Advanced therapy in thoracic surgery - part 8 pps
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (1.17 MB, 44 trang )
Lung Retransplantation
/
385
15. Novick RJ, Kaye MP, Patterson GA, et al. Redo lung trans-
plantation: a North American–European experience. J Heart
Lung Transplant 1993;12(Pt 1):5–15; discussion 15–6.
16. Struber M, Wilhelmi M, Harringer W, et al. Flush perfusion
with low potassium dextran solution improves early graft
function in clinical lung transplantation. Eur J
Cardiothorac Surg 2001;19:190–4.
17. Fischer S, Matte-Martyn A, De Perrot M, et al. Low-
potassium dextran preservation solution improves lung
function after human lung transplantation. J Thorac
Cardiovasc Surg 2001;121:594–6.
18. Pierre AF, Sekine Y, Hutcheon MA, et al. Marginal donor
lungs: a reassessment. J Thorac Cardiovasc Surg
2002;123:421–7; discussion 427–8.
CHAPTER
31
LUNG XENOTRANSPLANTATION:
LESSONS LEARNED AND
FUTURE
PERSPECTIVES
CARSTEN SCHRÖDER,
MD
PAOLO MACCHIARINI, MD, PhD
Success of clinical allotransplantation as a therapeutic
option for end-stage kidney, heart, lung, and liver
disease has resulted in the worldwide diffusion of this
life-saving treatment. However, since there are not
enough cadaveric organs to meet the present clinical
demand, it has also actualized the growing problem of
donor organ availability.
Despite this shortfall, which affects all organs, the
disparity between the supply and demand for organs is
most acute for the lung. According to the 2002 United
Network for Organ Sharing registry, 3,822 patients are
waiting on the recipient list in the United States for lung
transplantation, and only about 1,000 of these patients
will receive transplants. The reasons for this frustrating
scenario are the unique susceptibility of lungs to damage
induced by the brain-dead treatment, the marginal yearly
increase of donor lungs, and the growing number of
teams performing lung transplantation. As expected,
access to the waiting lists is currently very restricted,
patients in need of lungs are waiting approximately twice
as long today as in 1990 (the median waiting period is 7
months for patients younger than age 16 years and 23
months for those older than age 16 years), and many
patients die while awaiting lungs.
One solution to the shortage of donor lungs would be
to increase the supply of lung allografts from human
sources other than cadaveric donors
1
or use artificial
organs,
2
but the benefit of either as a temporary or
permanent alternative to allotransplantation remains to
be proven. Xenotransplantation, the transplantation into
humans of organs from other species, is regarded as an
important solution.
3
The advantages would be obvious.
An unrestricted number of donor lungs would be avail-
able for patients currently excluded from the waiting
lists, the procedure could be planned on routine operat-
ing lists and not as an emergency procedure, the lungs
would be harvested from healthy anesthetized animals
rather than from brain-dead human donors on life-
sustaining drugs and mechanical ventilation, the
ischemic time would be minimized, and donor lungs
could be genetically manipulated to minimize recipient
rejection responses.
4,5
History
The modern era of transplantation began in the early
twentieth century with the experiments of Alexis Carrel,
who transplanted a variety of vascularized organs into
different anatomic sites of the same animal (ie, auto-
transplantation) and between animals of the same (ie,
allotransplantation) and different (ie, xenotransplanta-
tion) species. The success observed with renal autografts
demonstrated that transplantation was indeed technically
feasible but also that other mechanisms were responsible
for the disappointing survival results. In the late 1950s,
the immunological mechanisms of the immune response
began to emerge and the subsequent advent of 6-mercap-
topurine, azathioprine, and prednisone made kidney
allotransplantation feasible.
This success suddenly generated a demand for renal
transplantation exceeding the organ supply and, as a
consequence, renewed attention toward the potential of
animal organ transplantation in humans. As shown in
Table 31-1,
6–15
pigs and nonhuman primates have been
used as sources of organs, and despite the early failures,
the 9 months’ functional survival of a chimpanzee kidney
transplanted into a human recipient
6
clearly suggested a
potential clinical application of xenografts.
Basic Immunobiology
Hyperacute Rejection
Experience with experimental lung xenotransplantation
is quite limited in comparison with other organs, and as
a consequence, the pathogenesis of lung HAR has not yet
been clearly defined. In other experimental discordant
models,
24
the factors initiating and sustaining HAR
involve an antigen–antibody interaction on the periph-
eral endothelium of the xenografts with subsequent
complement activation via the classical pathway. Once
activated, the mechanism by which HAR is promoted is
poorly understood; since lysis of xenograft endothelium
is usually not seen, it is most probably that individual
complement components, including C3a, C5a, and the
membrane attack complex, initiate rapid endothelial cell
(EC) activation, resulting in hemorrhage and edema of
interstitial tissues and thrombosis of xenogeneic vessels.
The lung was once considered relatively resistant to
HAR
25
until further investigations proved this to be false.
26
In 1995, Kaplon and colleagues reported short-term
survival of baboon into which pig single-lung was ortho-
topically transplanted, with evidence of modest rise in
pulmonary vascular resistance (PVR), acceptable gas
transfers, marginal decline of xenoreactive natural anti-
bodies (XNA), patchy deposition of immunoglobulin (Ig)
M and complement proteins along the pulmonary
endothelium.
27
Despite significant xenograft injury (eg,
intra-alveolar hemorrhage), the functional and histological
absence of HAR led the authors to conclude that the lung
was relatively resistant to HAR. A plausible explanation of
the lack of HAR would be, however, that their observations
were related to xenograft hypoperfusion, since following
transient occlusion of the contralateral pulmonary artery
or double-lung xenotransplantation,
28
all xenografts failed
within 3.5 hours as a result of a tenfold increase in PVR.
The lessons learned from these early experiences are that
studies of lung HAR should be performed with animals
models where recipient survival depends on xenograft
function.
29–32
By contrast, Pierson and colleagues, and
others, proved that pig lungs are rapidly damaged by
human blood via a XNA–complement interaction and a
consecutive loss of flow (PVR-related) and function.
26,33
In our experimental studies, we defined the functional
and histopathologic hallmarks of lung HAR using an ex
vivo perfusion-and-ventilation pig-to-human lung
model.
34,35
Pig lungs perfused with unmodified whole
human blood (WHB) showed severe pulmonary hyper-
tension and pulmonary dysfunction as early as 30
minutes into reperfusion, massive hemorrhagic pul-
monary edema, severe interstitial edema, alveolar hemor-
rhage, and several fibrin and platelet thrombi localized in
and obstructing the small vessels (arterioles, capillaries,
and venules) (Figure 31-2) but not the large (segmental
or lobar) pulmonary vessels. Upon immunofluorescence,
there were diffuse deposits of human IgG and IgM,
complement anaphylatoxins (C1q, C3a, C5a, C5b–9),
coagulation proteins, and fibrinogen on the alveolar
endothelial surfaces (Figure 31-3). All xenografts failed at
115 Ϯ 44.2 minutes into reperfusion. These observations
reinforce the paradigm that places the activation of
xenograft endothelium at the center of the HAR process
36
and provide evidence that pig lungs are equally suscepti-
ble to HAR as other solid organs upon reperfusion with
human blood and that the front-line target of the recipi-
ent effector system is the EC located in the peripheral
and not the proximal
28
pulmonary vasculature. Another
noteworthy facet of our findings is that the HAR
observed in the pig-to-human discordant model differs
completely from the rejection observed in the pig-to-
nonhuman primate
27,29–32,37
and in clinical allotrans-
plantation between human leukocyte antigen (HLA)-
incompatible patients.
38,39
388
/ Advanced Therapy in Thoracic Surgery
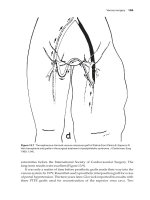
FIGURE 31-2. Histology of pig lungs perfused with human blood. A,
Fibrin and platelet thrombus (arrow) within a peripheral pulmonary
arteriole and located next to a terminal bronchiole (white arrow).
Original magnification ϫ360. B, Multiple fibrin and platelet thrombi in
an interalveolar capillary. Original magnification ϫ180.
390
/ Advanced Therapy in Thoracic Surgery
and anti-␣Gal IgG (63%) in the human blood within 30
minutes upon reperfusion. These findings, not observed
in pig lungs perfused with autologous blood, are in line
with previous observations suggesting that IgM and IgG
XNA bind the ␣Gal epitope on the xenograft’s endothe-
lial cells and initiate HAR.
4,21
However, the development
of lung HAR depends on the activation of complement
as well, as shown by the diffuse deposition of comple-
ments proteins C1q and C3 along the alveolar capillary
walls of pig lungs and the decrease of total complement
activity in the human blood shortly after reperfusion.
34,35
That this step is essential for the development of HAR is
suggested by the observations that inactivation of
complement by cobra venom factor (CVF), soluble
complement receptor type 1 (sCR1), C1 esterase
inhibitor (C1-Inh), or ␥-globulin prevents HAR and
prolongs discordant xenograft survival.
46
Most evidence
suggests that, in pig-to-primate xenografts, the comple-
ment system becomes activated through the classical
pathway (Figure 31-5) upon the binding of the XNA to
the Gal ␣1–3Gal epitopes. Except for situations in which
the alternative pathway is stimulated by ischemia-
reperfusion injury or ex vivo circuits, the alternative
pathway generally does not initiate tissue injury in pig-
to-primate models.
XNA and complement activate pig EC, a protein
synthesis–independent phase of the immune response,
referred to as type I EC activation, and hypothesized to
be the underlying cause of HAR.
22
Once ECs are acti-
vated, they retract from one other, leading to changes in
their physical and biologic characteristics with subse-
quent loss of the barrier function and normal anticoagu-
lant property of the vascular surface. This process
involves the occurrence of hemorrhage and edema and
exposure of the underlying collagen and subendothelial
molecules. Platelets adhere to and spread on the suben-
dothelial matrix by the interaction of platelets receptors
and von Willebrand’s factor (vWF), and this process is
accompanied by recruitment of cells facilitating coagula-
tion and vascular injury, such as P-selectin, platelet-
activating factor, thrombin, and leukotrienes. The end
result will thus be recruitment of platelets and promo-
tion of platelet thrombi and deposition of fibrin along
the surface of the activated ECs.
Vascular Rejection
If HAR can be overcome either by preventing the interac-
tion between XNA and epitopes on xenogeneic endothe-
lium or by interfering with the activation of complement,
a xenograft is subject over the ensuing days to weeks to a
rejection process characterized by EC dysfunction, inter-
stitial hemorrhage, focal necrosis, fibrin deposition, and
eventually thrombosis of the xenograft vessels. This
phenomenon, named “acute vascular xenograft
rejection”
47
or “delayed xenograft rejection,”
48
is also
observed in concordant xenografts and sometimes in
allografts and differs from HAR not only in the kinetics
of graft loss but also in the molecular and cellular mecha-
nisms leading to thrombosis.
That the vascular rejection is initiated by the binding
of XNA to the xenograft ECs has been established beyond
doubt. First, the onset of acute vascular rejection coin-
cides temporally with an increase in the synthesis of XNA
in subjects whose circulation is temporarily connected to
a pig organ. Second, XNA are diffusely deposited along
the xenograft endothelium. Third, removal of XNA from
a xenograft recipient delays the onset of acute vascular
rejection from days to weeks, and treatment of recipients
with agents suppressing XNA may delay rejection for
months or indefinitely.
On the binding of XNA to the ECs, there is a type II
EC activation that involves transcriptional induction of
genes and protein synthesis resulting in the expression of
adhesion molecules, cytokines, procoagulant molecules,
and complement components.
48
The main mechanisms
FIGURE 31-5. In pig-to-primate xenografts, complement becomes
activated through the classical pathway, and the cascade necessary
to the development of hyperacute rejection is the assembly of the
terminal components (C5b67, C5b-8, C5b-9). A key role is the forma-
tion of C3 convertase because it mediates opsonization and cell lysis
leading to loss of endothelial cell function. Under physiologic condi-
tions, regulators of complement activity (RCA) such as decay acceler-
ating factor (DAF) and membrane cofactor protein (MCP) regulate
complement activation by dissociating and degrading C3 convertase.
CD59 prevents formation of the membrane attack complex (MAC) by
blocking C9 binding to C8.
Lung Xenotransplantation: Lessons Learned and Future Perspectives
/
391
that underlie xenograft loss caused by acute vascular
rejection are thus the donor–organ EC activation and
infiltration into the graft of host monocytes, natural
killer cells, and the products of their activation, which
collectively promote intragraft inflammation and throm-
bosis.
48
Whether the total inhibition of XNA and comple-
ment would allow survival of discordant xenografts, if
the putative T cell response is suppressed, is questioned.
This suggests that other factors may potentially lead to
acute vascular rejection. Because HAR can now be
prevented in nearly all cases, vascular rejection is consid-
ered the major hurdle to the successful clinical applica-
tion of lung xenotransplantation.
Accommodation
Early attempts to transplant ABO-incompatible renal
allografts showed that temporary depletion of anti-A or
anti-B antibodies from the recipient in the pretransplan-
tation period allowed prolonged graft survival in some
patients even after the return of the antigraft antibodies
to the circulation and despite the presence of a functional
complement system. This process, called “accommoda-
tion,” denotes a sort of graft resistance to humoral injury
under conditions that would otherwise result in HAR or
vascular rejection. A similar phenomenon has been also
observed in xenografts, albeit infrequently.
49
The possible
causes for accommodation include morphologic and
functional differences between the XNA that return after
depletion and the preexisting XNA, alterations in antigen
expression or, more likely, an acquired resistance by
xenogeneic ECs to humoral immune injury after the
return of the XNA in the recipient’s circulation.
Cellular or Chronic Rejection
To date, no reports have been published where HAR or
vascular rejection have been indisputably overcome. It is
therefore uncertain as to whether these immune re-
sponses play an important role in xenotransplantation as
they do in lung allotransplantation. However, experi-
ments with murine skin and pancreatic-islet grafts,
which are not subject to HAR or vascular rejection, have
shown that T cell–mediated xenograft rejection is often
as vigorous, or more so, than T cell–mediated allograft
rejection and that conventional immunosuppressive
agents may be less effective in prolonging xenograft than
allograft survival.
25
Strategies to Overcome HAR
With the increasing understanding of its physiopathol-
ogy, four basic strategies to overcome HAR have
emerged, namely (1) prevention of the XNA–xenograft
endothelium interaction, (2) blockage of the early steps
of complement activation, (3) adhesive interactions in
the coagulation pathway, and (4) pig-donor genetic engi-
neering (Figure 31-6).
Prevention of XNA–Xenograft Endothelium
Interaction
This can be afforded either by depleting or inhibiting the
human XNA or by injecting soluble carbohydrate, satu-
rating the XNA binding sites before engrafting. The
rationale for depleting XNA from the circulation of a
potential xenograft recipient is the accommodation
whereby discordant or ABO-incompatible grafts
continue to survive despite a functional complement
system and in the presence of antidonor antibody if the
recipient has undergone a pretransplant depletion of
antidonor antibodies. Pretransplant removal of circulat-
ing XNA from potential xenotransplant recipients can be
obtained by (1) plasmapheresis, (2) perfusion of human
blood through pig donor organs, or (3) column
immunoabsorption.
The first two techniques prolong pig-to-primate
xenograft survival from minutes to many days.
50
During
plasmapheresis, red and white blood cells are isolated and
returned to the primate, but all other blood elements,
including the plasma containing XNA, are discarded.
During pig organ perfusion, the entire blood volume of a
primate is pumped into the pig organ vasculature, and
the XNA are removed because they adhere to the pig’s
endothelium. The major limitations of these techniques,
however, are that they remove also the primate’s
immunoglobulins and complement and coagulation
proteins, thus increasing the susceptibility to infection
and thrombogenic disorders. Moreover, neither tech-
nique can be continued indefinitely, and the ultimate risk
of immunological reactions once XNA reappear is still
there.
Specific depletion of human XNA has been obtained
by Rieben and colleagues with extracorporeal im-
munoabsorption (EIA) of human plasma through an
immunoaffinity column of a newly developed, synthetic
Gal␣1–3Gal disaccharide.
51
Based on these in vitro stud-
ies, 50 to 60% of the anti-␣Gal IgM and IgA were specifi-
cally absorbed and the cytotoxic effect of human serum
on pig kidney (PK15) cells was almost totally inhibited
after EIA; other plasma proteins were normal through
the process. Similarly, in vivo studies by Taniguchi and
colleagues suggested that in immunosuppressed, splenec-
tomized baboons, repeated EIA using the same
immunoaffinity column may reduce XNA levels and
serum cytotoxicity significantly for several days.
52
To t est the validity of the above-mentioned techniques
in the pig-to-human lung combination, we have devel-
oped in our laboratory an in vivo pig organ perfusion
colleagues.
57,58
They created a large polymer with several
␣Gal epitopes incorporated (␣1–3 galactose trisaccharide-
polyethylene glycol conjugate). This drug given intra-
venously before, during, and throughout a xenogenic
pig-to-baboon transplant diminished the ␣Gal-antibodies
to undetectable levels. The influence of ␣Gal-antibodies
could be controlled, but the remaining non-␣Gal-
antibodies were still present and played their role in
vascular rejection. The new substances seem capable to
overcome HAR and may lead to accommodation (not yet
shown). So far, none of these results are shown in lung
xenotransplantation, since there are major organ-specific
differences.
Prevention of the Early Steps of Complement
Activation
Although CVF prevents HAR following pig-to-baboon
heart transplantation,
59
it is unlikely that these strategies
will have clinical application since it is associated with
unacceptable morbidity and production of anti-CVF anti-
bodies. Since these antibodies have a ␣Gal oligosaccharide
as a terminal structure, there might be some anti-␣Gal
antibodies, which may preclude further therapy with CVF
and favor rapid xenograft rejection.
60
Other soluble
complement inhibitors injectable in the pretransplant
period are C1 Inh, which prevents the activation of C1 by
human XNA binding to pig EC and sCR1, which showed
marked inhibited total and alternative pathway serum
complement activity and prolonged xenograft survival in
an in vivo pig-to-primate cardiac xenotransplantation
model.
46
In addition, the lung is particularly sensitive to
ischemia and reperfusion, which is mediated in part
through activation of the complement cascade.
61,62
Since
the lung is particularly susceptible to complement injury,
and antibody-driven activation of the classical pathway is
the principle mediator of HAR in other organs,
63
we
reasoned that effective regulation of complement activa-
tion should be particularly effective for preventing HAR.
To date, there are no studies on the use of pharmacologic
complement inhibitors in discordant lung xenografting,
but one major disadvantage is that they must be given
systemically, and in addition to preventing complement-
mediated xenograft injury, they may also inhibit appro-
priate destruction of infectious pathogens.
64,65
Adhesive Interactions in the Coagulation Pathway
Adhesion molecules play a critical role in ischemia-
reperfusion injury and mediate the lung injury seen with
systemic complement activation,
66,67
Where they have
been examined, the interaction between most pig and
human integrin and selectin ligands appears to occur
under circumstances analogous to those described within
either species and may thus be considered to occur in an
appropriate “physiologic” manner. P-selectin and inter-
cellular adhesion molecule 1 (ICAM-1) are examples of
adhesion molecules whose function has been well char-
acterized in this species combination and found to func-
tion physiologically.
68,69
In the xenogeneic situation, other
“nonphysiologic” molecular interactions may also trigger
pathogenic adhesive interactions between porcine
endothelium and primate platelets and neutrophils.
Like complement activation, activation of the coagula-
tion cascade occurs most efficiently on activated cell
surfaces. Interestingly, coagulation pathway dysregulation
was recently shown to play a central role in clinical acute
lung injury, in that administration of activated protein C
was associated with decreased morbidity and mortality
from acute respiratory distress syndrome (ARDS)/systemic
inflammatory response syndrome (SIRS).
70–73
Several “nonphysiologic” interactions in the coagula-
tion pathway between porcine endothelium, human
platelets, and coagulation factors have been identified
that are potentially important to HAR.
74–78
Whereas
quiescent human platelets do not bind to human vWF,
porcine vWF binds to human platelets through a
nonphysiologic interaction via GP1b and the alpha1
domain of vWF.
79
Human thrombin activation is actively
inhibited by regulatory proteins on human endothelium,
but constitutive activation of human thrombin occurs
when human plasma is exposed to quiescent porcine
endothelium.
80
Thrombomodulin and ectoadeno-
sinediphosphatase, potent anticoagulant molecules
expressed by normal endothelium, are rapidly down-
regulated or lost after exposure of porcine endothelium
to human blood constituents, leading to a procoagulant
endothelial phenotype.
81,82
Porcine vWF appears to bind
human complement even in the absence of antipig anti-
body,
83
suggesting that pig vWF itself may serve as a
primary nidus for inflammation. In addition, high shear
stress, which occurs at sites of vasoconstriction, causes
platelet aggregation to vWF and shedding of procoagu-
lant microparticles.
84
Finally, aggregated platelets coated
with vWF, or vWF multimers released from the surface of
injured or activated ECs, may thus activate complement
in soluble phase, triggering productions of anaphylatox-
ins in the blood as well as where they are expressed in the
organ.
85
Thus even if pig endothelium is not activated by
other interactions, platelet adhesion and binding of
complement are likely to occur and to trigger prothrom-
botic and proinflammatory events in the graft and else-
where in the organ recipient.
Genetically Engineered Donor Pigs
The recent development of genetically engineered mice
and pigs has opened several alternative approaches for
the prevention of HAR.
17
One large step forward to xeno-
Lung Xenotransplantation: Lessons Learned and Future Perspectives
/
393
transplantation has been taken by generating transgenic
pigs that do not express the ␣Gal antigens on their ECs.
5
The expression of these antigens depends on the function
of a single gene encoding for the enzyme ␣1,3galactosyl-
transferase. This gene was “knocked out” by homologous
recombination, and the frontline targets for the human
XNA disappeared. Unfortunately, there are no data yet
published about these newly designed piglets. This very
promising news is hopefully not overestimated because it
is known from mouse ␣Gal-knockout strains that there is
still a remaining ␣Gal-epitope production (about 10%)
driven by an additional intracellular ␣1,3galactosyltrans-
ferase, which was recently discovered. Even though some
␣Gal-epitope will still be present in theses donor pigs an
important influence on HAR and vascular rejection will
be seen.
The most promising way appeared in the past to be
the development of genetically engineered pigs express-
ing one or more of the human C-reactive protein
(CRP).
17
They include (1) decay accelerating factor
(DAF), a phosphatidylinositol-linked integral
membrane protein that prevents assembly of the classi-
cal pathway C3 convertase, (2) membrane cofactor
protein (MCP), a membrane associate protein that
serves as a cofactor for factor I-mediated cleavage and
inactivation of C3b, (3) C4bBP, a soluble binding
protein with decay accelerating activity for the inactiva-
tion of C3 convertase, (4) CD59 or membrane inhibitor
of reactive lyses (MIRL), which prevents formation of
the membrane attack complex by blocking C9 binding
to C8. Because of the species-restricted molecular
incompatibilities, the membrane-associated CRPs
expressed on the surface of a given donor animal organ
are unable to effectively control the human complement
cascade, and this accounts for most of the inflammatory
response observed in HAR. By incorporating human
complement regulatory transgenes into the germline of
donor pigs, several groups have recently achieved
considerable prolongation of pig heart function after
heterotopic transplantation into primates.
86
Parallel
experience using lungs from animals transgenic for
human DAF (hDAF) or CD59 (hCD59) have produced
controversial results. Pierson and associates found
incomplete physiologic and histologic protection from
HAR using transgenic pigs expressing hDAF perfused ex
vivo with fresh human blood, except in two pigs
expressing very high levels of hDAF on their pulmonary
endothelium.
87
By contrast, Dagget and colleagues
found that pig lungs expressing hDAF and hCD59 func-
tioned better than nontransgenic pig lungs when
perfused (for 2 hours) with human plasma.
37
However,
in an earlier experience, Pierson and associates demon-
strated that by depleting the recipient’s complement
with CVF, profound pulmonary hypertension and HAR
still occurred, even when human XNA depletion was
added.
88
These and other
89
preliminary experiences
suggest that although transgenic pigs expressing CRPs
at physiologically appropriate levels may prolong
xenograft survival, other efforts directed to abrogate the
effects of the humoral and cellular response need to be
done.
Comment
Xenotransplantation has the potential to address the
acute problem of lung allograft shortage and may have
additional advantages over allotransplantation. Although
HAR has so far prevented the clinical use of pig lungs, a
combination of the outlined strategies and the new ␣Gal-
knockout pigs offer a realistic hope that lung xenografts
may survive in humans beyond the hurdle of HAR.
Unfortunately, while clinical trials are currently proposed
or underway to address whether pig kidneys, livers, and
hearts are suitable organs in humans, lung xenotrans-
plantation is still in its experimental childhood.
However, some clues are available; anatomic and
physiologic similarities between humans and pigs indi-
cate that pig lungs may function adequately, at least in
the short term. Pig lungs are hyperacutely rejected in a
similar fashion to other pig organs when perfused with
untreated human blood, and despite the fact that they do
not have the synthetic functional problems of pig kidneys
or livers, they are more prone to other nonxenogeneic
injuries (eg, ischemia-reperfusion injury) than are other
pig organs. There are several strategies that prevent lung
HAR, and there is optimism that the simplest one will be
useful in a future clinical setting.
Nevertheless, to be of significant clinical impact and
to solve the actual allograft shortage, lung xenograft
survival must be at least as good as allograft survival. In
this sense, major areas of consideration for laboratory
investigations beyond HAR need to be explored to
address the long-term xenograft survival.
Acknowledgment
This work was supported by the Immunology Concerted
Action (#3026PL950004) of the Immunology
Biotechnology Program from the European Union, an
East-West INSERM contract and the German Research
foundation (Deutsche Forschungsgemeinschaft, DFG).
References
1. Starnes V, Barr ML, Cohen RG, et al. Living-related lobar
lung transplantation experience: intermediate results. J
Thorac Cardiovasc Surg 1996;112:1284–91.
394
/ Advanced Therapy in Thoracic Surgery
2. Federspiel W, Sawzik P, Borovetz H, et al. Temporary
support of the lungs — the artificial lung. In: Cooper DKC,
Miller LW,Patterson GA, editors. The transplantation and
replacement of thoracic organs. Dordrecht (The
Netherlands): Kluwer Academic; 1996.
3. Fabre JW. Nudging xenotransplantation towards humans.
Nature Med 1995;1:403–4.
4. Cooper DKC, Oriol R. Glycobiology in xenotransplantation
research. In: Gabius HJ, Gabius S, editors. Glycosciences:
status and perspectives. London: Chapman & Hall; 1996.
5. Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the
alpha1,3-galactosyltransferase gene in cloned pigs. Nat
Biotechnol 2002;20:251–5.
6. Reemtsma K, McKracken K, Schlegel BH, et al. Renal
heterotransplantation in man. Ann Surg 1964;160:384–410.
7. Perper RJ, Najarian JL. Experimental renal heterotransplan-
tation. III. Passive transfer of transplantation immunity.
Tr ansplantation 1967;49:1000–1.
8. Hardy JD, Chavez CM, Kurrus FD. Heart transplantation in
man. JAMA 1964;188:1132–40.
9. Starzl TE, Marchioro TL, Peters GN. Renal heterotransplan-
tation from baboon to man: experience with 6 cases.
Tr ansplantation 1964;2:752–76.
10. Cooley DA, Hallman GL, Bloodwell RD. Human heart
transplantation: experience with 12 cases. Am J Cardiol
1968;22:804–10.
11. Barnard CN, Wolpitz A, Losman JG. Heterotopic cardiac
transplantation with a xenograft for assitance of the left
heart in cardiogenic shock after cardiopulmonary bypass. S
Afr Med J 1977;52:1035–8.
12. Bailey LL, Nehlsen-Cannarella SL, Concepcion W. Baboon-
to-human cardiac xenotransplantation in a neonate. JAMA
1985;254:3321–9.
13. Czaplicki J, Blonska B, Religa Z. The lack of hyperacute
xenogeneic heart transplant rejection in man. J Heart Lung
Tr anspl 1992;11:393–6.
14. Chen JM, Michler RE. Heart xenotransplantation: lessons
learned and future prospects. J Heart Lung Transpl
1993;12:869–75.
15. Chari RS, Collins BH, Mages JC, et al. Treatment of hepatic
failure with ex vivo pig-liver perfusion followed by liver
transplantation. N Engl J Med 1994;331:234–7.
16. Cooper DKC, Y Ye, LLJ Rolf. The pig as potential organ donor
for man. In: Cooper DKC, editor. Xenotransplantation: the
transplantation of organs and tissues between species.
Heidelberg (Germany): Springer; 1991.
17. Cozzi E, White DJG. Generation of transgenic pigs as
potential organ donors for humans. Nat Med 1996;1:964–6.
18. Perper RJ, Najarian JS. Experimental renal heterotransplan-
tation. I. In widely divergent species. Transplantation
1966;4:377–88.
19. Perper RJ, Najarian JS. Experimental renal heterotransplan-
tation. II. Closely related species. Transplantation 1966;4:700.
20. Calne RY. Organ transplantation between widley disparate
species. Transplant Proc 1970;2:550–6.
21. Platt JL, Bach FH. The barriers to xenotransplantation.
Tr ansplantation 1991;52:937–47.
22. Bach FH, Robson SC, Winkler H, et al. Barriers to xeno-
transplantation. Nat Med 1995;1:869–73.
23. Lawson JH, Platt JL. Molecular barriers to xenotransplanta-
tion. Transplantation 1996;62:303–10.
24. Platt JL, Fischel RJ, Matas AJ, et al. Immunopathology of
hyperacute xenograft rejection in a swine-to-primate
model. Transplantation 1991;52:214–20.
25. Dorling A, Riesbeck K, Warrens A, Lechler L. Clinical xeno-
transplantation of solid organs. Lancet 1997;349:867–71.
26. Pierson RN, Kasper-Konig W, Tew DN, et al. Hyperacute
lung rejection in a pig-to-human transplant model.
Tr ansplantation 1997;63:594–603.
27. Kaplon RJ, Platt JL, Kwiatkowski PA, et al. Absence of
hyperacute rejection in pig-to-primate orthotopic
pulmonary xenografts. Transplantation 1995;59:410–6.
28. Shah AS, O’Hair DP, Kaplon RJ. Absence of hyperacute
rejection in pig-to-primate double lung xenografts
[abstract]. J Heart Lung Transplant 1995;14:S83.
29. Yeatman M, Daggett CW, Lau CL, et al. Human comple-
ment regulatory proteins protect swine lungs from xeno-
geneic injury. Ann Thorac Surg 1999;67:769–75.
30. Yeatman M, Daggett CW, Parker W, et al. Complement-
mediated pulmonary xenograft injury: studies in swine-to-
primate orthotopic single lung transplant models.
Tr ansplantation 1998;65:1084–93.
31. Lau CL, Daggett WC, Yeatman MF, et al. The role of anti-
bodies in dysfunction of pig-to-baboon pulmonary trans-
plants. J Thorac Cardiovasc Surg 2000;120:29–38.
32. Daggett CW, Yeatman M, Logge AJ, et al. Total respiratory
support from swine lungs in primate recipients. J Thorac
Cardiovasc Surg 1998;115:19–27.
33. Pfeiffer S, Zorn GL III, Kelishadi S, et al. Role of anti
Gal␣1,3Gal and anti-platelet antibodies in hyperacute
rejection of pig lung by human blood. Ann Thorac Surg
2001;72:1681–9; discussion 1690.
34. Macchiarini P, Mazmanian GM, Oriol R, et al. Ex-vivo lung
model of pig to human hyperacute xenograft rejection. J
Thorac Cardiovasc Surg 1997;114:315–25.
35. Macchiarini P, Oriol R, Azimzadeh A, et al. Evidence of
human non-alpha-galactosyl antibodies involved in the
hyperacute rejection of pig lungs and their removal by pig
organ perfusion. J Thorac Cardiovasc Surg 1998;116:831–43.
36. Platt JL, Vercelotti GM, Dalmasso AP. Transplantation of
discordant xenografts: a review of progress. Immunol
To day 1990;11:450–6.
Lung Xenotransplantation: Lessons Learned and Future Perspectives
/
395
37. Dagget CW, Yeatman M, Lodge AJ, et al. Swine lungs express-
ing human complement-regulatory proteins are protected
against pulmonary dysfunction in a human plasma perfu-
sion model. J Thorac Cardiovasc Surg 1997;113:390–8.
38. Frost AE, Jammal CT, Cagle PT. Hyperacute rejection
following lung transplantation. Chest 1996;110:559–62.
39. Pierson RN III, Loyd JE, Goodwin A, et al. Successful
management of an ABO-mismatched lung allograft using
antigen-specific immunoadsorption, complement inhibi-
tion, and immunomodulatory therapy1. Transplantation
2002;74:79–84.
40. Cooper DKC. Clinical survey of heart transplantation
between ABO-blood group incompatible recipients and
donors. J Heart Transplant 1990;9:376–81.
41. Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique
natural human IgG antibody with anti-␣-galactosyl speci-
ficity. J Exp Med 1984;160:1519–31.
42. Galili U. Interaction of the natural anti-Gal antibody with
␣-galactosyl epitopes: a major obstacle for xenotransplan-
tation. Immunol Today 1993;14:480–2.
43. Oriol R, Ye Y, Koren E, Cooper DKC. Carbohydrate antigens
of pig tissues reacting with human natural antibodies as
potential targets for hyperacute vascular rejection in pig-to-
man organ xenotransplantation. Transplantation
1993;56:1433–42.
44. Galili U, Clark MR, Shohet SB, et al. Evolutionary relation-
ship between the natural anti-Gal antibody and the
Gal␣1→3Gal epitope in primates. Proc Natl Acad Sci U S A
1987;84:1369–73.
45. Galili U. The natural anti-gal antibody: evolution and
autoimmunity in man. Immunol Series 1991;55:355–73.
46. Pruitt SK, Kirk AD, Bollinger RR, et al. The effect of soluble
complement receptor type 1 on hyperacute rejection of
porcine xenografts. Transplantation 1994;57:363–70.
47. Leventhal JR, Matas AJ, Sun LH. The immunopathology of
cardiac xenograft rejection in the guinea pig-to-rat model.
Tr ansplantation 1993;56:1–8.
48. Bach FH, Winkler H, Ferran C, et al. Delayed xenograft
rejection. Review Immunol Today 1996;17:379–84.
49. Winkler H, Ferran C, Bach FH. Accommodation of
xenografts: a concept revisited. Xenotransplantation
1995;2:53–6.
50. Cooper DKC, Human PA, Lexer G. Effects of cyclosporin
and antibody adsorption on pig cardiac xenograft survival
in the baboon. J Heart Transplant 1988;7:238–46.
51. Rieben R, van Allmen E, Korchagina EY, et al. Detection,
immunoabsorption, and inhibition of cytotoxic activity of
anti-␣Gal antibodies using newly developed substances
with synthetic Gal ␣1–3Gal disaccharide epitopes.
Xenotransplantation 1995;2:98–106.
52. Taniguchi S, Neethling FA, Korchagina EY, et al. In vivo
immunoadsorption of antipig antibodies in baboons using
a specific Gal␣1–3Gal column. Transplantation
1996;62:1379–84.
53. Cooper DKC, Ye Y, Niekrasz M, et al. Specific intravenous
carbohydrate therapy. A new concept in inhibiting antibody-
mediated rejection — experience with ABO-incompatible
cardiac allografting in the baboon. Transplantation
1993;56:769–77.
54. Ghanekar A, Luo Y, Yang H, et al. The alpha-Gal analog
GAS914 ameliorates delayed rejection of hDAF transgenic
pig-to-baboon renal xenografts. Transplant Proc
2001;33:3853–4.
55. Cairns T, Lee J, Goldberg L, et al. Inhibition of the pig to
human xenograft reaction using soluble Gal␣1–3Gal and
Gal␣1–3Gal1–4GlcNac. Transplantation 1995;60:1202–7.
56. Galili U. The alpha-gal epitope (Gal alpha 1–3Gal beta
1–4GlcNAc-R) in xenotransplantation. Biochimie
2001;83:557–63.
57. Diamond LE, Byrne GW, Schwarz A, et al. Analysis of the
control of the anti-gal immune response in a non-human
primate by galactose alpha1–3 galactose trisaccharide-
polyethylene glycol conjugate. Transplantation
2002;73:1780–7.
58. Byrne GW, Schwarz A, Fesi JR, et al. Evaluation of different
alpha-galactosyl glycoconjugates for use in xenotransplan-
tation. Bioconjug Chem 2002;13:571–81.
59. Leventhal JR, Dalmasso AP, Cromwell JW. Prolongation of
cardiac xenograft by depletion of complement.
Tr ansplantation 1993;55:857–66.
60. Cooper DKC, Koren E, Oriol R. Manipulation of the anti-
aGal antibody-aGal epitope system in experimental discor-
dant xenotransplantation. Xenotransplantation
1996;3:102–11.
61. Heller T, Hennecke M, Baumann U, et al. Selection of a C5a
receptor antagonist from phage libraries attenuating the
inflammatory response in immune complex disease and
ischemia/reperfusion injury. J Immunol 1999;163:985–94.
62. Stammberger U, Hamacher J, Hillinger S, Schmid RA.
sCR1sLe ameliorates ischemia/reperfusion injury in experi-
mental lung transplantation. J Thorac Cardiovasc Surg
2000;120:1078–84.
63. Dalmasso AP, Vercellotti GM, Fischel RJ, et al. Mechanism
of complement activation in the hyperacute rejection of
porcine organs transplanted into primate recipients. Am J
Pathol 1992;140:1157–66.
64. Lu CY, Khaireldin TA, Dawidson IA, et al. Xeno-
transplantation. FASEB J 1994;8:1122–30.
65. Hecker JM, Lorenz R, Appiah R, et al. C1-inhibitor for
prophylaxis of xenograft rejection after pig to cynomolgus
monkey kidney transplantation. Transplantation
2002;73:688–94.
396
/ Advanced Therapy in Thoracic Surgery
Lung Xenotransplantation: Lessons Learned and Future Perspectives
/
397
66. Mulligan MS, Warner RL, Rittershaus CW, et al. Endothelial
targeting and enhanced antiinflammatory effects of
complement inhibitors possessing sialyl Lewisx moieties. J
Immunol 1999;162:4952–9.
67. Mulligan MS, Schmid E, Till GO, et al. C5a-dependent up-
regulation in vivo of lung vascular P-selectin. J Immunol
1997;158:1857–61.
68. Simon AR, Warrens AN, Sykes M. Efficacy of adhesive
interactions in pig-to-human xenotransplantation.
Immunol Today 1999;20:323–30.
69. Warrens AN, Simon AR, Theodore PR, Sykes M. Human-
porcine receptor-ligand compatibility within the immune
system: relevance for xenotransplantation. Xenotrans-
plantation 1999;6:75–8.
70. Bernard GR, Vincent JL, Laterre PF, et al. Recombinant
human protein C Worldwide Evaluation in Severe Sepsis
(PROWESS) study group. Efficacy and safety of recombi-
nant human activated protein C for severe sepsis. N Engl J
Med 2001;344:699–709.
71. Grey ST, Tsuchida A, Hau H, et al. Selective inhibitory
effects of the anticoagulant activated protein C on the
responses of human mononuclear phagocytes to LPS, IFN-
gamma, or phorbol ester. J Immunol 1994;153:3664–72.
72. Hirose K, Okajima K, Taoka Y, et al. Activated protein C
reduces the ischemia/reperfusion-induced spinal cord
injury in rats by inhibiting neutrophil activation. Ann Surg
2000;232:272–80.
73. Grinnell BW, Hermann RB, Yan SB. Human protein C
inhibits selectin-mediated cell adhesion: role of unique
fucosylated oligosaccharide. Glycobiology 1994;4:221–5.
74. Robson SC, Young VK, Cook NS, et al. Thrombin inhibi-
tion in an ex vivo model of porcine heart xenograft hypera-
cute rejection. Transplantation 1996;61:862–8.
75. Robson SC, Kaczmarek E, Seigel JB, et al. Loss of ATP
diphosphohydrolase activity with endothelial activation. J
Exp Med 1997;185:153–63.
76. Nagayasu T, Saadi S, Holzknecht RA, et al. Expression of
tissue factor mRNA in cardiac xenografts: clues to the
pathogenesis of acute vascular rejection. Transplantation
2000;69:475–82.
77. Alwayn IP, Appel JZ, Goepfert C, et al. Inhibition of platelet
aggregation in baboons: therapeutic implications for xeno-
transplantation. Xenotransplantation 2000;7:247–57.
78. Bustos M, Saadi S, Platt JL. Platelet-mediated activation of
endothelial cells: implications for the pathogenesis of trans-
plant rejection. Transplantation 2001;72:509–15.
79. Schulte am Esch J II, Cruz MA, Siegel JB, et al. Activation of
human platelets by the membrane-expressed A1 domain of
von Willebrand factor. Blood 1997;90:4425–37.
80. Siegel JB, Grey ST, Lesnikoski BA, et al. Xenogeneic
endothelial cells activate human prothrombin.
Tr ansplantation 1997;64:888–96.
81. Kalady MF, Lawson JH, Sorrell RD, Platt JL. Decreased
fibrinolytic activity in porcine-to-primate cardiac xeno-
transplantation. Mol Med 1998;4:629–37.
82. Saadi S, Holzknecht RA, Patte CP, et al. Complement-
mediated regulation of tissue factor activity in endothe-
lium. J Exp Med 1995;182:1807–14.
83. Holzknecht ZE, Coombes S, Blocher BA, et al.
Identification of antigens on porcine pulmonary microvas-
cular endothelial cells recognized by human xenoreactive
natural antibodies. Lab Invest 1999;79:763–73.
84. Miyazake Y, Momura S, Miyake T, et al. High shear stress
can initiate both platelet aggregation and shedding of
procoagulant containing microparticles. Blood; 88:3456–64.
85. Holzknecht ZE, Coombes S, Blocher BA, et al. Immune
complex formation after xenotransplantation: evidence of
type III as well as type II immune reactions provides clues
to pathophysiology. Am J Pathol 2001;158:627–37.
86. McCurry KR, Kooyman DL, Alvarado CG, et al. Human
complement regulatory proteins protect swine-to-primate
cardiac xenografts from humoral injury. Nat Med
1995;1:423–7.
87. Pierson RN, Pino-Chavez G, Young VK, et al. Expression of
human decay accellerating factor may protect pig lung from
hyperacute rejection by human blood. J Heart Lung
Tr anplant 1997;16:231–9.
88. Pierson RN, Kaspar-Konig W, Tew DN, et al. Profound
pulmonary hypertension characteristic of pig lung rejec-
tion by blood is mediated by xenoreactive antibodies inde-
pendent of complement. Transpl Proc 1995;27:274.
89. Hoopes CW, Platt JL. Molecular strategies for clinical xeno-
transplantation in cardiothoracic surgery. Sem Thorac
Cardiovasc Surg 1996;8:156–74.
CHAPTER
32
ARTIFICIAL LUNGS
ROBERT H. B
ARTLETT, MD
JONATHAN W. H
AFT, MD
Chronic respiratory failure represents a heterogeneous
group of diseases affecting millions of people worldwide
and is the third leading cause of death in the United
States, accounting for more than 350,000 deaths annually.
1
In addition, both the death rate and prevalence of lung
disease appear to be increasing, in large part related to the
rapid expansion of the aging population. Chronic respira-
tory failure includes, but is not limited to, chronic
obstructive pulmonary disease (COPD), idiopathic
pulmonary fibrosis, pulmonary sarcoidosis, cystic fibrosis,
and primary pulmonary hypertension. Emphysema and
chronic bronchitis affect approximately 16 million
Americans and has been steadily rising over the last
several decades, with nearly 120,000 deaths in 1999 attrib-
uted to COPD.
1
Idiopathic pulmonary fibrosis is a
progressively disabling illness characterized histologically
by fibrosis and architectural distortion. Its prevalence is
estimated to be around 13 to 20 per 100,000 in the United
States, and median survival from the time of diagnosis is
less than 3 years.
2
Pulmonary sarcoidosis is often asymp-
tomatic and associated with spontaneous regression, but
approximately 5% of affected individuals develop relent-
less pulmonary dysfunction leading to inevitable death
from respiratory failure.
3
Cystic fibrosis, a genetic condi-
tion with an autosomal recessive mode of transmission,
affects approximately 30,000 Americans,
4
with median
survival steadily rising but presently at approximately 30
years of age.
5
Primary pulmonary hypertension is a rare
and uniformly fatal disorder of unknown etiology.
Survival from the time of diagnosis remains less than 3
years.
6
Patients suffering from these diseases, as well as
several other less common conditions, typically progress
to end-stage respiratory failure. Long-term invasive
mechanical ventilation is often the only treatment strategy
as patients deteriorate. Lung transplant currently offers
the only hope for prolonged survival and reasonable qual-
ity of life under most circumstances.
Lung transplant has enjoyed increasing success over
the last 20 years, with 1- and 3-year survival rates of 76
and 56%.
7
However, transplant is necessarily limited by
the finite pool of suitable cadaveric organs. Between 1996
and 2002, the number of patients listed more than
doubled from 1,900 to nearly 4,000, while the number of
transplants performed has plateaued near 1,000. As a
result, the wait list time now exceeds 2 years at most busy
centers with a mortality rate for listed patients of over
20%. Because of the scarcity of this resource, relatively
strict criteria have been established for lung transplant
eligibility, thus excluding patients greater than 65 years of
age, obese or malnourished patients, and those in chronic
renal failure and strongly discouraging transplant in
patients who require temporary or permanent mechani-
cal ventilatory support.
8
Unfortunately, previously eligi-
ble patients who decompensate or those with end-stage
respiratory failure excluded from eligibility for transplant
because of age or underlying medical conditions cur-
rently have no treatment options. There clearly is a need
for new therapeutic strategies designed either as a bridge
to lung transplant for listed but acutely worsening
patients or as a transplant alternative, possibly serving
those individuals currently considered unsuitable for
transplant.
Recent successes have allowed mechanical cardiac
support to become standard practice in bridging patients
with severe heart failure to cardiac transplant. Since the
approval of ventricular assist devices by the Food and
Drug Administration as a bridge to transplant, more than
70% of patients have undergone successful implantation
and have survived until a suitable cadaveric organ could
be found.
9
In addition, there is increasing evidence
suggesting that the use of these devices allows rehabilita-
tion of the decompensated patient, thus improving
outcomes after transplant.
10
In fact, many patients have
elected to forgo transplant because their quality of life
while supported with a ventricular assist device was so
dramatically improved. In addition, mechanical cardiac
support may have a role in treating patients deemed inel-
igible for transplant by age or medical comorbidity, as
demonstrated by the much publicized and impressive
Rematch
11
trial and the anticipated Intrepid
12
trial.
Groups invested in the development and testing of artifi-
cial lungs hope to draw many parallels from the success
of mechanical cardiac support as they look for innovative
alternative treatment strategies for patients with end-
stage respiratory failure.
History
Attempts to treat respiratory failure with artificial lungs
have appeared since the 1970s.
13,14
These initial devices
also relied upon thin membranes to provide gas transfer,
but were limited by the quality and reproducibility of
these materials. As a result, oxygen transfer and carbon
dioxide elimination were highly variable and incapable of
providing meaningful support. In addition, several of the
initial prototypes attempted to avoid external communi-
cation with a continuous gas source by directly connect-
ing gas lines to the bronchial tree. Animal experiments
were fraught with ventilation problems as fluid and
fibrosis obstructed gas flow.
Nonetheless, these early reports demonstrate the need
and the feasibility of an implantable long-term artificial
respiratory support. As the use of extracorporeal oxy-
genation became more widespread in the setting of open
heart surgery on mechanical bypass, materials and tech-
niques improved dramatically. Hollow fiber oxygenating
membranes used today demonstrate improved gas-
exchange efficiency and are lower in profile, renewing
enthusiasm in the development of an artificial lung.
Current Designs
Features of a support device that can serve either as a
bridge or alternative to lung transplant in the treatment
of end-stage respiratory failure should include the capac-
ity to satisfy the necessary gas-exchange requirements,
eliminating or treating right heart failure, and to mini-
mize trauma to other organ systems. Furthermore, these
devices should be conceptually simple and reliable so as
to be capable of providing long-term support on an
ambulatory basis. Extracorporeal membrane oxygenation
(ECMO) involves percutaneous or surgical cannulation
of large peripheral or central vessels, circulation with
blood flow powered by servoregulated roller or centrifu-
gal blood pumps, and gas exchange delivered via microp-
orous hollow fiber or solid silicone oxygenators.
Extracorporeal life support (ECLS) has been used exten-
sively in the treatment of acute cardiac or respiratory fail-
ure both in children and adults, primarily successful as a
short-term bridge to recovery.
15
However, the cost and
complexity associated with ECLS using current systems,
in addition to the blood element trauma and inherent
infectious risks, make this modality unsuitable for
prolonged support. Furthermore, the need for continu-
ous monitoring by trained personnel in an intensive care
unit setting makes ambulatory support and physical
rehabilitation prohibitive.
Intravascular oxygenation has been used in several
clinical trials in the setting of acute respiratory failure as
an adjunct to conventional mechanical ventilation.
16–18
These devices consist of a network of hollow fiber oxy-
genating membranes connected by a manifold to the
sweep gas inflow and outflow lines and inserted into the
inferior vena cava percutaneously via the femoral vein.
Unfortunately, the effectiveness of these early devices was
limited largely because they could demonstrate being
capable of transferring only up to one-half of the total
gas-exchange requirements of an adult patient. Newer
technology has resurrected intravascular oxygenation,
with current efforts focusing on improving blood mixing
and thus the creation of secondary flows at the boundary
layer where blood and the gas exchange membranes
interface.
19
The most significant advances include the
incorporation of a mechanical balloon pump and a
system of fixed fiber matting, with in vivo testing
currently underway. Initial trials will again focus on
patients suffering from acute respiratory failure, serving
as a bridge to recovery. Whether this tactic has potential
in the treatment of chronic respiratory failure and is
feasible as a long-term ambulatory treatment remains to
be seen.
Several groups have focused their attention on a
pumpless implantable or wearable oxygenator because of
several theoretical advantages using this approach.
Advances in the efficiency of newer gas exchange mem-
branes have allowed the development of oxygenators
with low blood flow resistance. An example is the proto-
type device manufactured by Michigan Critical Care
Consultants, MC3 (Ann Arbor, MI).
20–26
Using a centrally
positioned inlet, this device takes advantage of radial
blood flow through a series of parallel wound micro-
porous hollow fibers potted at both ends into a manifold
for the sweep gas inlet and outlet (Figure 32-1). The
commercially available hollow fibers (Celgard Inc,
Charlotte, NC) that serve as the gas exchange substrate have
Artificial Lungs
/
399
Artificial Lungs
/
401
cific pathologic situation, such as the relative importance
of chronic right heart failure, deficiencies in oxygenation
or carbon dioxide removal, and infectious concerns.
The effect on right ventricular load under each of these
configurations has been investigated using a theoretical
lumped parameter model, taking into account the differ-
ences in outflow impedance.
28
Although the terms
pulmonary and systemic vascular resistances are most
commonly used to describe afterload, “input impedance”
defines cardiac load in a pulsatile system. Impedance is
the opposition to pulsatile flow and considers the
combined effect of vessel caliber, compliance, and pulse
wave reflections.
29
Pulse wave reflections occur in any
pulsatile system and primarily originate at locations
where there is a significant change in the flow path geom-
etry, such as narrowings or branch points. These pressure
wave reflections directly counter flow and affect imped-
ance. Determining input impedance involves mathemati-
cally reducing the instantaneous pressure and flow
waveforms in the aortic or PA into a mean term and a
series of sine waves using Fourier transformation
(Figure 32-3). These sine waves each have a characteristic
amplitude, or height, relative to the X-axis, a frequency,
and phase angle, or its position relative to the Y-axis. Each
sine wave represents a harmonic; the frequency at each
harmonic is an integer multiple of the fundamental
frequency, or the frequency of the pressure and flow wave-
forms. For example, when the heart rate is 60, a frequency
of 1 Hz, the frequency of the pressure and flow sine waves
at the first harmonic is 1 Hz, the frequency at the second
harmonic is 2 Hz, and so on. Impedance (Z) is the ratio of
the amplitudes of pressure and flow expressed as a func-
tion of harmonic. Although the zero harmonic imped-
ance (Z0) is the ratio of mean pressure to mean flow and
thus is analogous to resistance, impedance at the integer
harmonics represents the opposition to flow pulsations.
First harmonic impedance (Z
1
) is probably the most
important single indicator of pulsatile load because the
majority of flow occurs within the first harmonic.
Impedance is particularly important to consider in the
potential use of an artificial lung perfused by the right
ventricle. Although these devices have resistances that
approximate pulmonary vascular resistance, their geome-
try and compliance are drastically different from the
normal circulation. Therefore, the characteristics of the
device and its mode of attachment will have an enormous
impact on the impedance seen by the right ventricle, as
demonstrated by the theoretical lumped parameter study.
However, certain configurations may offer other advan-
tages despite significant increases in right ventricular
input impedance. Any mode of attachment may be more
appropriate under specific clinical scenarios and should
be considered for all its merits and shortcomings.
When the return of oxygenated blood is directed into
the left atrium, blood flow can be competitive with the
pulmonary circulation (see Figure 32-2A) or exclusively
through the device (see Figure 32-2B). The competitive
flow approach allows blood flow to travel in parallel,with
a fraction of the cardiac output shunted into the artificial
lung, and the remainder continuing through the native
pulmonary circulation. The magnitude of diverted blood
is dependent on the comparative impedance of the native
FIGURE 32-2. Schematics representing potential applications of a
pumpless artificial lung perfused by the right ventricle. A, Partial
respiratory support, with blood flow in parallel with the native
pulmonary circulation. B, Pulmonary replacement, diverting the entire
right sided cardiac output through the artificial lung. C, Total respira-
tory support, with flow in series with the native pulmonary circulation.
Artificial Lungs
/
403
demonstrated the consequences of high impedance in
the context of normal resistance on right ventricular load
and function. Although there was no change in cardiac
output or mean PA pressure, right ventricular ejection
flow patterns were severely altered, with persistent dias-
tolic flow and reduced peak and sustained systolic flow
(Figure 32-4). Whether these abnormalities will progress
to right heart failure and the effect on left ventricular
performance remain unclear but are under current active
investigation. We have also developed a prototype
compliance chamber, applying variable pneumatic
compression to the compliance reservoir (Figure 32-5),
which appears to reduce impedance and restore normal
cardiac function (see Figure 32-4).
31
The next potential mode of attachment involves creat-
ing a support circulation in series with the native lungs
(see Figure 32-2C). By creating inflow and outflow
conduit anastamoses to the proximal and distal main PA,
respectively, a snare placed around the intervening seg-
ment of PA can divert the entire right ventricular cardiac
output through the artificial lung for gas exchange. This
approach is capable of supporting all of the oxygenation
requirements of large animals, as demonstrated in acute
studies by clamping the endotracheal tube of anes-
thetized sheep,
22
and in chronic experiments using a
smoke inhalational model of respiratory failure.
24,25
The
PA-to-PA configuration has several inherent advantages,
other than its ability to provide total respiratory support.
First, directing the outflow of the artificial lung into the
distal PA, the diseased lungs can serve as an embolic trap
to prevent the inevitable microthrombi formed within
the extensive foreign surface from ejecting into the
systemic circulation. In addition, preserving native
pulmonary blood flow retains the metabolic and
endocrine lung functions. Lastly, delivering oxygenated
blood to chronically diseased lungs may allow some
recovery or slow the deterioration. Unfortunately, there
are several drawbacks to the PA-to-PA approach. Despite
its low resistance, application of the artificial lung in
series necessarily increases the total resistive load against
the right ventricle. As predicted by the theoretical
lumped parameter model, this application of a pumpless
artificial lung perfused by the right ventricle generates
the greatest magnitude of right heart strain.
28
Although
the incidence of right ventricular failure in large animal
series has been reduced with newer generations of
devices,
25
the in-series application will likely be prohibi-
tive in patients with respiratory failure associated with
cor pulmonale. Furthermore, anatomic considerations
may limit feasibility. The unusual length of the main PA
of sheep allows room for two large caliber anastamoses
with a flow occluder.
32
It is unclear if human anatomy
will be amenable to a similar construct.
Hematologic Compatibility
Flow through extracorporeal systems is associated with
problems involving various hematological components
and can be generalized into three major impediments:
(1) red cell hemolysis, (2) platelet activation and
consumption, and (3) activation of the coagulation
Pulmonary artery flow: native circulation
-5
0
5
10
15
20
25
30
L/min
Pulmonary artery flow: pulmonary replacement
-5
0
5
10
15
20
25
30
L/min
Pulmonary artery flow: pulmonary replacement with compliant device
-5
0
5
10
15
20
25
30
L/min
FIGURE 32-4. Pulmonary artery flow versus time. A, Normal circula-
tion, with rapid systolic upstroke and absent diastolic flow. B,
Pulmonary replacement with noncompliant artificial lung demonstrat-
ing arrested systolic upstroke with persistent diastolic flow. C,
Pulmonary replacement with artificial lung in series with a prototype
compliance chamber.
Durability
Membrane oxygenation using microporous hollow fibers
is associated with plasma leakage through the individual
micropores and eventual oxygenator failure. Mech-
anistically, plasma leakage results from the progressive
deposition and adsorption of phospholipids onto the
surface of the individual fibers.
38
These hydrophobic
lipids reduce the surface tension directly at the blood–gas
interface, allowing leakage to occur. The rate of progres-
sion towards device failure appears to be related to the
content and character of circulating bloodstream lipids.
Future prototypes will incorporate either solid silicone
fibers or microporous fibers coated with a nonporous
but gas-diffusible material, and oxygenators using such
nonporous membranes as the gas exchange substrate are
currently under study.
39
Clinical Trial Design
As with any clinical trial, success is largely dependent
upon patient selection. As the technology improves and
the potential for clinical utilization rapidly approaches,
planning the most appropriate clinical trial becomes
imperative. This includes not only patient selection, but
also trial size, the planned duration of support, and iden-
tifying achievable and meaningful outcomes. Several
bioengineering laboratories recently surveyed the nation’s
largest lung transplant centers to provide feedback in trial
design, in anticipation of future clinical application.
40
Not
surprisingly, 97% of all responding programs, including
70% of the high-volume centers, supported and would
actively participate in a clinical trial using an artificial
lung as a bridge to lung transplant. This degree of wide-
spread support is largely a result of the rising mortality on
the transplant wait list, the lack of alternatives for decom-
pensating patients, and the recently demonstrated success
of ventricular support as a bridge to cardiac transplant.
However, in order for the artificial lung to demonstrate
capability as a bridge to transplant, patients must receive
some degree of priority on the transplant wait list, as was
done during the initial trials of ventricular assist devices.
Current standing United Network for Organ Sharing
(UNOS) policy regarding the allocation of cadaveric lung
graft allografts relies entirely upon waiting time among
candidates of similar size and ABO blood type. It is prob-
ably unlikely to envision successful initial artificial lung
trials if expected to provide event-free support for as long
as 1 year. It is encouraging to the developers of artificial
lungs that more than one-half of lung transplant program
directors supported restructuring of the UNOS allocation
policy to allow priority allocation to patients enrolled in
an artificial lung trial. These changes must be enacted
before any clinical trial can be initiated.
In terms of patient selection, ideal candidates are
likely individuals currently awaiting transplant but wors-
ening to the point where they are unlikely to survive until
a suitable organ will become available. Patients with idio-
pathic pulmonary fibrosis and primary pulmonary
hypertension have the highest mortality on the wait list
and are typically younger and with fewer associated
comorbidities. The benefit of artificial lung support
would likely outweigh the risk of surgical implantation.
Cystic fibrosis patients also have high wait list mortality;
however, the risk of infectious complications leaves most
centers reluctant to include this group, at least in initial
trials. Patients with emphysema, although the most
frequent indication for transplant, are probably not ideal,
as it would be difficult to clearly demonstrate a survival
benefit, given their typically protracted and unpre-
dictable course.
Summary
Significant progress has been achieved in the develop-
ment of artificial lungs, capable of providing full or
partial gas-exchange support, for prolonged periods of
time, and on an ambulatory basis. These devices may be
capable of serving as a bridge to lung transplant for
patients with end-stage respiratory failure, a problem
that currently has no alternative remedy. With several
impending modifications and long-term animal testing,
along with support from the lung transplant community,
this technology will soon be available to satisfy a much-
needed solution to a difficult clinical problem.
References
1. American Lung Association. Available at: http://www.
lungusa.org/data/ (accessed June 1, 2002).
2. Gross TJ, Hunninghake GW. Medical progress: idiopathic
pulmonary fibrosis. N Engl J Med 2001;345:517–25.
3. Costabel U, Hunninghake GW. ATS/ERS/WASOG state-
ment on sarcoidosis. Eur Respir J 1999;14:735–7.
4. Cystic Fibrosis Foundation. Available at:
(accessed June 1, 2002).
5. Davis PB. Cystic fibrosis. Pediatr Rev 2001;22:257–64.
6. Bossone E, Paciocco G, Iarussi D, et al. The prognostic role
of the ECG in primary pulmonary hypertension. Clin
Chest Med 2002;121:513–8.
7. United Network for Organ Sharing. Available at:
(accessed June 1, 2002).
8. Maurer JR, Frost AE, Estenne M, et al. International guide-
lines for the selection of lung transplant candidates. J Heart
Lung Transplant 1998;17:703–9.
Artificial Lungs
/
405
9. Goldstein DJ, Oz MC, Rose EA. Medical progress-
implantable left ventricular assist devices. N Engl J Med
1998;339:1522–33.
10. Bank AJ, Mir SH, Nguyen DQ, et al. Effects of left ventricu-
lar assist devices on outcomes in patients undergoing heart
transplantation. Ann Thorac Surg 2000;69:1369–75.
11. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term
mechanical left ventricular assistance for end-stage heart
failure. N Engl J Med 2001;345:1435–43.
12. Mussivand T, Portner P, Jacobs D, et al. The INTRePID
trial-can mechanical circulatory support reduce mortality
and improve quality of life for end-stage heart failure
patients? ASAIO J 2002;48:151.
13. Bodell BR, Head JM, Head LR, Formolo AJ. An implantable
artificial lung: initial experiments in animals. JAMA
1965;191:301–3.
14. Shah-Mirany J, Head LR, Ghetzler R, et al. An implantable
artificial lung. Ann Thorac Surg 1972;13:381–7.
15. Bartlett RH, Roloff DW, Custer JR, et al. Extracoporeal life
support: the University of Michigan experience. JAMA
2000;283:904–8.
16. Mortensen JD, Berry G. Conceptual and design features of
a practical, clinically effective intravenous mechanical
blood oxygen/carbon dioxide exchange device (Ivox). Int J
Artif Organs 1989;12:384–9.
17. Kallis P, Al-Saady NM, Bennett ED, Treasure T. Early results
of intravascular oxygenation. Eur J Card Thorac Surg
1993;7:206–10.
18. Jurmann MJ, Demertzis S, Schaefers HJ, et al. Intravascular
oxygenation for advanced respiratory failure. ASAIO J
1992;38:120–4.
19. Federspiel WJ, Hewitt T, Hout MS, et al. Recent progress in
engineering the Pittsburgh Intravenous Membrane
Oxygenator. ASAIO J 1996;42:M435–42.
20. Fazzalari FL, Montoya JP, Bonnell MR, et al. The develop-
ment of an implantable artificial lung. ASAIO J
1994;40:M728–31.
21. Fazzalari FL, Bartlett RH, Bonnell MR, Montoya JP. An
intrapleural lung prosthesis: rationale, design, and testing.
Art Organs 1994;18:801–5.
22. Lynch WR, Montoya JP, Brant DO, et al. Hemodynamic
effect of a low-resistance artificial lung in series with the
native lungs of sheep. Ann Thorac Surg 2000;60:351–6.
23. Haft HW, Montoya P, Alnajjar O, et al. An artificial lung
reduces pulmonary impedance and improves right ventric-
ular efficiency in pulmonary hypertension. J Thorac
Cardiovasc Surg 2001;122:1094–100.
24. Lick SD, Zwischenberger JB, Alpard SK, et al. Development
of an ambulatory artificial lung in an ovine survival model.
ASAIO J 2001;47:486–91.
25. Lick SD, Zwischenberger JB, Wang D, et al. Improved right
heart function with a compliant inflow artificial lung in
series with the pulmonary circulation. Ann Thorac Surg
2001;72:899–904.
26. Lynch WR, Haft JW, Montoya JP, et al. Partial respiratory
support with an artificial lung perfused by the right ventri-
cle: chronic studies in an active animal model. ASAIO J
2000;46:202.
27. Conrad SA, Zwischenberger JB, Grier LR, et al. Total extra-
corporeal arteriovenous carbon dioxide removal in acute
respiratory failure: a phase I clinical study. Intensive Care
Med 2001;27:1340–51.
28. Boschetti F, Perlman CE, Cook KE, Mockros LF.
Hemodynamic effects of attachment modes and device
design of a thoracic artificial lung. ASAIO J 2000;46:42–8.
29. Milnor WR. Pulsatile blood flow. N Engl J Med
1972;287:27–34.
30. Cook KE, Makarewicz AJ, Backer CL, et al. Testing of an
intrathoracic artificial lung in a pig model. ASAIO J
1996;42:M604–9.
31. Haft JW, Bull JL, Rose R, et al. Design of an artificial lung
compliance chamber for pulmonary replacement. ASAIO J
2003;49:35–40.
32. Harper DD, Alpard SK, Deyo DJ, et al. Anatomic study of
the pulmonary artery as a conduit for an artificial lung.
ASAIO J 2000;46:184.
33. Hennessy VL, Hicks RE, Nierwiarowski S, et al. Function of
human platelets during extracorporeal circulation. Am J
Physiol 1977;232:H622–8.
34. Annich GM, Meinhardt JP, Mowery KA, et al. Reduced
platelet activation and thrombosis in extracorporeal
circuits coated with nitric oxide release polymers. Crit Care
Med 2000;28:915–20.
35. Miskulin J, Annich G, Gillian C, et al. NO flux determines
thromboresistance in NO-releasing extracorporeal circuits.
ASAIO J 2002;48:145.
36. Gorman RC, Ziats NP, Rao AK, et al. Surface-bound
heparin fails to reduce thrombin formation during clinical
cardiopulmonary bypass. J Thorac Cardiovasc Surg
1996;111:1–12.
37. Gartner MJ, Wilhelm CR, Gage KL, et al. Modeling flow
effects on thrombotic deposition in a membrane oxygena-
tor. Artif Organs 2000;24:29–36.
38. Montoya JP, Shanley CJ, Merz SJ, Bartlett RH. Plasma leak-
age through microporous membranes. ASAIO J
1992;38:M399–405.
39. Funakubo A, Higami T, Sakuma I, et al. Development of a
membrane oxygenator for ECMO using a novel fine sili-
cone hollow fiber. ASAIO J 1996;42:M837–40.
40. Haft JW, Griffith BP, Hirschl RB, Bartlett RH. Results of an
artificial-lung survey to lung transplant program directors.
J Heart Lung Transplant 2002;21:467–3.
406
/ Advanced Therapy in Thoracic Surgery
407
CHAPTER
33
SURGERY FOR MYASTHENIA
GRAVIS
RICHARD F. H
EITMILLER, MD
EUGENIE S. HEITMILLER
, MD
Clinical evidence continues to support the role of surgery
in the management of patients with myasthenia gravis.
The goal of surgery is complete thymectomy along with
resection of associated thymoma, if present. Successful
management of patients with myasthenia gravis depends
on multidisciplinary care involving neurology, surgery,
anesthesia, and critical care medicine. Diagnosis, preop-
erative preparation, intraoperative management, and
postoperative care are covered in this chapter. Since the
first edition of this text, staging criteria have been added,
new surgical techniques included, and surgical and anes-
thesia results updated. Predictors of treatment outcome
are also discussed.
Myasthenia gravis is a disorder characterized by
neuromuscular weakness and fatigue of voluntary
muscles. It is generally believed to be an autoimmune
disease that targets the postsynaptic acetylcholine recep-
tor, resulting in interference of signal transmission at the
neuromuscular junction. Supporting this theory is the
fact that circulating acetylcholine receptor antibodies are
identified in over 90% of patients with myasthenia
gravis. On the other hand, antibody levels do not change
or correlate with clinical response to therapy. Therefore,
the pathophysiology of this disease remains incompletely
worked out. Muscles supplied by the cranial nerves are
preferentially affected. The disease is twice as common in
women as in men, and the clinical course is variable. The
relationship of thymic abnormalities to myasthenia
gravis has long been appreciated and far antedated
successful thymectomy for this disease. Early surgical
management of patients with myasthenia involved
attempts at thymic gland devascularization or excision,
invariably without clinical improvement, and at the price
of exceedingly high mortality. Acceptance of thymectomy
for patients with myasthenia gravis is attributed to
Blalock and colleagues, who in 1939 reported successful
thymectomy in a 19-year-old woman with myasthenia
gravis and a 6 ϫ 5 ϫ 3cm benign thymoma.
1
Over half a
century later, although the benefits of thymectomy
remain undisputed, the indications for thymectomy and
the specific surgical approach that should be used remain
unresolved.
Diagnosis and Staging
The diagnostic tests available to establish the diagnosis of
myasthenia gravis are well established and are only
briefly covered in this chapter. Diagnostic tests include
(1) administration of anticholinesterase agents (eg,
Te nsilon test), (2) electrophysiology studies, (3) detection
of serum anticholine receptor antibodies, and (4) clinical
examination. Computed tomography of the chest is
recommended to detect occult thymic neoplasms. Once
the diagnosis is established, the disease is staged. Staging
classifies the severity of the disease and determines treat-
ment options. The most commonly used staging system
is that advocated by Osserman, or a variant of it, which
stages patients based on whether symptoms are ocular or
general, and on their severity (Table 33-1).
2
TABLE 33-1. Staging of Myasthenia Gravis
Grade Characteristic
I Ocular symptoms only
II Mild to moderate generalized symptoms only
III Severe generalized disease
IV Myasthenia crisis
Treatment: The Case for Thymectomy
The importance of thymectomy in the management of
patients with myasthenia gravis continues to be
supported by clinical results, and is no longer the contro-
versial issue it was in the past. It is still essential to under-
stand the evidence supporting the use of surgery in the
management of myasthenia patients.
There are no prospective treatment trials for patients
with myasthenia gravis from which to define practice
guidelines. The reasons for this are the variations in clini-
cal presentation of myasthenic patients with regard to
age, sex, muscle group involvement, degree of weakness,
and antireceptor antibodies. These variations have led
Drachman to question whether myasthenia gravis, in
fact, represents a homogeneous entity.
3
As a result, treat-
ment practice varies widely. Treatment options include
anticholinesterase agents, steroids, plasmapheresis,
immunosuppressive agents, and thymectomy. Only surgi-
cal therapy is covered in this chapter.
The evidence in favor of the safety and effectiveness of
thymectomy for myasthenia gravis is now extensive.
Operative mortality ranges from 0 to 2.7%,
4–10
with clini-
cal improvement in 62 to 100% of patients.
4,6–10
Remission,
defined as being symptom-free and off medication, is
achieved in 8 to 69% of patients.
4,6–10
The favorable surgi-
cal results appear to be a durable response. Crucitti and
coworkers reported a postsurgical 10-year survival rate of
78%.
11
Buckingham and colleagues used a computer-
assisted match in lieu of prospective randomized data to
compare medical and surgical therapy for myasthenia
gravis.
12
They found that overall improvement rate and 5-
and 10-year survival rates were all significantly better with
surgical therapy than with medical therapy alone
(Figure 33-1). Further, they showed that the surgical
advantages were not age-dependent.
The degree of acceptance of surgical therapy is
reflected in a report by Lanska,
13
who surveyed a group of
board-certified neurologists with an interest in myasthe-
nia to evaluate their referral practice with regard to
thymectomy. He found that 8% advocated the procedure
in less than one-third of their patients, 57% advocated it
for one-third to two-thirds of patients, and 35% advo-
cated it for more than two-thirds of their patients.
Among these physicians, there was general agreement
that thymectomy was indicated for (1) patients with
thymoma, (2) generalized disease unresponsive to
medical management, and (3) a small subset of patients
with ocular symptoms who fail nonoperative manage-
ment. The timing of surgery, preoperative preparation,
and recommended surgical technique are controversial,
but most accept the indications listed above. Further, the
trend in therapy is toward earlier surgical intervention.
Anatomy of the Thymus Gland
The mechanism by which the thymus gland affects symp-
toms in patients with myasthenia gravis is not known.
However, the principle of complete thymectomy in the
surgical management of myasthenic patients is widely
accepted. Therefore, an understanding of thymic
anatomy is essential to successful, complete thymectomy.
The gross anatomy of the thymus gland is well known
to thoracic surgeons as an H-shaped, gray-pink, lobu-
lated gland in the anterior superior mediastinum with a
variable arterial blood supply from branches of the inter-
nal mammary vessels and venous drainage through
larger, recognizable veins into the innominate vein. One
of the most common anatomic variations is for one of
the superior thymic limbs to pass posterior to the
innominate vein. Remarkably, Jaretzki and Wolff have
shown that thymic tissue is confined to the thymic
capsule in only 2% of patients.
14
As a result of a complex
embryologic migratory pattern, thymic tissue has been
identified widely throughout the neck and mediastinum.
Masaoka and colleagues reported ectopic thymic tissue in
“normal” anterior mediastinal fat.
15
Jaretzki and Wolff
found ectopic thymic tissue in the cervical and mediasti-
nal region in 32 and 98% of patients, respectively.
14
They
reported detailed “mapping” of sites where extracapsular
thymic tissue had been found, and on the basis of these
findings, they advocated a combined cervical and
transsternal approach to thymectomy, which they termed
maximal thymectomy. Fukai and colleagues identified
ectopic thymic tissue in the anterior mediastinal fat,
retrocarinal fat, and preaortic fat in 44%, 7.4%, and 0%
of patients, respectively.
16
Most recently, in a review of the
clinical significance of ectopic thymus, Ashour found
ectopic thymic tissue in 39.5% of patients.
17
In contrast
408
/ Advanced Therapy in Thoracic Surgery
FIGURE 33-1. Comparison of survival between myasthenic patients
treated surgically (thymectomy) and those treated medically. From
Buckingham JM et al.
12
to Jaretzki’s findings, the ectopic thymus was found pref-
erentially in the neck (63.2%). Proponents of complete
thymectomy have drawn on this data in support of their
respective operative techniques.
Preoperative Preparation
Although disagreement exists regarding the specifics of
preoperative preparation, there is general agreement that
a planned, systematic approach to stabilize patients
neurologically prior to surgery is important and yields
the best results. Wechsler has long been one of the
strongest advocates of a prospective management plan
for myasthenia patients.
18
The planned approach that he
advocates, which was developed at Duke University, uses
thymectomy as the sole therapy whenever possible.
Medications are used only if needed and not as a matter
of routine.
Plasmapheresis is used to stabilize the more acutely ill
patient with respiratory compromise. Goti and
colleagues showed that plasmaphersis improved forced
expiratory volume in 1 second (FEV
1
) and mean expira-
tory force and reduced functional residual capacity
(FRC), while pyridostigmine did not have these effects.
19
Plasmapheresis (4 to 8 exchanges) leads to remission of
myasthenia gravis in 45% of cases and lasts 1 to 2 weeks.
20
Plasmapheresis has been recommended for myasthenic
patients with vital capacity < 2 L.
21
The use of plasma-
pheresis has been shown to reduce postoperative
mechanical ventialtion and intensive care unit stay.
22
Preoperative infections, even those that may normally
seem clinically insignificant, are treated to resolution
prior to thymectomy. This is done for the theoretic
concern that a localized infection may affect systemic
immune function, which could therefore affect myas-
thenic symptoms and therapeutic outcome.
Several studies have examined preoperative risk
factors for postoperative respiratory failure. Leventhal
and colleagues proposed a scoring system to predict the
anesthetic risk in patients with myasthenia, assigning
points based on duration of disease, dose of pyridostig-
mine, presence of respiratory disease, and preoperative
vital capacity < 2.9 L.
23
However, subsequent studies by
other investigators attempting to validate this scoring
system found it to be of only limited value in patients
undergoing thymectomy via sternotomy.
24,25
The current
experience with myasthenics indicates that the risk
factors associated with the need for mechanical ventila-
tion after surgery are the severity of disease (ie,
Ostermann stage 3 or 4), borderline preoperative respira-
tory function, and the transsternal approach for thymec-
tomy.
26
Anesthetic Management
Premedication
Premedication is usually avoided. If the patient is
anxious, a small dose of a benzodiazepine, such as mida-
zolam or diazepam, is given. Controversy exists regarding
the administration of anticholinesterase on the morning
of surgery. The rationale for decreasing or withholding
the anticholinesterase is to prevent overdosing, since the
anticholinesterase requirement is usually decreased after
surgery. However, if the dose is withheld, the patient may
be weak on arrival to the operating room. The practice at
our institution and others is to have the patients take
their usual doses of anticholinesterase up to the time of
surgery.
20
Patients receiving chronic steroid therapy
usually receive additional coverage of hydrocortisone,
100 mg intravenously, prior to anesthetic induction and
then every 8 hours for an additional three doses.
Intraoperative Management
Standard basic monitoring (noninvasive blood pressure,
electrocardiographic monitoring, pulse oximetry, end-
tidal carbon dioxide, temperature, neuromuscular block-
ade, and oxygen concentration) is used for all patients
undergoing thymectomy. A precordial stethoscope used
during induction may need to be exchanged for an
esophageal stethoscope so as not to interfere with the
surgical field. An arterial catheter for hemodynamic and
arterial blood gas monitoring is placed in patients under-
going transsternal thymectomy or in patients who have
significant cardiovascular or respiratory disease. A single-
lumen endotracheal tube is appropriate unless a thoraco-
scopic approach is used, in which case a double-lumen
endotracheal tube may be required.
Anesthesia is usually induced using an intravenous
agent (such as thiopental, propofol, or etomidate) in
combination with an inhalational agent. Some anesthesi-
ologists avoid muscle relaxants, because the patient’s
baseline muscle weakness and the muscle-relaxing effect
of the volatile anesthetic are usually adequate for tracheal
intubation and anesthetic maintenance. However, other
anesthesiologists prefer to use a balanced technique with
carefully titrated muscle relaxants.
26
Neuromuscular
monitoring should be performed using the obicularis
oculi muscle, based on a study by Itoh and colleagues
that showed the obicularis oculi muscle to be more sensi-
tive than the adductor pollicis to neuromuscular block-
ing agents in myasthenic patients.
27
Patients receiving anticholinesterase drugs may have
an abnormal response to the depolarizing muscle relax-
ant, succinylcholine. A prolonged block can occur
because anticholinesterase therapy inhibits the activity of
true cholinesterase as well as plasma cholinesterase,
Surgery for Myasthenia Gravis
/
409
which is responsible for succinylcholine hydrolysis.
Resistance to succinylcholine has also been reported and
is most likely due to the decreased number of acetyl-
choline receptors in patients with active disease.
28
This
response to succinylcholine is usually normal in patients
who are in remission.
29
Patients who undergo plasma-
pheresis have reduced cholinesterase and will have a
delay in metabolism of succinylcholine as well as other
drugs metabolized by plasma cholinesterase such as
mivacurium and remifentanil.
20
Patients with active myasthenia gravis are usually
sensitive to the effects of nondepolarizing muscle relax-
ants (eg, vecuronium, rocuronium, and atracurium).
There may be both an increased response and a pro-
longed effect. Atracurium has been a recommended
nondepolarizer because of its short elimination half-life
and rapid breakdown independent of plasma
cholinesterase.
30
A study by Mann and colleagues showed
that patients with a preanesthetic fading after train-of-
four stimulation had a significantly decreased median
effective dose for atracurium while myasthenic patients
without fading had an median effective dose similar to
that of nonmyasthenic patients (0.24 mg/kg).
31
Vecuronium has also been successfully used in myas-
thenic patients. Incremental vecuronium doses of
0.005 mg/kg (one-tenth the normal dose), titrated to
effect with careful neuromuscular blocker monitoring is
the recommendation for these patients.
32
Neuromuscular blockade is reversed with neostigmine
or edrophonium (Tensilon). Using these anti-
cholinesterases for reversal has the theoretic potential of
producing a cholinergic crisis in a patient receiving anti-
cholinesterase therapy, but this is not commonly seen in
clinical practice. Caution is used in administering drugs
that have neuromuscular blocking properties as a side
effect. These include the aminoglycoside antibiotics,
calcium channel blockers, and antiarrhythmics such as
quinidine and procainamide.
An anticholinesterase infusion, usually neostigmine,
may be used during the perioperative period. The
patient’s daily dose of pyridostigmine is divided by 60,
and that amount of neostigmine is infused over 24 hours
(usually achieved by adding the dose of neostigmine to
1 L of Ringer’s lactate or normal saline, which is then
infused at 42 cc/h). When the patient is able to take oral
medications, the infusion is discontinued, and an oral
dose of pyridostigmine is restarted.
Postoperative Pain Relief
Thoracic epidural anesthesia in combination with light
general anesthesia has been reported to give excellent
postoperative pain relief after transsternal thymectomy.
33
In a study by Kirsch and colleagues, lumbar epidural
morphine administered preoperatively provided superior
postoperative pain relief and better respiratory mechan-
ics when compared with intravenous narcotics in
patients undergoing transsternal thymectomy.
34
No
difference was found between the groups for the duration
of postoperative intubation or ventilation.
Intrathecal (spinal) opioids are also frequently used
for postoperative pain relief. Intrathecal morphine
administered before incision reduces the amount of
parenteral narcotics needed for pain relief after surgery.
Patients who are not candidates for either intrathecal or
epidural analgesia are given reduced doses of parenteral
narcotics for postoperative pain relief.
Surgical Technique
Once a patient is stabilized, surgery may be considered.
The goals of surgery are to completely remove the
thymus gland and, if present, an associated thymoma.
Numerous incisional strategies have been developed to
accomplish this objective. Each has its advocates. Surgical
approaches include combined cervical exploration and
median sternotomy (maximal thymectomy), median
sternotomy (transsternal approach), partial sternotomy,
transcervical, infrasternal mediastinoscopy, and thora-
coscopy. To date, the evidence suggests that, as long as the
principle of complete thymectomy (with thymoma if
present) is adhered to, comparable results can be
obtained with the different approaches.
Combined Cervical Exploration and Transsternal
Thymectomy
The technique of combined cervical exploration and
median sternotomy (“maximal thymectomy”) is a fusion
of the principle of complete thymectomy with the
discovery that there is a high incidence of cervicomedi-
astinal ectopic thymic tissue. This incisional strategy is
designed to optimize cervicomediastinal exposure to
permit identification and removal of all thymic tissue.
The technique is credited to Jaretzki and colleagues.
4
General single-lumen endotracheal anesthesia is used
with the patient positioned supine. A separate generous
collar cervical incision and median sternotomy are
employed (Figure 33-2). The two incisions may be joined
as a “T,” for additional exposure for thymomas or for
reoperations. The cervical dissection extends from the
innominate vein inferiorly to the thyroid isthmus superi-
orly; the recurrent laryngeal nerve marks the lateral
borders. Jaretzki states that this incision provides expo-
sure superior to that provided with a cervical incision,
while avoiding the impact of a full sternotomy.
Contraindications include extensive thymic tumors,
especially those involving the lower mediastinum and
410
/ Advanced Therapy in Thoracic Surgery
the thymus to the inferior aspect of the thyroid gland are
identified. These are then ligated and divided. This inci-
sion does not need to be modified for thymoma. Again,
more aggressive resection may be required if local inva-
sion by thymoma is encountered.
Transcervical Thymectomy
Historically, there has been concern about the safety of
transsternal thymectomy in patients with myasthenia
gravis because of incisional pain and associated respira-
tory compromise, possible phrenic nerve injury, and
mediastinitis. In order to circumvent these possible
complications, Crile revived the technique, which ante-
dated open thoracic surgery, of transcervical thymec-
tomy.
35
Others, including Kark and Kirschner,
36
Cooper
and colleagues,
10
Ferguson,
37
and Deeb and colleagues,
38
also deserve credit for their contributions to the descrip-
tion of this technique.
Patients are positioned supine with the arms tucked at
the side and the neck extended. General endotracheal
anesthesia is used, and a collar cervical incision
employed. The superior thymic poles are identified and
dissected free. Dissection of the thymus continues inferi-
orly using upward traction on the superior poles
(Figure 33-4). A right-angled retractor is used to lift the
sternum anteriorly, enhancing exposure of the anterior
mediastinum. A fiberoptic headlight also helps. The
thymic veins are identified during the dissection and
clipped or ligated. Some find it easier to remove the ante-
rior mediastinal fat along with the thymus, others
remove the fatty tissue separately. The phrenic nerves are
protected and preserved. If additional exposure is
needed, a partial or complete sternotomy is added.
Most consider the presence of thymoma to be a
contraindication to using this approach. However, Deeb
and colleagues,
38
on the basis of their clinical experience,
concluded that the indications for intranscervical
thymectomy can safely be extended to include patients
with thymoma.
Infrasternal Mediastinoscopic Thymectomy
A recent report by Uchiyama and colleagues describing a
novel use of mediastinoscopy to accomplish thymectomy
underscores the variety of methods available to the
surgeon managing patients with myasthenia gravis.
39
The
procedure is performed under general anesthesia. Patients
are positioned supine with the neck extended as with
standard mediastinoscopy. For the first 18 patients, a
midline cervical incision was employed, the anterior
cervical muscles divided in the midline and the thymus
identified in the anterior mediastinal space. Using upward
traction, the thymus was dissected out and removed. For
the last five patients, the thymus was removed using a
subxiphoid, or infrasternal, mediastinoscopic thymec-
tomy. The sternum is retracted upward, video viewing
and fiberoptic lighting assist. The authors state that expo-
sure is adequate for control of thymic vessels and removal
of thymus and anterior mediastinal fat. Following dissec-
tion, a mediastinal drain is left in place.
The authors report one phrenic nerve injury and
successful thymectomy in 21 of 23 (91%) patients. The
remaining two patients were converted to open ster-
notomy. There were no deaths, and all patients showed
clinical improvement in their myasthenia gravis symp-
toms.
Partial Sternotomy with or without Cervical Incision
Cervicomediastinal exposure can be attained using a
partial sternotomy with or without a cervical incision.
This approach facilitates exposure of the anterior-
superior mediastinum without subjecting the patient to a
complete sternotomy. Patients are positioned as for a
standard sternotomy. A vertical midline incision is
employed down to the level of the second or third inter-
costal space. The upper sternum is split with a Lebsche
knife or oscillating saw, and the divided sternum spread
with a pediatric thoracotomy retractor to expose the
upper mediastinum (Figure 33-5). If needed, a collar
incision is added, resulting in a T-shaped incision. This
approach gives excellent exposure and avoids a full ster-
notomy; however, it is cosmetically unappealing.
LoCicero describes a modified approach to partial
sternotomy that has much more cosmetic appeal.
40
Contraindications to this approach include extensive
412
/ Advanced Therapy in Thoracic Surgery
FIGURE 33-4. Surgeon’s view of the operative field during transcervi-
cal thymectomy. The superior thymic lobes have been mobilized. From
Kark AE and Kirschner PA.
36
deaths, and complication rates were low (0 to 21%). At
least some improvement in clinical staging and decrease
in medication requirement was noted in 78 to 96% of
patients. Remission, defined as being asymptomatic and
off medication, occurred in 18 to 69% of patients.
Crucitti and colleagues reported a dramatic decrease
in operative mortality associated with thymectomy
during the study period 1969 to 1989.
11
This finding and
the recent low reported morbidity and mortality with
thymectomy are a tribute to a multidisciplinary approach
to this disease and advancements in care on all specialty
fronts.
Whereas the safety and effectiveness of thymectomy
for myasthenia are well established, the recommended
surgical approach remains controversial. There is general
agreement that successful surgical management of pa-
tients with myasthenia gravis requires complete thymec-
tomy.There is disagreement as to what surgical approach
is needed to achieve this goal. On one end of the spec-
trum, Jaretzki and coworkers, on the basis of studies
showing the incidence and wide cervicomediastinal
distribution of ectopic thymus and comparing postsurgi-
cal results by surgical technique (Figure 33-6) believe that
complete thymectomy can be accomplished only by a
combined cervical and median sternotomy incision
(maximal thymectomy).
4
At the other end of the surgical
spectrum are those who, citing the potential complica-
tions of median sternotomy in patients with myasthenia
who are often on immunosuppressive medications,
believe that complete thymectomy can be performed,
equally effectively and more safely, using transcervical or
videothoracoscopic approaches. The lack of prospective
controlled trials, the variation in clinical stage, and the
wide range of treatment plans used have prevented reso-
lution of the controversy regarding surgical approach. As
Table 33-2 clearly shows, there are excellent results
reported regardless of the surgical technique used. The
favorable effect of thymectomy on patients with general-
ized symptoms of myasthenia gravis has led some to
recommend surgery for patients with stage I (ocular
symptoms) disease. Roberts and colleagues have demon-
strated that ocular myathenia symptoms were cured or
improved in 70% if no thymoma was present and 67% in
patients with assciated thymoma.
47
Clinical improvement following surgery appears to be
durable. Jaretzki and colleagues showed postoperative
remission rates of 81% at 89 months of follow-up for
nonthymoma patients.
4
Frist and coworkers reported a 5-
year postsurgical survival of 100%,
9
and Crucitti and
colleagues reported a 10-year survival of 78% with a
recurrence rate of 3%.
11
There is disagreement as to which clinical factors are
predictive of favorable outcome to thymectomy. Jaretzki
and colleagues have shown that more severe preoperative
symptoms, thymoma, and the need for reoperative
thymectomy were factors predictive of a less favorable
outcome to surgical therapy.
4
On the other hand, multi-
variate analysis failed to show predictive effect of age, sex,
duration of symptoms, use of steriods, preoperative
plasmapheresis, thymic pathology (aside from thymoma),
or anticholinesterase antibody levels. Ashour found that
the presence or absence of ectopic thymic tissue was
414
/ Advanced Therapy in Thoracic Surgery
TABLE 33-2. Surgery for Myasthenia Gravis: Results
Author Number of Patients Approach Death Morbidity (%) Improvement (%) Remission (%)
Jaretzki et al
4
45 CX + MS 0 7.4 96, 87.5, 86* 46, 12.5, 13†
Frist et al
9
46 MS 0 ns 87 28
Cooper et al
10
65 CX 0 3.1 95 52
Nussbaum et al
6
48 MS 0 21 94 42
Detterbeck et al
8
100 MS 0 ns 78 69, 29†
Olanow et al
7
47 MS 0 0 83 61
DeFilippi et al
45
53 CX 0 ns 90 51
Mack et al
43
33 VATS 0 ns 88 18
Meyers and Cooper
46
100 CX 0 8 63 35
CX = cervical; MS = median sternotomy; ns = not significant; VATS = video-assisted thoracoscopy.
*Improvement and remission rates for thymectomy without thymoma, reoperation, and with thymoma, respectively.
†Remission rates are shown for patients with mild and severe disease, respectively.
FIGURE 33-6. Comparison of remission rates after thymectomy
according to surgical technique. Thymoma patients are excluded. From
Jaretzki A et al.
4
predictive of postoperative outcome.
17
He reported
complete, postthymectomy remission rates for patients
with and without ectopic thymus to be 13.3% and 47.8%,
respectively. This difference was highly significant. Frist
and colleaguesreviewed their experience with thymectomy
in 46 patients specifically to determine predictors of
outcome and found three predictive factors: (1) age less
than 45 years, (2) female sex, and (3) preoperative stage.
9
More recently, Budde and colleagues identified gender,
patient age, and presence of thymoma as factors which
affect postthymectomy outcome in myasthenia patients.
48
Wo men fared better than men, younger patients did better
than older patients, and absence of thymoma was more
favorable than presence of thymoma. Abt and colleagues
analyzed their 20-year experience with thymectomy for
myasthenia gravis in older patients.
49
In their study, older
patients were defined as greater than or equal to 55 years
of age. They concluded that older patients had a similar
response-rate and need for postoperative medications as
younger patients, but with higher short-term morbidity.
On the other hand Sanders and colleagues challenged
those conclusions and detailed standards for future
myathenia gravis treatment outcome studies to follow.
50
There are not many data concerning reoperative or
completion thymectomy in myasthenia patients.
However, the available data suggest that repeat surgery
for patients without thymoma is both safe and effective.
Jaretzki reported finding residual thymus tissue in all his
patients undergoing reoperation despite the fact that a
previous thymectomy had been performed and preoper-
ative chest computed tomography was deemed normal or
inconclusive.
4
There were no operative deaths in these
patients. Some clinical improvement was noted in 87.5%
of patients. No patient’s condition worsened. Complete
remission was seen in 12.5% of patients.
References
1. Blalock A, Mason MF, Morgan HJ, Riven SS. Myasthenia
gravis and tumors of the thymic region. Ann Surg
1939;110:544–61.
2. Osserman KE. Myasthenia gravis. New York: Grune and
Stratton; 1958. p. 79–86.
3. Drachman DB. Myasthenia gravis. N Engl J Med
1978;298:136–42,186–93.
4. Jaretzki A, Penn AS, Younger DS, et al. “Maximal” thymec-
tomy for myasthenia gravis. J Thorac Cardiovasc Surg
1988;95:747–57.
5. Blossum GB, Erstoff RM, Howells GA, et al. Thymectomy
for myasthenia gravis. Arch Surg 1993;128:855–62.
6. Nussbaum MS, Rosenthal GJ, Samaha FJ, et al. Management
of myasthenia gravis by extended thymectomy with anterior
mediastinal dissection. Surgery 1992;112:681–8.
7. Olanow CW, Wechsler AS, Roses AD. A prospective study of
thymectomy and serum acetylcholine receptor antibodies
in myasthenia gravis. Ann Surg 1982;196:113–21.
8. Detterbeck FC, Scott WW, Howard JF Jr, et al. One hundred
consecutive thymectomies for myasthenia gravis. Ann
Thorac Surg 1996;62:242–5.
9. Frist WH, Thirumalai S, Doehring CB, et al. Thymectomy
for the myasthenia gravis patient: factors influencing
outcome. Ann Thorac Surg 1994;57:334–8.
10. Cooper JD, Al-Jilaihawa N, Pearson FG, et al. An improved
technique to facilitate transcervical thymectomy for myas-
thenia gravis. Ann Thorac Surg 1988;45:242–7.
11. Crucitti F, Doglietto GB, Bellantone R, et al. Effects of
surgical treatment in thymoma with myasthenia gravis: our
experience in 103 patients. J Surg Oncol 1992;50:43–6.
12. Buckingham JM, Howard FM Jr, Bernatz PE, et al. The
value of thymectomy in myasthenia gravis. Ann Surg
1993;184:453–8.
13. Lanska DJ. Indications for thymectomy in myasthenia
gravis. Neurology 1990;40:1828–9.
14. Jaretzki A III, Wolff M. “Maximal” thymectomy for myas-
thenia gravis. Surgical anatomy and operative results. J
Thorac Cardiovasc Surg 1988;96:711–76.
15. Masaoka A, Nagaoka Y, Kotake Y. Distribution of thymic
tissue at the anterior mediastinum: current procedures in
thymectomy. J Thorac Cardiovasc Surg 1975;70:747–54.
16. Fukai I, Funato Y, Mizuno T, et al. Distribution of thymic
tissue in the anterior mediastinal adipose tissue. J Thorac
Cardiovasc Surg 1991;101:1099–102.
17. Ashour M. Prevalence of ectopic thymic tissue in myasthe-
nia gravis and its clinical significance. J Thorac Cardiovasc
Surg 1995;109:632–5.
18. Wec hsler AS. Surgical management of myasthenia gravis. In:
Sabiston DC Jr, Spencer FC, editors. Surgery of the chest.
6th ed. Philadelphia (PA): W.B. Saunders; 1995. p. 1100–22.
19. Goti P, Spinelli A, Marconi G, et al. Comparative effects of
plasma exchange and pyridostigmine on respiratory muscle
strength and breathing patterns in patients with myasthenia
gravis. Thorax 1995;50:1080–6.
20. Krucylak PE, Naunheim KS. Preoperative preparation and
anesthetic management of patients with myasthenia gravis.
Semin Thorac Cardiovasc Surg 1999;11:47–53.
21. Drachman DB. Myasthenia gravis. N Engl J Med
1994;330:1791–810.
22. d’Empaire G, Hoaglin DC, Perlo VP, et al. Effect of
prethymectomy plasma exchange on postoperative respira-
tory function in myasthenia gravis. J Thorac Cardiovasc
Surg 1985;89:592–6.
23. Leventhal SR, Orkin FK, Hirsh RA. Prediction of the need
for postoperative mechanical ventilation in myasthenia
gravis. Anesthesiology 1980;53:26–30.
Surgery for Myasthenia Gravis
/
415
24. Eisenkraft JB, Papatestas AE, Kahn CH, et al. Predicting the
need for postoperative mechanical ventilation in myasthe-
nia gravis. Anesthesiology 1986;65:79–82.
25. Grant RP, Jenkins LC. Prediction of the need for postopera-
tive mechanical ventilation in myasthenia gravis: thymec-
tomy compared to other surgical procedures. Can Anaesth
Soc J 1982;29:112–6.
26. Baraka A. Anesthesia and critical care of thymectomy for
myasthenia gravis. Chest Surg Clin N Am 2001;11:337–61.
27. Itoh H, Shibata K, Yoshida M, Yamamoto K.
Neuromuscular monitoring at the obiculari oculi may
oversestimate the blockade in myasthenic patients.
Anesthesiology 2000;93:1194–7.
28. Eisenkraft JB, Book WJ, Mann SM, et al. Resistance to
succinylcholine in myasthenia gravis: a dose-response
study. Anesthesiology 1988;69:760–3.
29. Abel M, Eisenkraft JB, Paten N. Response to suxametho-
nium in a myasthenic patient during remission.
Anesthesiology 1991;46:30–2.
30. Vacanti CA, Ali HH, Schweiss JF, Scott RP. The response of
myasthenia gravis to atracurium. Anesthesiology
1985;62:692–4.
31. Mann R, Blobner M, Jelen-Esselborn S, et al. Preanesthetic
train-of-four fade predicts the atracurium requirement of
myasthenia gravis patients. Anesthesiology 2000;93:346–50.
32. Nilsson E, Meretoya DA. Vecuronium dose-reponse and
maintentance requirements in patients with myasthenia
gravis. Anesthesiology 1990;73:28–32.
33. Burgess FW, Wilcosky B Jr. Thoracic epidural anesthesia for
transsternal thymectomy in myasthenia gravis. Anesth
Analg 1989;69:529–31.
34. Kirsch JR, Diringer MN, Borel CO, et al. Preoperative
lumbar epidural morphine improves postoperative analge-
sia and ventilatory function after transsternal thymectomy
in patients with myasthenia gravis. Crit Care Med
1991;19:1474–9.
35. Crile G Jr. Thymectomy through the neck. Surgery
1966;59:213–5.
36. Kark AE, Kirschner PA. Total thymectomy by the transcer-
vical approach. Br J Surg 1971;58:323–6.
37. Ferguson MK. Transcervical thymectomy. Chest Surg Clin
N Am 1996;6:105–15.
38. Deeb ME, Brinster CJ, Kucharzuk J, et al. Expanded indica-
tions for transcervical thymectomy in the management of
anterior mediastinal masses. Ann Thorac Surg
2001;72:208–11.
39. Uchiyama A, Shimizu S, Murai H, et al. Infrasternal medi-
astinoscopic thymectomy in myasthenia gravis: surgical
results in 23 patients. Ann Thorac Surg 2001;72:1902–5.
40. LoCicero J. The combined cervical and partial sternotomy
approach for thymectomy. Chest Surg Clin N Am
1996;6:85–93.
41. Kaiser LR. Thoracoscopic resection of mediastinal tumors
and the thymus. Chest Surg Clin N Am 1996;6:41–52.
42. Yim APC, Kay RLC, Issat MB, Ng SK. Video-assisted thora-
coscopic thymectomy for myasthenia gravis. Semin Thorac
Cardiovasc Surg 1999;11:65–73.
43. Mack MJ, Landreneau RJ, Yim AP, et al. Results of video-
assisted thymectomy in patients with myasthenia gravis. J
Thorac Cardiovasc Surg 1996;112:1352–9.
44. Kas J, Kiss D, Simon V, et al. Decade-long experience with
surgical therapy of myasthenia gravis: early complications
of 324 transsternal thymectomies. Ann Thorac Surg
2001;72:1691–7.
45. DeFilippi VJ, Richman DP, Ferguson MK. Transcervical
thymectomy for myasthenia gravis. Ann Thorac Surg
1994;57:194–7.
46. Meyers BF, Cooper JD. Transcervical thymectomy for myas-
thenia gravis. Ferguson MK, section editior. General
thoracic experts’ techniques. CTSNet experts’ techniques.
Cardiothoracic Surgery Network. Available at:
/>47. Roberts PF, Venuta F, Rendina E, et al. Thymectomy in the
treatment of ocular myasthenia gravis. J Thorac Cardiovasc
Surg 2001;122:562–8.
48. Budde JM, Morris CD, Gal AA, et al. Predictors of outcome
in thymectomy for myasthenia gravis. Ann Thorac Surg
2001;72:197–202.
49. Abt PL, Patel HJ, Marsh A, Schwartz SI. Analsysis of
thymectomy for myasthenia gravis in older patients: a 20-
year single institution experience. J Am Coll Surg
2001;192:459–64.
50. Sanders DB, Kaminski HJ, Jaretzki A, Phillips LH.
Thymectomy for myasthenia gravis in older patients. J Am
Coll Surg 2001;193:340–1.
416
/ Advanced Therapy in Thoracic Surgery
417
CHAPTER
34
MANAGEMENT OF GERM CELL
TUMORS OF THE M
EDIASTINUM
ISAN CHEN,
MD
CHRISTOPHER L
OGOTHETIS, MD
Germ cell tumors (GCTs) comprise a clinically and
morphologically diverse group of tumors. The name
stems from its origin in the primordial germ cells. More
than 90% of the malignant GCTs arise from the testis;
occasionally (in 5 to 10%) they arise from extragonadal
sites, along the body’s midline, from the cranium to the
presacral area. This midline corresponds to the embrio-
logic urogenital ridge, and it is presumed that extrago-
nadal GCT originates from the malignant transformation
of germ cells that abnormally migrated during the
embriogenesis.
1,2
GCTs affect mainly young adults, and its
natural history, biological behavior, and prognostic
factors are now well established.
The treatment of GCT is based on histology, clinical
stage, primary site of the tumor, and well-defined prog-
nostic factors.
3
It is a highly curable malignancy when
properly treated, although primary mediastinal nonsemi-
nomatous GCT entails a worse prognosis.
The management of this disease has improved
dramatically over the last 20 years, since the introduction
of cisplatin-containing combination chemotherapy in
advanced disease. Delays in the diagnosis of GCT have
significant impact on the prognosis, stage, and outcome
of this disease; therefore, heightened clinical suspicion
for GCT in patients with anterior mediastinal masses is
important.
4,5
Primary mediastinal GCTs have features that are
histologically and serologically similar to their gonadal
counterparts. They share the same chromosomal marker
in the form of an isochromosome of the short arm of
chromosome 12, i(12p), or increased 12p genetic mater-
ial. Although similarities exist in histology and genotype,
there are also clinically significant differences, such as the
presence of higher rate of pure choriocarcinoma and
endodermal sinus tumors in the mediastinal GCT rela-
tive to testicular GCTs. The greatest difference of all is the
significantly worse prognosis of patients with true
extragonadal GCTs when compared with patients with
primary testicular germ cell cancer. In fact, nonsemino-
matous GCT with mediastinal primary is classified as a
poor prognosis subgroup in the 1997 International Germ
Cell Consensus Classification (IGCCC) with 5-year
progression-free survival of 41% and overall survival of
48% (Table 34-1).
3
Incidence and Epidemiology
An estimated 7,200 new cases of GCT with 400 disease-
related deaths were reported in the United States in
2001.
6
Fewer than 10% of GCTs arise from extragonadal
primary site.
7
Only 2 to 5% percent of GCTs have medi-
astinum as their primary site. GCTs account for 10 to
15% of all primary mediastinal tumors. It is, with
retroperitoneum, the most common site of extragonadal
GCT, accounting for 50 to 70% in some series.
8
Testicular GCTs occur more frequently in young adult
Caucasians, with reported ratios between whites and
African-Americans of approximately 5:1 to 4:1.
9
In
contrast, mediastinal GCTs occur equally in all races.
2
Mediastinal GCTs affect more commonly patients in
their third decade of life, but they can occur in patients as
old as 60 years of age. In the adult population, there is no
gender predilection for the occurrence of teratomas. In
contrast, up to 90% of malignant GCTs occur in males.
Extragonadal GCTs occur with equal distribution in both
genders in the pediatric population.
10
Klinefelter’s syndrome is associated with nonsemino-
matous GCTs arising in the mediastinum. The 47, XXY