1985;7(4):409–17.
19. Reyes BA, Beutner KR, Cullen SI, et al. Butenafine, a fungicidal benzylamine
derivative, used once daily for the treatment of interdigital tinea pedis. Int J
Dermatol 1998;37(6):450–3.
20. Savin R, De Villez RL, Elewski B, et al. One-week therapy with twice-daily
butenafine 1% cream versus vehicle in the treatment of tinea pedis: a multicenter,
double-blind trial. J Am Acad Dermatol 1997;36(2 Pt 1):S15–9.
21. Syed TA, Hadi SM, Qureshi ZA, et al. Butenafine 1% versus terbinafine 1% in
cream for the treatment of tinea pedis. A placebo-controlled, double-blind, comparative study. Clin Drug Investig 2000;19(6):393–7.
22. Tschen E, Elewski B, Gorsulowsky DC, et al. Treatment of interdigital tinea pedis
with a 4-week once-daily regimen of butenafine hydrochloride 1% cream. J Am
Acad Dermatol 1997;36(2 Pt 1):S9–14.
23. Barnetson RS, Marley J, Bullen M, et al. Comparison of one week of oral terbinafine (250 mg/per day) with four weeks of treatment with clotrimazole 1% cream
in interdigital tinea pedis. Br J Dermatol 1998;139:675–8.
24. Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial
mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea
manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy
of Dermatology. J Am Acad Dermatol 1996;34(2 Pt 1):282–6.
25. Chang CH, Young-Xu Y, Kurth T, et al. The safety of oral antifungal treatments
for superficial dermatophytosis and onychomycosis: a meta-analysis. Am J Med
2007;120(9):791–8.